Abstract
Nevoid basal cell carcinoma syndrome (NBCCS) is an autosomal dominant disorder characterized by multiple basal cell carcinomas (BCC), mainly caused by PTCH1 gene mutations. Our current study aimed to establish (1) PTCH1 germinal and somatic mutational status, (2) component and Hedgehog (HH) pathway targets gene expression patterns, and (3) profile variations according to the genetic background in BCC and normal surrounding skin (NSS). We collected 23 blood and 20 BCC patient samples and analyzed the PTCH1 gene using bidirectional sequencing and multiplex ligation-dependent probe amplification. Quantitative PCR was used to determine the mRNA expression levels of PTCH1, SMO, GLI3, and CCND1 in paired samples of BCC and NSS from 20 patients and four non-NBCCS skin controls (C). Our analyses identified 12 germline and five somatic sequence variants in PTCH1. mRNA levels of PTCH1, SMO, and GLI3 were higher in NSS compared to C samples, reaching maximum values in BCC samples (p < 0.05). NSS with PTCH1 germline mutations had modified SMO, PTCH1, and GLI3 mRNA levels compared to samples without mutation (p < 0.01). Two PTCH1 mutations in BCC led to an increase in PTCH1, SMO, and GLI3, and a decrease in CCND1 mRNA levels (p < 0.01 vs. BCC with germline mutation only). These results indicate that besides PTCH1, other genes are responsible for NBCCS and BCC development in a population exposed to high UV radiation. Additionally, the mutational events caused increased expression of HH-related genes, even in phenotypically normal skin.
Keywords: Gorlin–Goltz syndrome, PTCH1 mutation, Hedgehog pathway, basal cell carcinoma
1. Introduction
Nevoid basal cell carcinoma syndrome (NBCCS; OMIM #109400)—also known as the Gorlin–Goltz Syndrome—is a multisystem genetic disorder with an autosomal dominant inheritance pattern, complete penetrance, and variable expression [1]. NBCCS patients are characterized by numerous malformations and a high susceptibility for developing multiple basal cell carcinomas (BCC)—the most common type of cancer worldwide—at an early age. PTCH1 is the human homolog of the Drosophila segment polarity gene, with 23 exons that encode a transmembrane glycoprotein composed of 1447 amino acids, and is responsible for most NBCCS cases. PTCH1 acts as a receptor in the Hedgehog signaling pathway [2,3]. Its ligands, Hedgehog (HH), are morphogens that physiologically drive the inhibitory signaling effects of PTCH1 over smoothened, which in turn activates the GLI effectors. GLI proteins are transcription factors that stimulate their target genes to induce cell cycle effects. These proteins have alternate activating and repressing functions depending on HH concentration and gradient [4]. In several cell types, the HH pathway activation increases the expression of key regulators from the G1/S and G2/M cell cycle phases, promoting the transition from the quiescent to the proliferative state. Mutations in PTCH1 cause constitutive activation of the HH pathway, which in turn leads to the development of NBCCS [5].
Ponti et al. proposed that normal cells become tumorigenic via the Knudson’s two-hit mechanism, based on evidence showing loss of heterozygosity in genetic studies in human odontogenic keratocysts [6]. Based on this hypothesis, the addition of somatic mutations to inherited germline mutations was associated with the development of these and other tumors, such as BCC and meningiomas [1]. It has also been described that the progressive allele inactivation of PTCH1 over-activates the HH pathway, leading to cell cycle progression [7]. However, systematic analysis of pathway over-activation and the presence of mutations in these patients have not been entirely clarified. Therefore, we systematically looked for mutations in the PTCH1 gene in peripheral blood and BCC tissue of patients with NBCCS. In addition, we quantified the expression of Hedgehog pathway genes and its effectors in BCC and phenotypically normal adjacent tissue, to evaluate expression profile variations according to the mutational status.
2. Materials and Methods
Twenty-three patients from 20 unrelated families, who complied with the diagnostic criteria defined by Kimonis for NBCCS, were included in the study [8].
Genomic DNA and total RNA were isolated from BCC and normal surrounding epithelial tissue (NSS) samples following a routine biopsy. Genomic DNA was also extracted from blood samples obtained by venipuncture, using 0.5% EDTA as an anticoagulant.
2.1. Extraction of Nucleic Acids
Genomic DNA and total RNA were sequentially isolated using an SV Total RNA Isolation System kit (Promega Corporation, Madison, WI, USA) [9], whereas DNA from blood was obtained using a High Pure PCR Template Preparation kit (Roche Applied Bioscience, Roche Diagnostic GmbH, Mannheim, Germany), following the manufacturers’ instructions. The DNA and RNA samples were stored at −20 °C and −70 °C, respectively.
