Abstract
Pathogenic Leptospira spp. is the etiological agent of leptospirosis. The high diversity among Leptospira species provides an array to look for important mediators involved in pathogenesis. Toxin-antitoxin (TA) systems represent an important survival mechanism on stress conditions. vapBC modules have been found in nearly one thousand genomes corresponding to about 40% of known TAs. In the present study, we investigated TA profiles of some strains of Leptospira using a TA database and compared them through protein alignment of VapC toxin sequences among Leptospira spp. genomes. Our analysis identified significant differences in the number of putative vapBC modules distributed in pathogenic, saprophytic, and intermediate strains: four in L. interrogans, three in L. borgpetersenii, eight in L. biflexa, and 15 in L. licerasiae. The VapC toxins show low identity among amino acid sequences within the species. Some VapC toxins appear to be exclusively conserved in unique species, others appear to be conserved among pathogenic or saprophytic strains, and some appear to be distributed randomly. The data shown here indicate that these modules evolved in a very complex manner, which highlights the strong need to identify and characterize new TAs as well as to understand their regulation networks and the possible roles of TA systems in pathogenic bacteria.
Keywords: Toxin-antitoxin, VapBC, VapC, Leptospira
1. Introduction
Leptospirosis is caused by pathogenic spirochetes of the genus Leptospira. It is a zoonotic disease affecting humans and a wide range of animals worldwide with significant impact. The genus Leptospira comprises saprophytic and pathogenic species (family Leptospiraceae, order Spirochaetales) and were named based on their spiral shape. They are mobile and measure 6 to 20 μm in length by 0.1 μm in diameter [1,2]. The most serious manifestation of pathogenic leptospirosis results in a syndrome known as Weil’s disease, which is characterized by a devastating kidney and liver failure [2,3].
Based on 16S rRNA phylogeny, DNA-DNA hybridization, pathogenicity, virulence, and in vitro growth characteristics, the genus Leptospira includes at least 21 species arranged in three groups: pathogenic, intermediate pathogenic, and non-pathogenic or saprophytic [4,5], which in turn are divided into over 200 serovars defined by agglutination with homologous antigen [3]. Group I pathogens produce disease in people, mostly severe, caused by bacteria belonging to the evolutionarily-related species L. interrogans, L. kirschneri, and L. noguchii. Group II intermediate pathogens grow better in culture and cause predominantly mild self-resolving illnesses without fatal complications. Group III saprophytic Leptospira are free-living environmental microorganisms [4].
Toxin-antitoxin (TA) systems represent an important mechanism of bacteria survival during stress conditions, such as starvation or antibiotic pressure. TA operons are widely distributed among bacteria and are characterized by a pair of genes encoding for a stable toxin and an unstable antitoxin. The antitoxin acts as an antagonistic regulator that prevents the toxin from exerting its toxicity, except when some environmental conditions determine a decrease in antitoxin concentration, exposing the cell to toxic effects, leading to a reversible cessation of growth [6,7]. TA systems have been involved in potentially harmful aspects of an infection, such as antimicrobial resistance, persistence, and biofilm formation [8]. In some pathogens, including L. interrogans [9], Mycobacterium tuberculosis [10,11], Escherichia coli [12], Haemophilus influenzae [13], and Salmonella enterica [14], they participate in the bacterial physiology during the infection.
Experimental data of toxin-antitoxin and in silico analysis on prokaryotic chromosomes have shown the widespread presence of TA modules among bacteria with few exceptions, like the spirochetes Borrelia burgdorferi, Treponema pallidum, and other obligated host associated bacteria [15,16,17,18]. TAs are classified into types based on the nature—nucleic acid or protein—of their toxin and antitoxin and on the kind of interaction between them. To date, six types of TA systems have been described [19,20], with the type II system being the most abundant. Type II TA systems are composed of an inhibitory proteic antitoxin that interacts with the toxic protein [21].
Type II TA modules are grouped into different families according to the toxin structure and protein sequence similarity [6,22]. VapBC is the main TA type II family, with about 1900 VapBC modules identified in 960 genomes, corresponding to 30–40% of the known TA modules (URL:http://bioinfo-mml.sjtu.edu.cn/TADB/) [23]. They are classified based on the presence of a PIN domain (PilT N-terminal) VapC, which is presumed to confer ribonuclease activity to the toxin. Like VapCs, toxins of the RelBE, MazEF, and HicAB families have been described as endoribonucleases, also called interferase RNAs [20,24], which hydrolyze different and specific RNA targets. The RelE toxin cleaves mRNA in the ribosomal A site with high codon specificity [25]. The HicA toxins also cleave mRNA, but independently of the ribosome [26]. Toxins of MazF family have been shown to cleave mRNA [24], rRNA [27], and also tRNA [28]. Most of the few characterized VapC toxins have been shown to exert their activities on the initiator tRNA in a very specific manner. Up until now, the initiator tRNA (tRNAfMet) has been the specific biological target found in the largest number of bacterial species: Leptospira interrogans [29], Salmonella enterica [30], Shigella flexneri [30], and Haemophilus influenzae [31]. Other specific tRNA, such as tRNACys-GCA, tRNALeu-CAG, tRNASer-TGA, CGA, and tRNATrp-CCA, have been identified as substrates of VapCs of Mycobacterium tuberculosis [32]. Additionally, two VapCs of M. tuberculosis cleave 23S rRNA at the sarcin-ricin loop (SRL) [32,33].
The substantial progress of genomic and proteomic studies has allowed for high-throughput studies of leptospiral proteins aimed mainly at the identification of potential antigens for vaccine and diagnostic development [34]. Most of the studies on the genome of leptospiral species have focused mainly on the search for surface-exposed antigens or important proteins to vital metabolic routes [35,36,37,38]. More recently, TA modules have achieved more prominence [39,40]. The high diversity among Leptospira species makes their studies very complex and challenging, providing a rich ground to look for specific mediators with importance for bacterial virulence and pathogenesis. In this work, we have investigated the diversity among toxin-antitoxin type II systems among Leptospira species, with a focus on the toxin of the VapBC family. We searched for and compared the putative TA operons of the whole sequenced genomes of pathogenic L. interrogans and L. borgpetersenii, intermediate-pathogenic L. licerasiae, and saprophytic L. biflexa strains within 20 Leptospira ssp., classified according to the pathogenicity phylogenetic tree [5]. This extensive analysis aimed to study the conservation of these TA modules and to evaluate a possible correlation of their presence in the three phenotypes of pathogenicity.
2. Materials and Methods
2.1. Analysis of Type II TA Modules
The analyses of bacterial type II toxin-antitoxin loci were performed using TADB 2.0 database (http://bioinfo-mml.sjtu.edu.cn/TADB2/) [23]. To explore the whole set of putative TA operons of each Leptospira we have used the tool TAfinder (http://202.120.12.133/TAfinder/TAfinder.php) from TADB by entering the Refseq Accession Number of the following bacteria: L. interrogans serovar Copenhageni strain Fiocruz L1-130 (Refseq NC_005823), L. interrogans serovar Lai strain 56601 (Refseq NC_004342), L. borgpetersenii serovar Hardjo-bovis strain JB197 (Refseq NC_008510) and strain L550 (Refseq NC_008508), L. biflexa serovar Patoc strain Patoc 1 (Ames) (Refseq NC_010842) and strain Patoc 1 (Paris) (Refseq NC_010602). The set of TA modules of L. licerasiae serovar Varillal strain VAR010 was obtained from the manuscript of Ricaldi et al. [39].