2.2. Mutation Search
2.2.1. PTCH1 Gene Sequencing
Blood (23/23) and BCC (20/23) samples were used as starting materials to amplify the 23 exons from the PTCH1 coding sequence. The experiment was conducted in 20 PCR reactions using published primer sequences [10,11], following the protocols described in References [12,13]. The PCR conditions were as follows: Initial denaturalization at 95 °C for 5 min; followed by 35 cycles of denaturalization at 95 °C for 30 s; annealing at a specific temperature depending on the primers for 30 s; elongation at 72 °C for 55 s for fragments under 700 bp, or for 90 s for longer fragments; and final elongation at 72 °C for 5 min. Blood DNA samples from four healthy subjects were included as controls in each experiment.
Bidirectional sequencing was conducted in an automated sequencer ABI PRISM® 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), using BigDye™ Terminator v3.1 Cycle Sequencing kit (Applied Biosystems), according to our previously published procedure [12,13]. We used 5–10 ng of the material purified using PureLink Quick Gel Extraction kit (Invitrogen, Life Technologies, Carlsbad, CA, USA).
2.2.2. PTCH1 Gene Analysis using Multiplex Ligation-dependent Probe Amplification (MLPA)
The detection of major sequence duplications or deletions was performed via MLPA using the commercial kit P067-B1 PTCH1 (MRC—Holland, The Netherlands), following published procedures [12,13]. Each MLPA reaction generated a combination of 23 amplified fragments in one single PCR, which were identified and quantified using capillary electrophoresis with an ABI PRISM® 310 Genetic Analyzer (Applied Biosystems). Patterns of the generated peaks were assessed using the Coffalyser.Net software [14]. The heterozygous deletion profile showed a reduction in 30–50% of the peak area compared to controls, whereas profile duplication increased the peak area by 35–55%.
2.2.3. Genetic Variant Analysis
The functionality of variants that were not synonymous was predicted using SIFT (http://siftdna.org/www/Extended_SIFT_chr_coords_submit.html) [15], and PolyPhen2 (http://genetics.bwh.harvard.edu/pph2/) [16] software programs. In the case of variants that changed their nucleotide sequence, Mutalyzer version 2.0.29 (https://mutalyzer.nl/) was used to assess this change and verify the nomenclature [17]. Variants, including a change in nearby regions or in splicing regions, were assessed using the Human Splicing Finder Analyze Mutation software (http://www.umd.be/HSF/) [18].
Variants found in both patients and controls, which were predicted as nonpathogenic using bioinformatics analysis, were considered benign. Whereas, variants that did not exist in control samples, and were predicted as pathogenic were considered mutations. Using these analyses results, a mutation was assigned to a germline status if it was found in the same patient’s BCC and blood samples. On the other hand, a somatic nature was assigned to the mutations found only in patient tumors.
2.3. Gene Expression Analysis
PTCH1 (gene ID: 5727), SMO (ID: 6608), GLI3 (ID: 2737), and cyclin D1 (CCND1, ID: 595) gene expressions were evaluated by quantitative real-time PCR, using total RNA from BCC and NSS of NBCCS patients, and normal skin (NS) from four healthy subjects, as starting material. One NSS and one BCC sample per subject were obtained from 20 out of 23 patients. RNA was retrotranscribed to cDNA using Illustra Ready-To-Go RT-PCR Beads kit (GE Healthcare UK Limited, Little Chalfont, UK), as described previously [9,19]. Real-time PCR was performed using LightCycler® FastStart DNA Master SYBR Green I kit (Roche Applied Bioscience, Roche Diagnostic GmbH, Germany) with a LightCycler® 2.0 carrousel system (Roche Diagnostic GmbH, Germany). The primers were designed with the Primer 3 software (http://bioinfo.ut.ee/primer3-0.4.0) [20], using NM_000264.3, NM_005631.4, NM_000168.5, and NM_053056.2 as the reference target mRNA sequences for PTCH1, SMO, GLI3, and CCND1, respectively; the GAPDH gene was employed as a housekeeping normalizer gene using the primers reported by Rubio et al. [21]. The primer sequences are available upon request. The real-time PCRs were run in duplicate for each sample, and the gene expression was calculated according to Pfaffl [22], where Ct represents the first cycle at which the output signal exceeds the threshold level.