2.2. Evaluation of the Presence of vapBC among Leptospira spp.
To evaluate the presence of putative vapBC operons in the 20 Leptospira spp. comprised in this study, we used the program Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi), specifically the protein-protein BLAST tool (BLASTp), to compare the protein sequences of the putative VapC toxins. The VapC query sequences were obtained directly from the TADB database or through locus identification and numbered according to the order of appearance on each genome (base pair numbering) of L. interrogans serovar Copenhageni strain Fiocruz L1-130 (LIC), L. borgpetersenii serovar Hardjo-bovis strain JBL97 (LBJ), L. licerasiae serovar Varillal strain VAR 010 (LEP1GSC185), and L. biflexa serovar Patoc strain Patoc 1 (Ames) (LBF). The subject taxonomic ID (taxid) of the Leptospira used to browse homologous proteins were: Leptospira interrogans serovar Copenhageni str. Fiocruz L1-130 (taxid:267671), L. interrogans serovar Lai str. Lai (taxid:1049911), L. borgpetersenii serovar Hardjo-bovis str. JB197 (taxid:355277), L. borgpetersenii serovar Hardjo-bovis str. L550 (taxid:355276), L. biflexa serovar Patoc strain ‘Patoc 1 (Paris)’ (taxid:456481), L. biflexa serovar Patoc strain ‘Patoc 1 (Ames)’ (taxid:355278), Leptospira (taxid:171), L. interrogans (taxid:173), L. kirschneri (taxid:29507), L. noguchii (taxid:28182), L. borgpetersenii (taxid:174), L. weilii (taxid:28184), L. santarosai (taxid:28183), L. alexanderi (taxid:100053), L. alstoni (taxid:28452), L. kmetyi (taxid:408139), L. wolffii (taxid:409998), L. licerasiae (taxid:447106), L. inadai (taxid:29506), L. fainei (taxid:48782), L. broomii (taxid:301541), L. wolbachii (taxid:29511), L. meyeri (taxid:29508), L. biflexa (taxid:172), L. vanthielii (taxid:293085), L. terpstrae (taxid:293075), and L. yanagawae (taxid:293069).
“Conservation Value” Index
In order to easily visualize the conservation between two VapCs sequences, we established the “Conservation value” (C-value) formed from values provided by BLASTp analyses, expressed as a frequency between 0 and 1. For each query protein, the C-value was calculated as follows:
| (1) |
+ where “i” is the amino acid identity, “p” refers to a positive or similar amino acid, and “c” is the amino acid coverage sequence, given by the ratio between the length of the highest scoring matching sequence and the query length. Arbitrarily, we considered sequences with the result of “p” and “c” > 0.5 as having a considerable degree of “C-value”.
2.3. Protein Alignment
Alignment between two amino acid sequences and identity (%) were performed using the tool Global Align from BLAST, and multiple sequences alignment was computed using COBALT (Constraint-based Multiple Alignment Tool) (https://www.ncbi.nlm.nih.gov/tools/cobalt/re_cobalt.cgi), which considers the proteic conserved domain and sequence similarity information.
3. Results
We have been working on the genomics and proteomics of L. interrogans serovar Copenhageni strain Fiocruz L1-130 [5,35,41]. Therefore, this strain was chosen as the basis for the comparative studies presented here. Furthermore, we have studied the TA profile of the sequenced genomes of the pathogenic species L. interrogans serovar Lai [37] and two strains of L. borgpetersenii [42], as well as two strains of the saprophytic L. biflexa [43], available in the TADB database. In the case of the intermediate pathogenic L. licerasiae, a detailed TA profile was described in its genome analysis manuscript [39]. Briefly, 28 TA modules were identified, which code for 15 VapBC, one HigAB, one ChpIK, one MazEF, five ArsR/Aha1, one HTH/Aha1, one XRE/COG2856, one RHH/UNK, one RHH/COG2929, and one RHH/DUF497. We have focused this work on the study of the VapBC family because it is the most abundant type II TA family, comprising about 40% of the identified TA modules in bacterial genomes, allowing us to discuss the variability of TA systems in Leptospira. Moreover, data from the International Leptospira Genome Project [5], made it possible to relate TAs of the genome sequences of 20 Leptospira species in order to build up several comparative tables.
3.1. Analysis and Comparison of TA Profiles within Leptospira Species
3.1.1. L. interrogans Serovar Copenhageni Fiocruz L1-130 and L. interrogans Serovar Lai Strain 56601
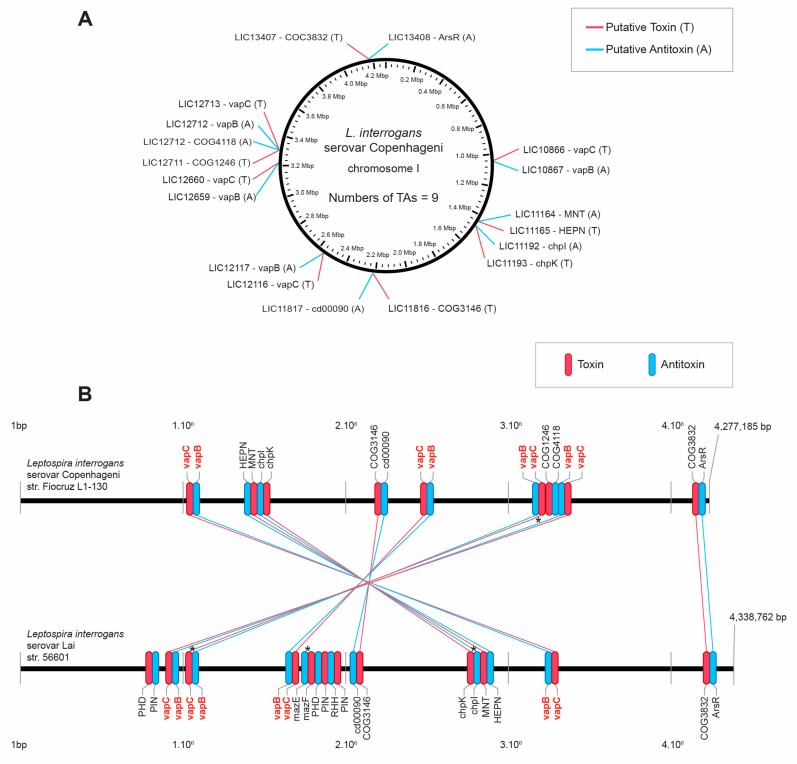
Investigation of type II TA modules in the genome of L. interrogans serovar Copenhageni strain Fiocruz L1-130 and L. interrogans serovar Lai strain 56601 by the TAfinder tool of TADB resulted in the identification of nine modules in the strain Fiocruz L1-130 (Figure 1A) coding for four VapBC, one MazEF (ChpIK), and four TA modules of unclassified families (according TADB), in which the domains are: HEPN/MNT, cd00090/COG3146, COG4118/COG1246, and ArsR/COG3832. The L. interrogans serovar Lai strain 56601 was shown to have all the operons identified in the strain Fiocruz L1-130, except for the TA pair LIC12711/12712. In addition, the serovar Lai strain presented three TA modules, one MazEF, and two extra possible VapBC, inserted in the genome between the modules 4 and 5 of the L. interrogans serovar Copenhageni. The large chromosomal inversion described in the genome of the two L. interrogans strains investigated [44] can be observed by the inverted position of seven TAs of the serovar Copenhageni in relation to serovar Lai, except for the last TA of both chromosomes LIC 13407/13408–LA4258/4259 that are parallelly located (Figure 1B).
Figure 1.
(A) Location map of toxin-antitoxin (TA) type II systems on the chromosome of L. interrogans serovar Copenhageni. (B) Comparison of the TAs sets of L. interrogans serovar Copenhageni and L. interrogans serovar Lai. In this schema, horizontal black lines represent the chromosomes of Copenhageni and Lai serovars. Red and blue lines indicate the amino acid sequence correspondence of toxins and antitoxins, respectively. Identity between linked toxins is greater than 90%. Toxins and antitoxins are identified by family or domains, as they appear in TAfinder. * denotes TA modules that were experimentally characterized and published.
3.1.2. L. borgpetersenii Serovar Hardjo-bovis Strain JB197 and Strain L550
Search by TAfinder and BLASTp comparison identified nine conserved TA modules in both strains of L. borgpetersenii analyzed here. They code for three VapBC, one MazEF, and five TA modules of unclassified families, in which the domains are: HEPN/MNT, PRK13696/cd09981, cd00090/COG3146, RHH/COG2929, and ArsR/COG3832. TAs of both L. borgpetersenii strains are displayed in completely inverted positions in their genomes, as shown in supplementary results (Table S1).
3.1.3. L. biflexa Serovar Patoc Strain Patoc 1 (Ames) and Strain Patoc 1 (Paris)
The same analysis (TAfinder and BLASTp) of TAs of the genomes of L. biflexa resulted in the identification of 23 conserved TA modules. They code for eight VapBC, two MazEF, six RelBE, one phd/doc, and six TA modules of unclassified families, in which the domains are: HEPN/MNT, RHH-COG2929, and four ArsR/COG3832. The TA positionings in the genomes of both strains studied here are fully parallel (Table S2).