2.4. Statistical Analysis
Continuous variables are shown as means ± standard error or percentage/frequency, as applicable. A p-value < 0.05 was considered significant. The Chi-square test was used for dichotomous comparisons, and the Mann–Whitney test or Student t-test was used for continuous comparisons. The results were analyzed using a factorial MANOVA, with the main variables (mRNA expression of PTCH1, SMO, GLI3, and CCND1) representing the dependent outcomes to be analyzed. After finding the significant MANOVA for the main variables, or for their respective interactions, differences between the groups were explored according to the dependent variable. Statistical analyses were conducted using IBM SPSS Statistics 21.0.0.0.
Ethical approval for this study was obtained from each participant hospital-authorized Ethics Committees. All patients provided written informed consent. The study was conducted according to the Declaration of Helsinki Principles.
3. Results
Due to the autosomal dominant nature of NBCCS, our mutation search focused on the 20 index cases from each family. The most frequent clinical manifestations (Table 1) were BCCs (18/20), odontogenic cysts (18/20), and palmoplantar pits (18/20). Among the infrequent findings, we found two patients without BCC—one of them with agenesis of the corpus callosum, and the other with NBCCS with concomitant autosomal dominant polycystic kidney disease [12,13].
Table 1.
Attributes found in nevoid basal cell carcinoma syndrome probands [8].
| Patient | Age | Gender | Major Criteria | Minor Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >5 BCCs 1 or 1 BCC < 30 years | Odontogenic Keratocysts | ≥3 palmoplantar Pits | Falx Cerebri Calcification | Bifurcated Ribs | First-Degree Relative | Macrocephaly | Congenital Abnormalities | Skeletal Abnormalities | Radiological Abnormalities | Ovarian/Cardiac Fibromas | |||
| SG1 | 41 | M | X | X | X | X | |||||||
| SG2 | 12 | M | X | X | X | X | X | ||||||
| SG3 | 34 | F | X | X | X | X | X | ||||||
| SG6 | 73 | F | X | X | X | ||||||||
| SG7 | 12 | M | X | X | X | X | |||||||
| SG8 | 40 | M | X | X | X | X | |||||||
| SG9 | 68 | F | X | X | X | X | X | ||||||
| SG10 | 66 | F | X | X | X | X | X | X | |||||
| SG11 | 49 | F | X | X | X | X | |||||||
| SG12 | 59 | F | X | X | X | X | X | X | |||||
| SG13 | 55 | M | X | X | X | X | X | X | |||||
| SG14 | 70 | M | X | X | X | X | X | ||||||
| SG15 | 46 | M | X | X | X | X | |||||||
| SG16 | 51 | F | X | X | X | X | X | ||||||
| SG17 | 33 | F | X | X | X | X | X | ||||||
| SG18 | 41 | M | X | X | X | ||||||||
| SG19 | 60 | F | X | X | X | ||||||||
| SG20 | 34 | F | X | X | X | X | X | ||||||
| SG21 | 14 | F | X | X | X | X | |||||||
| SG22 | 31 | F | X | X | X | X | X | ||||||
1 BCCs: Basal cell carcinomas.
3.1. PTCH1 Gene Mutations
PTCH1 gene sequencing and MLPA analysis identified 12 germinal and five somatic mutations (Table 2), distributed throughout the gene. Among these mutations, 10 were frameshift mutations causing premature stop codons; three were missense changes resulting in harmful or pathogenic effects, as shown in the analysis performed by the prediction software; c.1068-2A>G led to a cryptic splice site abolition, according to the Human Splicing Finder Analyze Mutation prediction, which resulted in a frameshift with a premature stop codon at 16 bp from the mutation site; and one mutation generated an amino acid change in a premature stop codon.
Table 2.
Mutations, according to their nature, found in index cases from each family examined.
| Patient | Exon | Mutation | Effect on Sequence | Blood | Tumor | Nature | |
|---|---|---|---|---|---|---|---|
| SG1 | 10 22 |
c.1392_1405del c.3587C>T |
p.(Lys464Asnfs*28) p.(Pro1196Leu) |
Frameshift Missense |
X – |
X X |
Germline Somatic |
| SG2 | 15 | c.2309_2312del | p.(Val771Glufs*34) | Frameshift | X | X | Germline |
| SG6 | 19 | c.3277G>A [23,24] c.3277G>A |
p.(Gly1093Arg) LOH1 |
Missense |
X – |
X X |
Germline Somatic |
| SG7 | 10 | c.1375_1399dup [12] | p.(Gly467Alafs*38) | Frameshift | X | – | Germline |
| SG11 | 3 | c.513del | p.(Thr172Glnfs*48) | Frameshift | X | X | Germline |
| SG12 | 14 – |
c.2012dup [25,26] Allelic deletion |
p.(His671Glnfs*10) |
Frameshift Allelic loss |
X – |
X X |
Germline Somatic |
| SG13 | Intron7 | c.1068-2A>G c.1068-2A>G |
– LOH1 |
Frameshift |
X – |
X X |
Germline Somatic |
| SG17 | 4 22 |
c.652dup c.3580C>T |
p.(Gln218Argfs*2) p.(Pro1194Ser) |
Frameshift Missense |
X – |
X X |
Germline Somatic |
| SG18 | 9 | c.1286del | p.(Asp429Alafs*3) | Frameshift | X | X | Germline |
| SG20 | 3 | c.573C>G [13] | p.(Tyr191*) | Nonsense | X | – | Germline |
| SG21 | 16 | c.2677del | p.(Arg893Alafs*10) | Frameshift | X | X | Germline |
| SG22 | 2 | c.290del | p.(Asn97Thrfs*20) | Frameshift | X | X | Germline |
1 LOH: Loss of heterozygosity.
3.1.1. Localization of Mutations Found in NBCCS Patients
According to the protein structure, based on data from the ProtParam database (https://web.expasy.org/protparam/), five of the 12 germline mutations (c.513del, c.573C>G, c.652dup, c.1068-2A>G, and c.1286del) were located at the first extracellular loop, two at the first cytoplasmic loop and the sterol-sensing domain (c.1375_1399dup and c.1392_1405del), and two at the fourth extracellular loop (c.2309_2312del and c.2677del). The remaining mutations were located at the amino-terminal end (c.290del), and the third and fifth intracytoplasmic loops (c.2012dup and c.3277A>G, respectively).
With respect to somatic mutations, we identified a second somatic mutation in two patients that resulted in the loss of PTCH1 gene heterozygosity. In patient SG12, the MLPA technique identified the loss of an entire allele (Figure 1), and two somatic mutations (c.3580C>T and c.3587C>T) were located at the protein carboxy-terminal end. No germline-independent somatic mutations were detected in the PTCH1 gene. According to our results (Table 2), five patients showed germline and somatic mutations in the same gene, which supports the Knudson’s two-hit hypothesis for tumor development [27].
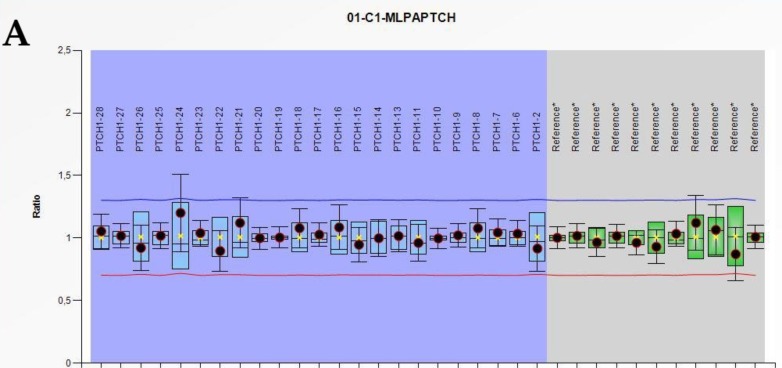
Figure 1.
Representations of wild type (A) and allelic PTCH1 deletion (B) in the BCC of patient SG12 obtained from multiplex ligation-dependent probe amplification (MLPA) analysis. The blue and green boxes represent the interquartile range of normal (N = 3) values for 23 PTCH1 exons and 11 reference non-PTCH1 probes, respectively, of the P067-B1 PTCH1 kit. The blue and red lines represent duplication and deletion cut-off values, respectively. Patient median values for each probe and their standard deviations are shown as circles filled with red (abnormal), yellow (indeterminate), or black (normal).
In conclusion, 83% of the detected germline mutations generated a truncated protein. Among the germline mutations detected in our study population, three have been previously described [23,24,25,26], and nine represent novel mutations, which have not been identified in other families or the NBCCS-associated bibliography.