3.2. Variability of Amino Acids Sequences of VapCs within Leptospira Strains
Based on the fact that the toxins of TA modules are the elements responsible for the function and substrate specificity, and also for the family classification of these systems, we have focused this study on the distribution and conservation of the VapC toxins, from the VapBC family, which are known to have poor conservation of the primary sequences but high conservation of the PIN domain structural fold [45].
According to the search conducted on TAfinder, the L. interrogans serovar Copenhageni strain Fiocruz L1-130 has four vapBC loci, all on chromosome I, which encode four toxin-antitoxin modules of the VapBC family. Based on the numerical order of the genes in the chromosome, only VapBC-3 (LIC12660–12659) was experimentally characterized and thus considered a bona fide element [29], while the others remain to be elucidated. The alignment of the amino acid sequences of these four VapCs (Figure 2) shows the diversity between their primary sequences, but with conservation of the set of three or four acid residues responsible for coordinating Mg2+ or Mn2+ ions at the catalytic site. The cognate VapB antitoxins partners comprise different types of protein domains (RHH, AbrB, and PHD) and therefore cannot have their primary structures compared.
Figure 2.
Alignment of the VapCs of L. interrogans serovar Copenhageni. Alignment was done using the COBALT tool (Constraint-based Multiple Alignment Tool), which aligns the sequences via considering the amino acid sequence and protein domains. The numbers of VapCs in parentheses are based on the numerical order of the genes in the chromosome and refer to the loci described in Table 1. The highlighted acidic amino acids comprise the residues essential to the catalytic activity and classification within the PIN (PilT N-terminal) domain.
In order to investigate whether the amino acid sequences of the toxins from the VapBC modules are divergent within their own bacterial strain, we have evaluated the VapCs’ identities using the tool “Global Align” for the four VapCs of L. interrogans serovar Copenhageni Fiocruz L1-130, the 15 VapCs L. licerasiae serovar Varillal strain VAR010, and the eight VapCs L. biflexa serovar Patoc 1 (Ames) (Table 1, Figure S1). The analysis of the sequence identities within each of the strains were found to be mostly very low, varying from 13% to 46%, with most of them being around 20%, corroborating the well-known low amino-acid identity among VapC toxins.
Table 1.
Amino acid sequence identities of VapCs within the same Leptospira strains.
| A—L. interrogans Serovar Copenhageni str. Fiocruz L1-130—Identity (%) | B—L. biflexa Serovar Patoc Strain Patoc 1 (Ames)—Identity (%) | ||||||||||||||||
| VapC-1 | VapC-2 | VapC-3 | VapC-4 | VapC-1 | VapC-2 | VapC-3 | VapC-4 | VapC-5 | VapC-6 | VapC-7 | VapC-8 | ||||||
| VapC-1 | 100 | 19 | 23 | 18 | VapC-1 | 100 | 17 | 13 | 24 | 19 | 19 | 27 | 21 | ||||
| VapC-2 | 100 | 19 | 17 | VapC-2 | 100 | 18 | 23 | 19 | 22 | 20 | 17 | ||||||
| VapC-3 | 100 | 26 | VapC-3 | 100 | 27 | 22 | 16 | 16 | 19 | ||||||||
| VapC-4 | 100 | VapC-4 | 100 | 22 | 22 | 22 | 28 | ||||||||||
| VapC-5 | 100 | 36 | 19 | 46 | |||||||||||||
| VapC-6 | 100 | 17 | 38 | ||||||||||||||
| VapC-7 | 100 | 22 | |||||||||||||||
| VapC-8 | 100 | ||||||||||||||||
| C—L. licersiae Serovar Varillal str. VAR 010—Identity (%) | |||||||||||||||||
| VapC-1 | VapC-2 | VapC-3 | VapC-4 | VapC-5 | VapC-6 | VapC-7 | VapC-8 | VapC-9 | VapC-10 | VapC-11 | VapC-12 | VapC-13 | VapC-14 | VapC-15 | |||
| VapC-1 | 100 | 26 | 18 | 19 | 28 | 17 | 22 | 14 | 24 | 18 | 17 | 14 | 23 | 21 | 14 | ||
| VapC-2 | 100 | 13 | 19 | 31 | 21 | 18 | 17 | 19 | 16 | 19 | 17 | 20 | 21 | 17 | |||
| VapC-3 | 100 | 21 | 14 | 17 | 19 | 17 | 20 | 17 | 24 | 17 | 22 | 23 | 17 | ||||
| VapC-4 | 100 | 18 | 21 | 28 | 25 | 21 | 18 | 22 | 25 | 20 | 31 | 25 | |||||
| VapC-5 | 100 | 23 | 18 | 21 | 24 | 20 | 18 | 21 | 21 | 19 | 21 | ||||||
| VapC-6 | 100 | 16 | 18 | 32 | 19 | 16 | 18 | 21 | 21 | 18 | |||||||
| VapC-7 | 100 | 32 | 18 | 19 | 18 | 32 | 18 | 22 | 32 | ||||||||
| VapC-8 | 100 | 23 | 19 | 20 | 100 | 15 | 23 | 100 | |||||||||
| VapC-9 | 100 | 17 | 20 | 23 | 19 | 25 | 23 | ||||||||||
| VapC-10 | 100 | 20 | 19 | 12 | 21 | 19 | |||||||||||
| VapC-11 | 100 | 20 | 20 | 17 | 20 | ||||||||||||
| VapC-12 | 100 | 15 | 23 | 100 | |||||||||||||
| VapC-13 | 100 | 20 | 15 | ||||||||||||||
| VapC-14 | 100 | 23 | |||||||||||||||
| VapC-15 | 100 | ||||||||||||||||
(A) VapCs of L. interrogans serovar Copenhageni Fiocruz L1-130. (B) VapCs of L. biflexa serovar Patoc 1 (Ames). (C) VapCs of L. licerasiae serovar Varillal strain VAR010.
3.3. Distribution of VapCs of Pathogenic, Intermediate, and Saprophytic Leptospira Strains
In order to study how the amino acid sequences of VapCs are conserved among leptospiral pathogenicity groups (pathogenic, intermediate, and saprophytic) and to infer functional and evolutionary relationships between sequences, we have carried out an extensive study using the BLAST tool, in which we submitted the identified VapCs sequences of two pathogenic strains (L. interrogans and L. borgpetersenii), one intermediate strain (L. licerasiae), and one saprophytic strain (L. biflexa) to compare with sequence database of the Leptospira taxid for each of the species described in the methods section. To enable this comparison, we developed a parameter to indicate “conservation” between sequences named C-value (Cv) (see the methods section), which takes into account three results provided by BLAST analysis: the coverage (“c”), the number of identical amino acids (“i”), and also the number of conserved amino acids designed as positive (“p”), which varies from 0 to 1. We did not use the e-value given by BLAST because it varies according to protein size and it does not consider conservative mutations, which are known to be important to structure and function, as previously indicated for VapC-3 [29]. We present here the results comparing the four strains mentioned above. All tables were colored to indicate, through color intensity, the degree of conservation among the toxins evaluated by C-value. Results for L. interrogans serovar Copenhageni are shown in Table 2. Detailed results for the remaining three strains are shown in Tables S3–S5. In addition to the C-values, the results also include the “i”, “p”, and “c” values. It is important to consider that BLAST searches for similar sequences based on a large number of entries from several isolates and that each of the analyzed species have (for the most part) many entries too, and that some of the entries of specific strains do not have the serovar group defined. With that said, it should be noted that when one cell of the Table 2, Table 3, Table 4 and Table 5 shows that a particular VapC is present in a given species (e.g., L. interrogans or L. noguchii), it is not necessarily present in all members of this species, but in one or some isolates of them.
Table 2.