3.1.2. Analysis of NBCCS Families with PTCH1 Mutations
The 12 patients with known germline mutations were re-called for update interviews to receive feedback on their analyses, to obtain more family data, and to offer the possibility of extending the genetic study to their relatives. Family trees in Figure 2 correspond to a detailed assessment of the patients enrolled and their relatives. Using this analysis, we identified two patients with singular clinical attributes that were directly or indirectly linked to the primary cilium [12,13].
Figure 2.
Genealogy tree of families with nevoid basal cell carcinoma syndrome (NBCCS) and known germline mutation: (A) SG6 family, (B) SG7 family, (C) SG12 family, (D) SG13 family, (E) SG20 family, and (F) SG21 family. The black arrows indicate the studied proband. Symbols filled in black indicate affected members with clinical manifestations. Symbols with “?” indicate that no clinical diagnosis was made. Symbols filled in grey indicates the family member affected by Autosomal Dominant Polycystic Kidney Disease (ADPKD) whereas the stripped ones indicate the family members affected by NBCCS and ADPKD. M: Mutation found in PTCH1; No M: No mutation in PTCH1.
Patient SG7 and his family were examined, as this patient’s neurologic image did not show the corpus callosum (a criterion that is not particularly sought in these patients), along with a negative family history of BCC (one of the typical criteria for NBCCS). The discussion involved the HH pathway in the primary cilium, which participates in the embryonic commissural plate’s development pattern that originates from the corpus callosum [12]. Patient SG20, who inherited NBCCS from her paternal side without BCCs at the time of enrollment, presented an inheritance pattern of autosomal dominant polycystic kidney disease via the maternal line. Polycystic kidney disease is a ciliopathy, since the anomalous protein responsible is also located in the primary cilium, as are the HH pathway components [13].
3.2. Hedgehog Pathway Gene Expression
To investigate the effects of detected mutations on the Hedgehog pathway gene expression, we quantified the expression of genes PTCH1, SMO, GLI3, and CCND1 in BCC (N = 20), NSS samples (N = 20) from NBCCS patients, and NS from healthy controls (N = 4).
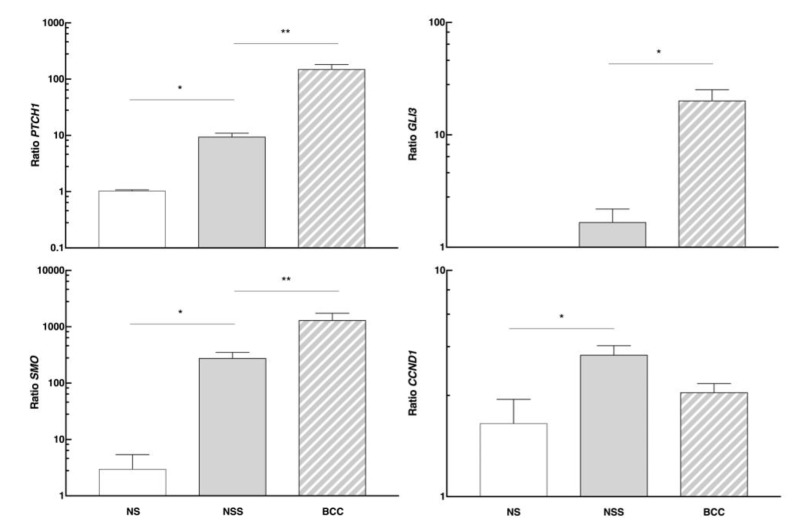
PTCH1, SMO, and the GLI3 effector—whose expression is almost undetectable in NS—had an increased expression in patients’ NSS compared to the NS group (Figure 3). This difference was even more pronounced in the BCCs, indicating overexpression of the pathway in this tissue. Furthermore, we analyzed CCND1 expression levels as one of the transcriptional targets in the HH pathway, and observed an increase in NSS of patients compared to the NS control group.
Figure 3.
mRNA levels of PTCH1, SMO, GLI3, and CCND1 genes studied in normal skin (NS) of controls, as well as in the normal surrounding skin (NSS) and basal cell carcinomas (BCC) of NBCCS patients. * p < 0.05 and ** p < 0.03.
PTCH1 Gene Mutations Affecting the Hedgehog Pathway Gene Expression
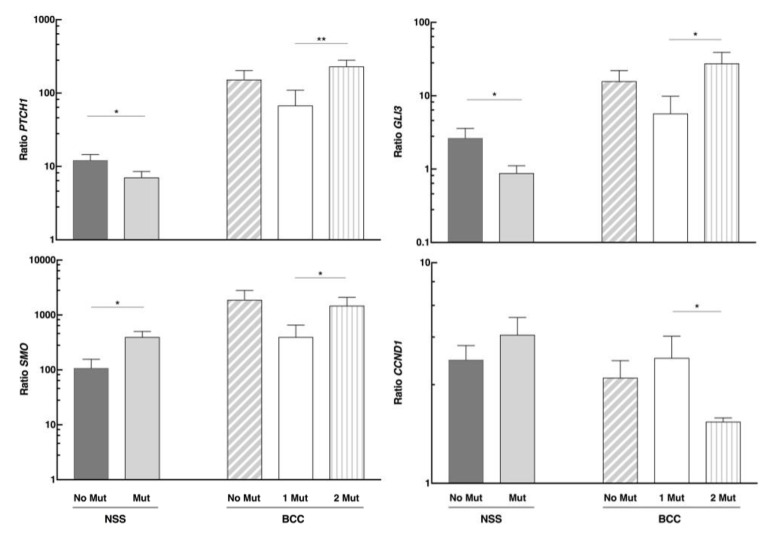
We analyzed the same gene expression in different tissue according to our patients’ PTCH1 genetic status. The expression of this pathway-repressing gene decreased in the NSS of the group with a germline mutation in PTCH1 (N = 11) compared to those without the mutation (N = 9) (Figure 4). The same expression pattern was observed when we studied the GLI3 effector. However, SMO had the opposite effect—its expression in the NSS of the group with PTCH1 mutation was higher than in those without mutation. In the case of CCND1, there was no significant change in the NSS with respect to the genetic status.
Figure 4.
mRNA levels of PTCH1, SMO, GLI3, and CCND1 genes in normal surrounding skin (NSS) and basal cell carcinomas (BCC) of NBCCS patients, according to their genetic status. No Mut: No mutation found in PTCH1; Mut: Tissue with the germline mutation in PTCH1; BCC 1 Mut and 2 Mut: Tissue with germline or germline plus somatic mutations in PTCH1, respectively. * p < 0.05 and ** p < 0.01.
Expression levels of PTCH1, SMO, and GLI3 were higher in tumors with germline and somatic mutations in PTCH1 compared to those with germline mutations only, whereas CCND1 levels reflected the opposite behavior. Therefore, it is clear that the second mutational event further stimulates the expression of the pathway participants.
4. Discussion
This study provides a new mutational analysis of the PTCH1 gene, distinguished by its germinal and somatic status, and the Hedgehog pathway gene expression profile in Argentine patients with NBCCS. We describe herein the largest Latin American cohort that belongs to a population exposed to larger UV radiation, due to its geographical location. We also found that the Hedgehog pathway was found to be active in these patients, both in their healthy skin and in their BCCs. Furthermore, the co-occurrence of two mutations modified these expression levels, suggesting that the cells genetic status affects the phenotypic destination of different tissues.
A significant proportion of patients did not have PTCH1 mutations (even after an extensive mutation search), which supports the hypothesis of NBCCS’s multigenic nature. This hypothesis has already been proposed by other authors [11], and indicates the involvement of defects in genes other than PTCH1. The percentage of germline mutations detected for the PTCH1 gene, within the range described in other series, also highlights the genetic heterogeneity of the syndrome [2,26]. Genetic diagnosis, despite its cost, is a valuable tool for affected families, particularly in those cases with inconclusive clinical signs. It, therefore, becomes essential for identifying other carriers in the family to aid clinicians when giving genetic advice. For example, genetic diagnosis may help the patient, and provide parents with a strong awareness of the severe consequences that ultraviolet radiation and/or X-ray exposure may have from the moment of the diagnosis.
Likewise, the somatic mutation discovery made in 5/20 patients was within the range reported by Pellegrini [28], whereas the lack of somatic mutations in half the BCCs of patients, who had a germline mutation, pointed towards other target genes as potentially being responsible for tumorigenesis. In this respect, germline mutations in other genes of the HH pathway have been detected, such as SUFU and PTCH2, in isolated reports of families with NBCCS [29,30]. Moreover, somatic mutations in SMO and SUFU have been found in sporadic BCCs [28].