Basic Local Alignment Search Tool (BLAST) analysis of VapCs of L. interrogans serovar Copenhageni Fiocruz L1-130.
| VapC of L. interrogans Serovar Copenhageni str. Fiocruz L1-130 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptospira spp. | LIC10866 VapC-1 | LIC12116 VapC-2 | LIC12660 VapC-3 | LIC12713 VapC-4 | |||||||||||||
| Identity (%) | Positives (%) | Cover (%) | C-value | Identity (%) | Positives (%) | Cover (%) | C-value | Identity (%) | Positives (%) | Cover (%) | C-value | Identity (%) | Positives (%) | Cover (%) | C-value | ||
| Pathogenic |
L. interrogans
L. interrogans |
100 | 100 | 100 | 1.00 | 100 | 100 | 100 | 1.00 | 100 | 100 | 100 | 1.00 | 100 | 100 | 100 | 1.00 |
| L. kirschneri | 99 | 99 | 100 | 0.99 | 97 | 98 | 100 | 0.98 | 50 | 66 | 97 | 0.56 | 97 | 97 | 100 | 0.97 | |
| L. noguchii | - | - | - | - | 97 | 99 | 100 | 0.98 | 44 | 64 | 97 | 0.52 | - | - | - | - | |
| L. borgpetersenii | - | - | - | - | - | - | - | - | 48 | 68 | 97 | 0.56 | - | - | - | - | |
| L. weilii | - | - | - | - | 87 | 95 | 97 | 0.88 | 90 | 95 | 100 | 0.93 | 48 | 66 | 100 | 0.57 | |
| L. santarosai | - | - | - | - | 83 | 90 | 100 | 0.87 | 66 | 83 | 100 | 0.75 | 64 | 75 | 100 | 0.70 | |
| L. alexanderi | 93 | 95 | 100 | 0.94 | 85 | 94 | 100 | 0.90 | 89 | 94 | 100 | 0.92 | - | - | - | - | |
| L. alstoni | - | - | - | - | 86 | 95 | 100 | 0.91 | 90 | 96 | 100 | 0.93 | 61 | 73 | 97 | 0.65 | |
| L. kmetyi | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Intermediary | L. wolffii | - | - | - | - | - | - | - | - | 44 | 68 | 97 | 0.54 | 45 | 62 | 100 | 0.54 |
| L. licerasiae | - | - | - | - | - | - | - | - | 70 | 87 | 99 | 0.78 | 44 | 60 | 100 | 0.52 | |
| L. inadai | - | - | - | - | - | - | - | - | 63 | 84 | 99 | 0.73 | 65 | 78 | 100 | 0.72 | |
| L. fainei | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| L. broomii | - | - | - | - | - | - | - | - | - | - | - | - | 63 | 75 | 100 | 0.69 | |
| Saprophytic | L. wolbachii | - | - | - | - | - | - | - | - | 66 | 83 | 99 | 0.74 | 64 | 75 | 100 | 0.70 |
| L. meyeri | 44 | 70 | 95 | 0.54 | - | - | - | - | 66 | 84 | 99 | 0.74 | 62 | 74 | 100 | 0.68 | |
| L. biflexa | - | - | - | - | - | - | - | - | 62 | 82 | 99 | 0.71 | 28 | 55 | 93 | 0.39 | |
| L. vanthielii | 46 | 69 | 95 | 0.55 | - | - | - | - | 51 | 69 | 98 | 0.59 | 61 | 75 | 100 | 0.68 | |
| L. terpstrae | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| L. yanagawae | - | - | - | - | - | - | - | - | 48 | 70 | 98 | 0.58 | - | - | - | - | |
Conservation values (C-values) were expressed as a frequency between 0 and 1. The colors indicate:  very highly conserved (0.85 ≤ C-value ≤ 1.0);
very highly conserved (0.85 ≤ C-value ≤ 1.0);  highly conserved (0.7 ≤ C-value ≤ 0.84);
highly conserved (0.7 ≤ C-value ≤ 0.84);  moderately conserved (0.4 ≤ C-value ≤ 0.69);
moderately conserved (0.4 ≤ C-value ≤ 0.69);  poorly conserved (C-value ≤ 0.39);
poorly conserved (C-value ≤ 0.39);  no hits. Positive and cover values below 50% were not included.
no hits. Positive and cover values below 50% were not included.
Table 3.
Conservation analysis of VapCs of L. borgpetersenii serovar Hardjo-bovis strain JB197.
| VapC of L. borgpetersenii Serovar Hardjo-bovis str. JB197—Conservation value (C-value) | ||||
|---|---|---|---|---|
| Leptospira spp. | LBJ_0624 VapC-1 | LBJ_0764 VapC-2 | LBJ_2077 VapC-3 | |
| Pathogenic |
L. interrogans
L. interrogans |
0.95 | 0.90 | 0.39 |
| L. kirschneri | 0.49 | 0.89 | 0.38 | |
| L. noguchii | 0.93 | - | 0.39 | |
| L. borgpetersenii | 1.00 | 1.00 | 1.00 | |
| L. weilii | 0.94 | 0.89 | 0.96 | |
| L. santarosai | 0.49 | 0.87 | 0.40 | |
| L. alexanderi | 0.52 | 0.48 | - | |
| L. alstoni | 0.95 | 0.88 | 0.44 | |
| L. kmetyi | - | - | - | |
| Intermediary | L. wolffii | 0.49 | - | - |
| L. licerasiae | 0.49 | - | 0.43 | |
| L. inadai | - | - | 0.38 | |
| L. fainei | - | - | - | |
| L. broomii | - | - | - | |
| Saprophytic | L. wolbachii | 0.51 | - | 0.38 |
| L. meyeri | 0.83 | - | - | |
| L. biflexa | 0.53 | - | 0.38 | |
| L. vanthielii | 0.82 | 0.47 | 0.38 | |
| L. terpstrae | - | - | 0.35 | |
| L. yanagawae | 0.51 | - | - | |
Conservation values (C-values) were expressed as a frequency between 0 and 1. The colors indicate:  very highly conserved (0.85 ≤ C-value ≤ 1.0);
very highly conserved (0.85 ≤ C-value ≤ 1.0);  highly conserved (0.7 ≤ C-value ≤ 0.84);
highly conserved (0.7 ≤ C-value ≤ 0.84);  moderately conserved (0.4 ≤ C-value ≤ 0.69);
moderately conserved (0.4 ≤ C-value ≤ 0.69);  poorly conserved (C-value ≤ 0.39);
poorly conserved (C-value ≤ 0.39);  no hits. Positive and cover values below 50% were not included.
no hits. Positive and cover values below 50% were not included.
Table 4.
Conservation analysis of VapCs of L. licerasiae serovar Varillal strain VAR010.