The main objective of our study was to determine the underlying tumorigenic mechanisms in BCCs of patients with NBCCS. Unden et al. formulated the two-hit theory for tumor suppressor genes [31], which was later applied to odontogenic cysts [32,33,34]. In our patient groups, the proposed mechanism explains tumorigenesis in ~50% of the tumors with a known germline mutation. Thus, tumor suppressor genes-associated mutations phenotypically behave as recessive, meaning that to observe their effect it is necessary for both copies of the gene to be inactive in the cell [31,35]. However, in a particular group of tumors, we cannot dismiss the hypothesis of haploinsufficiency, as has been reported in some brain tumors [36,37]. It should be noted that we only detected somatic PTCH1 mutations in patients who had a germline mutation in that gene, suggesting that allelic instability leads to somatic mutations in the wild allele, promoting normal tissue progression to tumor tissue. Likewise, we cannot rule out that those patients with germline PTCH1 mutations could have somatic mutations in other genes of the HH pathway or another pathway involved in cell proliferation or differentiation.
Gene expression profile analysis showed that the skin of patients with NBCCS exhibited activation of the HH pathway even when phenotypically normal, and levels reached a maximum after malignant transformations. This finding conclusively showed fundamental involvement of HH in the syndrome, and changes at the level of expression. PTCH1 and GLI3 overexpression could be caused by a self-inhibiting mechanism of the pathway, since they are a tumor suppressor and a repressor effector, respectively [26,35]. On the other hand, SMO would increase as a result of its activating function at the beginning of the pathway cascade. Considering that PTCH1 is a known target gene of the pathway [5], our data suggest that SMO and GLI3 increase their expression either directly or indirectly in the assessed signaling cascade. The role of CCND1 is less evident, as it is elevated in normal tissue and seems to decrease in tumors. Considering its key role in the G1-S phase transition, by increasing cell proliferation, it is likely that other effectors of HH pathway could be also modulated, thus indicating that it is not the only final effector involved in the tumor phenotype.
Perhaps the most interesting and revealing information from our study arises from the analysis of gene expression levels according to the presence of mutations in the PTCH1 gene. These results show that normal skin with germline mutation releases pathway inhibitors (PTCH1 and GLI3) together with the stimulation of SMO. The results in tumors suggest that the suppressors are even more activated with the addition of a mutation in the other allele, generating an effective repression of the CCND1 effector. This result would indicate that—at least within the gene spectrum assessed in this study—there is a differential regulation in the tumor transformation that depends on the accumulated mutations. Thus, the genetic context would help define the expression profile that leads to such a condition and, consequently, it is extremely useful to know what factors and mechanisms are involved in its modification. It would also be interesting to know if, through the process of tumor transformation, the cell could eventually reach a different phenotype, or if whether the tumoral processes determine treatment outcomes.
In summary, our study explored the physiopathological and genetic understanding of the syndrome. Our findings contribute to knowledge regarding the events taking place inside the cell with an aberrant activation of the HH pathway. This aspect is essential for characterizing the study population, taking into consideration the development and implementation of future prevention and even treatment strategies. Likewise, from these outcomes, we can extrapolate important information to further the understanding of complex cell proliferation and differentiation mechanisms in other cancers and diseases, which apply to embryonic abnormalities, growth injuries, etc., in which the Hedgehog signaling pathway is involved.
Acknowledgments
We gratefully acknowledge our patients and their families, as well as Maria Fernanda Rubio and Mónica Alejandra Costas for their kind gift of GAPDH primers, and Matias Maskin, Alejandra Abeldaño, Paula Carolina Luna, and Patricia Della Giovanna for their assistance in the enrollment of patients for the study.
Author Contributions
P.J.A., F.M.S., and L.D.M. conceived and designed the experiments; M.F.M. and M.V.R. performed the experiments; L.D.M., A.G., and A.P.M. contributed reagents and materials; C.M. contributed to data analysis; P.J.A., M.F.M., and L.D.M. analyzed the data and wrote the paper.