| VapC of L. licerasiae Serovar Varillal str. VAR 010—Conservation value (C-value) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leptospira spp. | LEP1GSC185_0307—VapC-1 | LEP1GSC185_0418—VapC-2 | LEP1GSC185_0630—VapC-3 | LEP1GSC185_1922—VapC-4 | LEP1GSC185_2251—VapC-5 | LEP1GSC185_2580—VapC-6 | LEP1GSC185_3193—VapC-7 | LEP1GSC185_3530—VapC-8 * | LEP1GSC185_3550—VapC-9 | LEP1GSC185_3553—VapC-10 | LEP1GSC185_3557—VapC-11 | LEP1GSC185_3561—VapC-13 | LEP1GSC185_3566—VapC-14 | ||
| Pathogenic |
L. interrogans
L. interrogans |
0.48 | 0.36 | 0.66 | - | 0.53 | 0.80 | 0.54 | 0.64 | 0.78 | - | 0.87 | 0.82 | 0.92 | |
| L. kirschneri | - | - | 0.66 | - | 0.53 | 0.41 | 0.41 | 0.64 | 0.56 | - | 0.82 | - | 0.90 | ||
| L. noguchii | - | - | 0.65 | - | 0.20 | 0.46 | 0.42 | 0.63 | - | - | 0.54 | 0.85 | 0.92 | ||
| L. borgpetersenii | - | - | 0.48 | - | 0.20 | 0.79 | 0.46 | 0.67 | 0.55 | - | - | - | 0.92 | ||
| L. weilii | - | - | 0.48 | - | 0.67 | 0.80 | 0.45 | 0.66 | 0.93 | 0.91 | 0.86 | - | 0.77 | ||
| L. santarosai | - | 0.37 | 0.46 | - | 0.54 | - | 0.42 | 0.63 | 0.89 | - | 0.54 | 0.94 | 0.72 | ||
| L. alexanderi | - | - | 0.65 | - | 0.20 | 0.80 | 0.41 | 0.64 | 0.78 | - | 0.86 | - | - | ||
| L. alstoni | 0.65 | 0.40 | 0.48 | - | 0.50 | 0.79 | 0.75 | 0.66 | 0.78 | - | 0.87 | - | 0.77 | ||
| L. kmetyi | - | - | - | - | - | - | - | - | - | - | - | - | 0.92 | ||
| Intermediary | L. wolffii | 0.38 | 0.78 | - | 0.81 | 0.80 | 0.42 | 0.41 | - | 0.53 | - | 0.96 | - | 0.97 | |
| L. licerasiae | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| L. inadai | 0.77 | 0.38 | - | - | 0.55 | - | 0.39 | 0.87 | 0.88 | - | 0.94 | - | 0.91 | ||
| L. fainei | - | - | - | - | - | - | - | - | - | - | 0.93 | - | - | ||
| L. broomii | - | 0.41 | - | - | 0.56 | - | - | 0.40 | - | - | - | - | - | ||
| Saprophytic | L. wolbachii | - | 0.39 | - | - | 0.52 | 0.42 | 0.42 | 0.64 | 0.85 | - | 0.81 | - | 0.37 | |
| L. meyeri | - | 0.38 | 0.65 | - | 0.54 | 0.78 | 0.41 | 0.64 | 0.85 | - | 0.55 | - | 0.73 | ||
| L. biflexa | - | 0.42 | - | - | 0.37 | 0.77 | 0.40 | 0.66 | 0.80 | - | - | - | 0.73 | ||
| L. vanthielii | - | 0.39 | 0.61 | - | 0.53 | 0.78 | 0.41 | 0.65 | 0.56 | - | 0.54 | 0.75 | 0.37 | ||
| L. terpstrae | - | - | - | - | - | - | 0.41 | 0.66 | - | - | - | - | - | ||
| L. yanagawae | - | - | - | - | - | 0.42 | - | - | 0.56 | - | 0.55 | 0.74 | - | ||
Conservation values (C-values) were expressed as a frequency between 0 and 1. The colors indicate:  very highly conserved (0.85 ≤ C-value ≤ 1.0);
very highly conserved (0.85 ≤ C-value ≤ 1.0);  highly conserved (0.7 ≤ C-value ≤ 0.84);
highly conserved (0.7 ≤ C-value ≤ 0.84);  moderately conserved (0.4 ≤ C-value ≤ 0.69);
moderately conserved (0.4 ≤ C-value ≤ 0.69);  poorly conserved (C-value ≤ 0.39);
poorly conserved (C-value ≤ 0.39);  no hits. Positive and cover values below 50% were not included. * VapCs 8, 12, and 15 (LEP1GSC185:2580, LEP1GSC185:3559, and LEP1GSC185:3880) share the same amino-acid sequence. VapC-12 and VapC-15 were not shown here.
no hits. Positive and cover values below 50% were not included. * VapCs 8, 12, and 15 (LEP1GSC185:2580, LEP1GSC185:3559, and LEP1GSC185:3880) share the same amino-acid sequence. VapC-12 and VapC-15 were not shown here.
Table 5.
Conservation analysis of VapCs of L. biflexa serovar Patoc 1 (Ames).
| VapC of L. biflexa serovar Patoc strain Patoc 1 (Ames)—Conservation value (C-value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Leptospira spp. | LBF_0418 VapC-1 | LBF_2142 VapC-2 | LBF_ 2175 VapC-3 | LBF_2183 VapC-4 | LBF_2185 VapC-5 | LBF_2276 VapC-6 | LBF_2813 VapC-7 | LBF_3285 VapC-8 | |
| Pathogenic |
L. interrogans
L. interrogans |
0.40 | 0.60 | - | 0.78 | 0.72 | 0.83 | 0.83 | 0.59 |
| L. kirschneri | 0.40 | - | - | 0.78 | 0.55 | - | 0.83 | 0.73 | |
| L. noguchii | - | 0.61 | - | 0.77 | 0.48 | 0.47 | 0.54 | 0.52 | |
| L. borgpetersenii | 0.35 | - | 0.27 | 0.78 | 0.55 | 0.89 | - | 0.73 | |
| L. weilii | 0.42 | 0.59 | 0.60 | 0.78 | 0.83 | 0.89 | 0.83 | 0.73 | |
| L. santarosai | 0.41 | 0.62 | - | 0.78 | 0.78 | 0.55 | 0.53 | 0.75 | |
| L. alexanderi | 0.35 | 0.59 | 0.60 | 0.77 | 0.72 | 0.88 | 0.84 | 0.72 | |
| L. alstoni | - | - | 0.59 | 0.79 | 0.72 | 0.85 | 0.83 | 0.73 | |
| L. kmetyi | - | - | - | - | - | - | - | - | |
| Intermediary | L. wolffii | - | - | - | - | 0.52 | 0.44 | 0.80 | 0.75 |
| L. licerasiae | 0.44 | - | - | 0.56 | 0.80 | 0.79 | 0.82 | 0.55 | |
| L. inadai | 0.41 | - | - | 0.63 | 0.79 | 0.33 | 0.80 | 0.50 | |
| L. fainei | - | - | - | - | - | - | 0.79 | - | |
| L. broomii | 0.42 | - | - | - | - | - | - | - | |
| Saprophytic | L. wolbachii | 0.40 | 0.94 | 0.99 | 0.88 | 0.92 | 0.45 | 0.87 | 0.92 |
| L. meyeri | 0.43 | 0.95 | 0.96 | 0.96 | 0.84 | 0.97 | 0.52 | 0.91 | |
| L. biflexa | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| L. vanthielii | 0.43 | 0.95 | - | 0.91 | 0.56 | 0.97 | 0.53 | 0.93 | |
| L. terpstrae | - | - | - | 0.91 | - | - | - | - | |
| L. yanagawae | - | 0.95 | 0.97 | - | 0.57 | 0.44 | 0.53 | 0.95 | |
Conservation values (C-values) were expressed as a frequency between 0 and 1. The colors indicate:  very highly conserved (0.85 ≤ C-value ≤ 1.0);
very highly conserved (0.85 ≤ C-value ≤ 1.0);  highly conserved (0.7 ≤ C-value ≤ 0.84);
highly conserved (0.7 ≤ C-value ≤ 0.84);  moderately conserved (0.4 ≤ C-value ≤ 0.69);
moderately conserved (0.4 ≤ C-value ≤ 0.69);  poorly conserved (C-value ≤ 0.39);
poorly conserved (C-value ≤ 0.39);  no hits. Positive and cover values below 50% were not included.
no hits. Positive and cover values below 50% were not included.
3.3.1. L. interrogans Serovar Copenhageni Fiocruz L1-130
BLAST analysis of the four VapC sequences showed that VapC-3, previously described and characterized [29], is the toxin most widely distributed, with high C-value, among the 20 Leptospira species studied, followed by VapC-4 (Table 2). In contrast, VapC-1 amino acid sequence shows high C-value only for the pathogenic species L. kirschneri and L. alexanderi. Interestingly, VapC-2 is the only one of the four toxins that is highly conserved exclusively among most of the pathogenic species with the exception of L. borgpetersenii and L. kmetyi species. It should be noted that these two pathogenic species only showed conservation within the VapCs of L. interrogans serovar Copenhageni Fiocruz L1-130.
In parallel, we evaluated the similarity of these four VapCs among 12 other important human pathogenic bacteria species, such as Mycobacterium tuberculosis and Shigella sonnei (see Supplementary Material—Table S6), but no conclusions could be taken from it. Interestingly, as for the Leptospira species, VapC-3 is the toxin most distributed among investigated bacteria, followed by VapC-4. VapC-1 and VapC-2 showed medium conservation only with P. aeruginosa and M. tuberculosis.
3.3.2. L. borgpetersenii Serovar Hardjo-Bovis Strain JB197
We also analyzed the conservation of the VapCs of pathogenic L. borgpetersenii (Table 3), whose genome was completely sequenced. From the nine TA modules identified by TAfinder, three belong to the VapBC family. VapC1 shows the highest distribution among the species analyzed, concentrating high C-value among the pathogenic species. Similar to VapC-2 of L. interrogans serovar Copenhageni, L. borgpetersenii VapC-2 is highly conserved only among pathogenic species. VapC-3 amino acid sequence is highly conserved only in L. weilii, with diffuse distribution and low C-value among others.
3.3.3. L. licerasiae Serovar Varillal strain VAR010
Among the 28 TAs identified in the intermediate pathogenic L. licerasiae [39], 15 are VapBC modules (Table 4). We observed a diffuse pattern of conservation to the toxins. VapCs 1, 2, 4, and 10 appear with low C-values among most species, while VapCs 3, 5, 7, and 8 exhibit wide distribution and medium conservation C-values. VapCs 6, 9, 11, 13, and 14 present a larger distribution with high C-values indicating higher conservation of sequences. It should be noted that VapCs 8, 12, and 15 (LEP1GSC185:2580, LEP1GSC185:3559, and LEP1GSC185:3880) share the same amino-acid sequence, probably stemming from genetic duplication events.
3.3.4. L. biflexa Serovar Patoc 1 (Ames)
When the nonpathogenic L. biflexa strain was analyzed (Table 5), we observed a more homogenous pattern of distribution (i.e., we noted that, with the exception of VapC1, all the others displayed high C-values in the other saprophytic strains, and the VapCs 4, 5, 6, 7, and 8 appear with relatively high conservation in the pathogenic species as well). It is interesting to note that among the eight VapCs of L. biflexa, three of them (VapC-4, VapC-5, and VapC-6) resemble VapC-3 of L. interrogans Copenhageni, which might suggest some evolutionary link and explain their strong presence among the pathogenic species.
Taken all together, our results describing the distribution of these TA modules among leptospiral strains of varied phenotypes of pathogenicity showed that VapCs are unequally distributed, with some toxins apparently being randomly disseminated, to others present in unique species or specific pathogenicity groups.
4. Discussion
Toxin-antitoxin systems have been involved in potentially harmful aspects of an infection, such as antimicrobial resistance, persistence, and biofilm formation, and therefore have been the subject of intensive efforts to elucidate biochemical and functional effects besides their role on the physiology of infection. Nevertheless, due to the high number of types, families, and high divergence among them, all these efforts on the elucidation of biochemical and biological functions are still incipient and remain controversial.
Currently, three type II TAs were described and experimentally characterized in the genome of L. interrogans serovar Copenhageni strain Fiocruz L1-130 and serovar Lai strain Lai: one VapBC [29,46], one ChpIK, and one MazEF [9,47], and therefore can be considered bona fide elements, all the others need to be confirmed. It should be pointed out that the ChpKI module has also been called as MazEF [48] since both ChpI and MazF toxins share the structurally and functionally similar domain PemK, which is a sequence-specific endoribonuclease [49]. Even though both chpIK and mazEF, found in L. interrogans serovar Lai strain Lai, have been described as extensively distributed and the sequences conserved in different pathogenic Leptospira [9], the mazEF operon (LA1780/1781) was not identified in the L. interrogans serovar Copenhageni strain Fiocruz L1-130 using BLAST analysis, but it is present only in the L. interrogans serovar Copenhageni strain HAIO156 from the 19 isolates available of this species-serovar. Considering the strict curation of Fiocruz L1-130 and Lai genomes, this fact indicates that the function of MazEF in pathogenesis is not essential or, more likely, redundant, and could be replaced by other TAs in these strains.
The analysis of the whole genomes of Leptospira has shown that there is considerable genomic plasticity even within the same species, as in the case of the large inversion in chromosome I and an ~54 kb genomic island that differentiates the genomes of L. interrogans serovars Lai and Copenhageni, which share ~99% similarity at amino acid level of ortholog genes [44]. Our data shows that the disposition of seven TAs along the chromosome of both bacteria is inverted as expected, but more significantly, have the addition of three TA modules. Similarly, we have found that the genomes of both strains of L. borgpetersenii analyzed (JB197 and L550) displayed total inversion of the seven TA sequences along the chromosome, which share exactly the same amino acid sequences (data not shown). Differently, the analysis of the 23 TA modules of the genomes of the two strains of L. biflexa (Ames and Paris) did not show any variation between them, even in their position on the chromosome, which is in agreement with data in the literature describing the high conservation in the genomes within this species [43].
The low intra-species amino acid sequence identity among VapCs of the L. interrogans, L. licerasiae, and L. biflexa strains evaluated in our study might indicate that they act on different substrates producing distinct effects, however, no link between toxin homologs and a specific physiological function has been established [50,51]. Additionally, it remains controversial whether such TA systems are redundant or not in relation to their physiological functions. It has been postulated that TA systems are responsive to environmental changes, both within their hosts or in free-living conditions. In the case of type II TAs, such changes would trigger the activity of enzymes such as Lon protease [52], which would cause antitoxin degradation, de-repressing transcription, increasing TA expression, and finally releasing the toxins to perform their functions. TAs have been proposed to participate in bacterial pathogenicity based on the idea that adaptation of pathogenic bacteria to the host is intrinsically linked to the expression of virulence genes. VapBC modules have been suggested to be involved with bacterial phenotypes both under intra-host and free-living conditions, which was shown by the loss of adaptability to stressing conditions when knocking out the operons vapBC2ST of Salmonella typhimurium [53], vapBC-1 of Haemophilus influenzae [13], and vapBC of the thermophilic Sulfolobus solfataricus [54]. In L. interrogans Copenhageni, VapBC-3 has been biochemically characterized to act on the lysis of the initiator tRNAfMet and therefore to inhibit translation [29], but information of its role in the physiology of the bacteria is still lacking.
TAs are represented by a highly variable number of modules in several bacteria, as highly variable as the extreme environmental or host differences to which these organisms must adapt to live, suggesting it might contribute to their diversification and evolution. Unsurprisingly, the resulting phenotype of each species is affected by a diversity of features including the whole genomic background, among which the diversity of TA modules can be included and may interfere in the response of individual TA systems during adaptation stresses [55]. In the case of Leptospira, the overall set of TA modules characteristic among species living in saprophytic or pathogenic conditions reveals that the L. biflexa has a considerable larger number of TAs then L. interrogans and L. borgpetersenii, which can indicate a relatively large subset of adaptive facilities. However, the intermediate L. licerasiae, whose genomic analysis led to the conclusion of its greater proximity to pathogenic strains than to saprophytic [39], has an even greater set of TA systems, in general, and vapBC specifically. Although the data available to date are very incipient, we could hypothesize that this larger number of modules found in the intermediate pathogenic L. licerasiae, which could also be referred as “intermediate saprophytic” [56], would be related to its ability to survive in both the environment and animal hosts. There are examples in the literature showing that proposed TAs, exhibiting a typical TA genetic organization (e.g., XreA-Ant and Bro-XreB of S. pneumonia [57] and VapBC of M. tuberculosis [58]), did not appear to act as bona fide TAs when experimentally tested. Therefore, it is important to reinforce that the results and hypothesis discussed here are based on in silico analysis and questions related to the possible redundancy and functionality of the VapBC modules analyzed here need to be confirmed by in vivo experiments.
It is not clear how acquisition by horizontal gene transfer of the set of TA loci influenced the evolution of each bacterial species in its broad set of genes, and whether TAs may play roles in determining phenotypic aspects such as virulence and persistence [14,51]. It has been suggested that TAs should more likely be considered as just selfish elements that would improve the fitness of bacteria to eventual stressing conditions, driving the evolution of bacterial genomes [21].
By providing an overview of the distribution of vapBC operons among Leptospira species, through the analysis of the conservation of their toxic elements, we could outline a rather complex picture indicating that these modules evolved differently. It is remarkable that, out of all VapC sequences of pathogenic species submitted to BLAST analysis, VapC-2 of L. interrogans serovar Copenhageni and VapC-2 of L. borgpetersenii serovar Hardjo-bovis are, at the same time, widely and strictly highly conserved only among pathogenic species. Interestingly, proteomic analysis showed an increase of the antitoxin component from VapBC-2 of L. interrogans serovar Copenhageni during stress induced by antibiotic treatment (Ciprofloxacin) [59]. Thus, they might represent potential candidates for participating in the adaptation of the pathogen to the infection host and have a role in pathogenesis.
Even though in recent years we have accumulated an increase in the knowledge about TA systems, information on how they have evolved is still missing. One reason that may help to explain this lack of knowledge is the fact that most TA loci are acquired by horizontal gene transfer [39,60] and thus are often not conserved in different isolates belonging to the same bacterial species. According to that explanation and corroborating our results, a study dealing with dissemination type II TAs in Escherichia coli showed that they are unevenly distributed among E. coli phylogroups and are not a universal feature of the species [61]. In parallel studies, our attempts at using molecular phylogenetic analysis to try to identify some branching points and thus infer some correlation of VapCs with pathogenic, intermediate, and saprophytic species of Leptospira has failed. Except in the specific cases mentioned above, our data were inconclusive and unable to infer this kind of correlation, most likely due to the high diversity of their amino acid sequences. Moreover, it is very important to consider that protein primary sequences are the groundings for the three-dimensional structure folding, which ultimately is responsible for toxin and antitoxin activities.
The large variability in numbers and the low sequence conservation among VapCs shown here emphasizes the strong need to identify and characterize new TAs as well to understand the regulation nets and roles of TA systems in pathogenic bacteria.
Acknowledgments
We would like to thank Marcelo A. Ventura for helping with the design of the figures. We also thank Martin Wesley for his help with English revisions.
Supplementary Materials
The following are available online at http://www.mdpi.com/2076-2607/7/2/56/s1.
Author Contributions
Conception of the work: A.P.Y.L., A.L.T.O.N., and G.C.B.; Data acquisition: A.P.Y.L., B.O.P.A., R.C.E., and D.K.D.; Interpretation: A.P.Y.L., B.O.P.A., R.C.E., D.K.D., A.L.T.O.N., and G.C.B.; Writing and revising: A.P.Y.L., B.O.P.A., R.C.E., D.K.D., A.L.T.O.N., and G.C.B.
Funding
This work was financially supported by FAPESP (grant 14/50981-0), CNPq (grant 301229/2017-1), and Fundação Butantan—Brazil. This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Plank R., Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2000;2:1265–1276. doi: 10.1016/S1286-4579(00)01280-6. [DOI] [PubMed] [Google Scholar]
- 2.Bharti A.R., Nally J.E., Ricaldi J.N., Matthias M.A., Diaz M.M., Lovett M.A., Levett P.N., Gilman R.H., Willig M.R., Gotuzzo E., et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003;3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann J.S., Matthias M.A., Vinetz J.M., Fouts D.E. Leptospiral pathogenomics. Pathogens. 2014;3:280–308. doi: 10.3390/pathogens3020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouts D.E., Matthias M.A., Adhikarla H., Adler B., Amorim-Santos L., Berg D.E., Bulach D., Buschiazzo A., Chang Y.F., Galloway R.L., et al. What Makes a Bacterial Species Pathogenic?:Comparative Genomic Analysis of the Genus Leptospira. PLoS Negl. Trop. Dis. 2016;10:e0004403. doi: 10.1371/journal.pntd.0004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerdes K., Christensen S.K., Løbner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi Y., Park J.H., Inouye M. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 2011;45:61–79. doi: 10.1146/annurev-genet-110410-132412. [DOI] [PubMed] [Google Scholar]
- 8.Lioy V.S., Machon C., Tabone M., Gonzalez-Pastor J.E., Daugelavicius R., Ayora S., Alonso J.C. The zeta toxin induces a set of protective responses and dormancy. PLoS ONE. 2012;7:e30282. doi: 10.1371/journal.pone.0030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komi K.K., Ge Y.M., Xin X.Y., Ojcius D.M., Sun D., Hu W.L., Zhao X., Lin X., Yan J. ChpK and MazF of the toxin-antitoxin modules are involved in the virulence of Leptospira interrogans during infection. Microbes Infect. 2015;17:34–47. doi: 10.1016/j.micinf.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Ramage H.R., Connolly L.E., Cox J.S. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: Implications for pathogenesis, stress responses, and evolution. PLoS Genet. 2009;5:e1000767. doi: 10.1371/journal.pgen.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-García L., Blasco L., Lopez M., Bou G., García-Contreras R., Wood T., Tomas M. Toxin-Antitoxin Systems in Clinical Pathogens. Toxins. 2016;8 doi: 10.3390/toxins8070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norton J.P., Mulvey M.A. Toxin-antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog. 2012;8:e1002954. doi: 10.1371/journal.ppat.1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren D., Walker A.N., Daines D.A. Toxin-antitoxin loci vapBC-1 and vapXD contribute to survival and virulence in nontypeable Haemophilus influenzae. BMC Microbiol. 2012;12:263. doi: 10.1186/1471-2180-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De la Cruz M.A., Zhao W., Farenc C., Gimenez G., Raoult D., Cambillau C., Gorvel J.P., Méresse S. A toxin-antitoxin module of Salmonella promotes virulence in mice. PLoS Pathog. 2013;9:e1003827. doi: 10.1371/journal.ppat.1003827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Sharma B., Brown A.V., Matluck N.E., Hu L.T., Lewis K. Borrelia burgdorferi, the Causative Agent of Lyme Disease, Forms Drug-Tolerant Persister Cells. Antimicrob. Agents Chemother. 2015;59:4616–4624. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey D.P., Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díaz-Orejas R., Espinosa M., Yeo C.C. The Importance of the Expendable: Toxin-Antitoxin Genes in Plasmids and Chromosomes. Front. Microbiol. 2017;8:1479. doi: 10.3389/fmicb.2017.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S.M., Kim D.H., Jin C., Lee B.J. A Systematic Overview of Type II and III Toxin-Antitoxin Systems with a Focus on Druggability. Toxins. 2018;10 doi: 10.3390/toxins10120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kędzierska B., Hayes F. Emerging Roles of Toxin-Antitoxin Modules in Bacterial Pathogenesis. Molecules. 2016;21 doi: 10.3390/molecules21060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerdes K., Maisonneuve E., Gottesman S., Harwood C., Schneewind O. Bacterial Persistence and Toxin-Antitoxin Loci. Annu. Rev. Microbiol. 2012;66:103–123. doi: 10.1146/annurev-micro-092611-150159. [DOI] [PubMed] [Google Scholar]
- 21.Ramisetty B.C.M., Santhosh R.S. Endoribonuclease type II toxin-antitoxin systems: Functional or selfish? Microbiology. 2017;163:931–939. doi: 10.1099/mic.0.000487. [DOI] [PubMed] [Google Scholar]
- 22.Shao Y., Harrison E.M., Bi D., Tai C., He X., Ou H.Y., Rajakumar K., Deng Z. TADB: A web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res. 2011;39:D606–D611. doi: 10.1093/nar/gkq908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y., Wei Y., Shen Y., Li X., Zhou H., Tai C., Deng Z., Ou H.Y. TADB 2.0: An updated database of bacterial type II toxin-antitoxin loci. Nucleic Acids Res. 2018;46:D749–D753. doi: 10.1093/nar/gkx1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Zhang J., Hoeflich K., Ikura M., Qing G., Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell. 2003;12:913–923. doi: 10.1016/S1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen K., Zavialov A.V., Pavlov M.Y., Elf J., Gerdes K., Ehrenberg M. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell. 2003;112:131–140. doi: 10.1016/S0092-8674(02)01248-5. [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen M.G., Pandey D.P., Jaskolska M., Gerdes K. HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J. Bacteriol. 2009;191:1191–1199. doi: 10.1128/JB.01013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schifano J.M., Edifor R., Sharp J.D., Ouyang M., Konkimalla A., Husson R.N., Woychik N.A. Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal A site. Proc. Natl. Acad. Sci. USA. 2013;110:8501–8506. doi: 10.1073/pnas.1222031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schifano J.M., Cruz J.W., Vvedenskaya I.O., Edifor R., Ouyang M., Husson R.N., Nickels B.E., Woychik N.A. tRNA is a new target for cleavage by a MazF toxin. Nucleic Acids Res. 2016;44:1256–1270. doi: 10.1093/nar/gkv1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes A.P.Y., Lopes L.M., Fraga T.R., Chura-Chambi R.M., Sanson A.L., Cheng E., Nakajima E., Morganti L., Martins E.A.L. VapC from the Leptospiral VapBC Toxin-Antitoxin Module Displays Ribonuclease Activity on the Initiator tRNA. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winther K.S., Gerdes K. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc. Natl. Acad. Sci. USA. 2011;108:7403–7407. doi: 10.1073/pnas.1019587108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walling L.R., Butler J.S. Homologous VapC Toxins Inhibit Translation and Cell Growth by Sequence-Specific Cleavage of tRNA. J. Bacteriol. 2018;200 doi: 10.1128/JB.00582-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winther K., Tree J.J., Tollervey D., Gerdes K. VapCs of Mycobacterium tuberculosis cleave RNAs essential for translation. Nucleic Acids Res. 2016;44:9860–9871. doi: 10.1093/nar/gkw781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winther K.S., Brodersen D.E., Brown A.K., Gerdes K. VapC20 of Mycobacterium tuberculosis cleaves the Sarcin-Ricin loop of 23S rRNA. Nat. Commun. 2013;4:2796. doi: 10.1038/ncomms3796. [DOI] [PubMed] [Google Scholar]
- 34.Kositanont U., Saetun P., Krittanai C., Doungchawee G., Tribuddharat C., Thongboonkerd V. Application of immunoproteomics to leptospirosis: Towards clinical diagnostics and vaccine discovery. Proteom. Clin. Appl. 2007;1:400–409. doi: 10.1002/prca.200600805. [DOI] [PubMed] [Google Scholar]
- 35.Nascimento A.L., Verjovski-Almeida S., Van Sluys M.A., Monteiro-Vitorello C.B., Camargo L.E., Digiampietri L.A., Harstkeerl R.A., Ho P.L., Marques M.V., Oliveira M.C., et al. Genome features of Leptospira interrogans serovar Copenhageni. Braz. J. Med. Biol. Res. 2004;37:459–477. doi: 10.1590/S0100-879X2004000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler B., Lo M., Seemann T., Murray G.L. Pathogenesis of leptospirosis: The influence of genomics. Vet. Microbiol. 2011;153:73–81. doi: 10.1016/j.vetmic.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Ren S.X., Fu G., Jiang X.G., Zeng R., Miao Y.G., Xu H., Zhang Y.X., Xiong H., Lu G., Lu L.F., et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422:888–893. doi: 10.1038/nature01597. [DOI] [PubMed] [Google Scholar]
- 38.Gamberini M., Gómez R.M., Atzingen M.V., Martins E.A., Vasconcellos S.A., Romero E.C., Leite L.C., Ho P.L., Nascimento A.L. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol. Lett. 2005;244:305–313. doi: 10.1016/j.femsle.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Ricaldi J.N., Fouts D.E., Selengut J.D., Harkins D.M., Patra K.P., Moreno A., Lehmann J.S., Purushe J., Sanka R., Torres M., et al. Whole genome analysis of Leptospira licerasiae provides insight into leptospiral evolution and pathogenicity. PLoS Negl. Trop. Dis. 2012;6:e1853. doi: 10.1371/journal.pntd.0001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llanes A., Restrepo C.M., Rajeev S. Whole Genome Sequencing Allows Better Understanding of the Evolutionary History of Leptospira interrogans Serovar Hardjo. PLoS ONE. 2016;11:e0159387. doi: 10.1371/journal.pone.0159387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nascimento Filho E.G., Vieira M.L., Teixeira A.F., Santos J.C., Fernandes L.G.V., Passalia F.J., Daroz B.B., Rossini A., Kochi L.T., Cavenague M.F., et al. Proteomics as a tool to understand Leptospira physiology and virulence: Recent advances, challenges and clinical implications. J. Proteom. 2018;180:80–87. doi: 10.1016/j.jprot.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Bulach D.M., Zuerner R.L., Wilson P., Seemann T., McGrath A., Cullen P.A., Davis J., Johnson M., Kuczek E., Alt D.P., et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. USA. 2006;103:14560–14565. doi: 10.1073/pnas.0603979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picardeau M., Bulach D.M., Bouchier C., Zuerner R.L., Zidane N., Wilson P.J., Creno S., Kuczek E.S., Bommezzadri S., Davis J.C., et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE. 2008;3:e1607. doi: 10.1371/journal.pone.0001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nascimento A.L., Ko A.I., Martins E.A., Monteiro-Vitorello C.B., Ho P.L., Haake D.A., Verjovski-Almeida S., Hartskeerl R.A., Marques M.V., Oliveira M.C., et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 2004;186:2164–2172. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arcus V.L., McKenzie J.L., Robson J., Cook G.M. The PIN-domain ribonucleases and the prokaryotic VapBC toxin-antitoxin array. Protein Eng. Des. Sel. 2011;24:33–40. doi: 10.1093/protein/gzq081. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y.X., Li J., Guo X.K., Wu C., Bi B., Ren S.X., Wu C.F., Zhao G.P. Characterization of a novel toxin-antitoxin module, VapBC, encoded by Leptospira interrogans chromosome. Cell Res. 2004;14:208–216. doi: 10.1038/sj.cr.7290221. [DOI] [PubMed] [Google Scholar]
- 47.Picardeau M., Le Dantec C., Richard G.F., Saint Girons I. The spirochetal chpK-chromosomal toxin-antitoxin locus induces growth inhibition of yeast and mycobacteria. FEMS Microbiol. Lett. 2003;229:277–281. doi: 10.1016/S0378-1097(03)00848-6. [DOI] [PubMed] [Google Scholar]
- 48.Gerdes K. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress. J. Bacteriol. 2000;182:561–572. doi: 10.1128/JB.182.3.561-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda Y., Miyakawa K., Nishimura Y., Ohtsubo E. chpA and chpB, Escherichia coli chromosomal homologs of the pem locus responsible for stable maintenance of plasmid R100. J. Bacteriol. 1993;175:6850–6856. doi: 10.1128/jb.175.21.6850-6856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harms A., Brodersen D.E., Mitarai N., Gerdes K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell. 2018;70:768–784. doi: 10.1016/j.molcel.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Sierra R., Viollier P., Renzoni A. Linking toxin-antitoxin systems with phenotypes: A Staphylococcus aureus viewpoint. Biochim. Biophys. Acta Gene Regul. Mech. 2018 doi: 10.1016/j.bbagrm.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Winther K., Gerdes K. Regulation of Enteric vapBC Transcription: Induction by VapC Toxin Dimer-Breaking. Nucleic Acids Res. 2012;40:4347–4357. doi: 10.1093/nar/gks029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobato-Márquez D., Moreno-Córdoba I., Figueroa V., Díaz-Orejas R., García-del Portillo F. Distinct type I and type II toxin-antitoxin modules control Salmonella lifestyle inside eukaryotic cells. Sci. Rep. 2015;5:9374. doi: 10.1038/srep09374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper C.R., Daugherty A.J., Tachdjian S., Blum P.H., Kelly R.M. Role of vapBC toxin-antitoxin loci in the thermal stress response of Sulfolobus solfataricus. Biochem. Soc. Trans. 2009;37:123–126. doi: 10.1042/BST0370123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolic N. Autoregulation of bacterial gene expression: Lessons from the MazEF toxin-antitoxin system. Curr. Genet. 2019;65:133–138. doi: 10.1007/s00294-018-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Victoria B., Ahmed A., Zuerner R.L., Ahmed N., Bulach D.M., Quinteiro J., Hartskeerl R.A. Conservation of the S10-spc-alpha locus within otherwise highly plastic genomes provides phylogenetic insight into the genus Leptospira. PLoS ONE. 2008;3:e2752. doi: 10.1371/journal.pone.0002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan W.T., Yeo C.C., Sadowy E., Espinosa M. Functional validation of putative toxin-antitoxin genes from the Gram-positive pathogen Streptococcus pneumoniae: Phd-doc is the fourth bona-fide operon. Front. Microbiol. 2014;5:677. doi: 10.3389/fmicb.2014.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahidjo B.A., Kuhnert D., McKenzie J.L., Machowski E.E., Gordhan B.G., Arcus V., Abrahams G.L., Mizrahi V. VapC toxins from Mycobacterium tuberculosis are ribonucleases that differentially inhibit growth and are neutralized by cognate VapB antitoxins. PLoS ONE. 2011;6:e21738. doi: 10.1371/journal.pone.0021738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malmström J., Beck M., Schmidt A., Lange V., Deutsch E.W., Aebersold R. Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature. 2009;460:762–765. doi: 10.1038/nature08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramisetty B.C., Santhosh R.S. Horizontal gene transfer of chromosomal Type II toxin-antitoxin systems of Escherichia coli. FEMS Microbiol. Lett. 2016;363 doi: 10.1093/femsle/fnv238. [DOI] [PubMed] [Google Scholar]
- 61.Fiedoruk K., Daniluk T., Swiecicka I., Sciepuk M., Leszczynska K. Type II toxin-antitoxin systems are unevenly distributed among Escherichia coli phylogroups. Microbiology. 2015;161:158–167. doi: 10.1099/mic.0.082883-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.