Funding
This research was funded by the Ministerio de Ciencia y Tecnología, grant Préstamo BID-PICT 2013-2461, and the Fundación del Cancer de Piel Argentina. M.F.M. and L.D.M. are fellows of the Instituto Nacional de Cáncer and Fundación Florencio Fiorini, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gorlin R.J. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet. Med. 2004;6:530–539. doi: 10.1097/01.GIM.0000144188.15902.C4. [DOI] [PubMed] [Google Scholar]
- 2.Lindström E., Shimokawa T., Toftgård R., Zaphiropoulos P.G. PTCH mutations: Distribution and analyses. Hum. Mutat. 2006;27:215–219. doi: 10.1002/humu.20296. [DOI] [PubMed] [Google Scholar]
- 3.Johnson R.L., Rothman A.L., Xie J., Goodrich L.V., Bare J.W., Bonifas J.M., Quinn A.G., Myers R.M., Cox D.R., Epstein E.H., et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 4.Liu A., Wang B., Niswander L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 5.Dahmane N., Lee J., Robins P., Heller P., Ruiz I., Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/37408. [DOI] [PubMed] [Google Scholar]
- 6.Ponti G., Pollio A., Mignogna M.D., Pellacani G., Pastorino L., Bianchi-Scarrà G., Di Gregorio C., Magnoni C., Azzoni P., Greco M., et al. Unicystic ameloblastoma associated with the novel K729M PTCH1 mutation in a patient with nevoid basal cell carcinoma (Gorlin) syndrome. Cancer Genet. 2012;205:177–181. doi: 10.1016/j.cancergen.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Adolphe C., Hetherington R., Ellis T., Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006;66:2081–2088. doi: 10.1158/0008-5472.CAN-05-2146. [DOI] [PubMed] [Google Scholar]
- 8.Kimonis V.E., Goldstein A.M., Pastakia B., Yang M.L., Kase R., DiGiovanna J.J., Bale A.E., Bale S.J. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 1997;69:299–308. doi: 10.1002/(SICI)1096-8628(19970331)69:3<299::AID-AJMG16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Azurmendi P.J., Oddo E.M., Toledo J.E., Martin R.S., Ibarra F.R., Arrizurieta E.E. Sexual hormones modulate compensatory renal growth and function. Medicina. 2013;73:513–519. [PubMed] [Google Scholar]
- 10.Klein R.D., Dykas D.J., Bale A.E. Clinical testing for the nevoid basal cell carcinoma syndrome in a DNA diagnostic laboratory. Genet. Med. 2005;7:611–619. doi: 10.1097/01.gim.0000182879.57182.b4. [DOI] [PubMed] [Google Scholar]
- 11.Soufir N., Gerard B., Portela M., Brice A., Liboutet M., Saiag P., Descamps V., Kerob D., Wolkenstein P., Gorin I., et al. PTCH mutations and deletions in patients with typical nevoid basal cell carcinoma syndrome and in patients with a suspected genetic predisposition to basal cell carcinoma: A French study. Br. J. Cancer. 2006;95:548–553. doi: 10.1038/sj.bjc.6603303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzuoccolo L.D., Martínez M.F., Muchnik C., Azurmendi P.J., Stengel F. Nevoid basal cell carcinoma syndrome with corpus callosum agenesis, PTCH1 mutation and absence of basal cell carcinoma. Medicina. 2014;74:307–310. [PubMed] [Google Scholar]
- 13.Martínez M.F., Mazzuoccolo L.D., Oddo E.M., Iscoff P.V., Muchnik C., Neumann H.P.H., Martin R.S., Fraga A.R., Azurmendi P.J. Co-inheritance of autosomal dominant polycystic kidney disease and naevoid basal cell carcinoma syndrome: Effects on renal progression. Nephron. 2018;140:282–288. doi: 10.1159/000490771. [DOI] [PubMed] [Google Scholar]
- 14.Coffa J., van den Berg J. Modern Approaches to Quality Control. IntechOpen; London, UK: 2011. Analysis of MLPA data using novel software coffalyser.NET by MRC-Holland; pp. 125–151. [Google Scholar]
- 15.Sim N.-L., Kumar P., Hu J., Henikoff S., Schneider G., Ng P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildeman M., van Ophuizen E., den Dunnen J.T., Taschner P.E.M. Improving sequence variant descriptions in mutation databases and literature using the Mutalyzer sequence variation nomenclature checker. Hum. Mutat. 2008;29:6–13. doi: 10.1002/humu.20654. [DOI] [PubMed] [Google Scholar]
- 18.Desmet F.O., Hamroun D., Lalande M., Collod-Bëroud G., Claustres M., Béroud C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:1–14. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.di Ciano L.A., Azurmendi P.J., Vlachovsky S.G., Celía A.F., Oddo E.M., Arrizurieta E.E., Silberstein C.M., Ibarra F.R. Gender differences in blood pressure, renal function and response to high-sodium diet in wistar rats, Diferencias de género en presión arterial, función renal y respuesta a la dieta hipersódica en ratas wistar. Rev. Nefrol. Dial. y Traspl. 2018;38:1–27. [Google Scholar]
- 20.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubio M.F., Fernandez P.N.L., Alvarado C.V., Panelo L.C., Grecco M.R., Colo G.P., Martínez-Noel G.A., Micenmacher S.M., Costas M.A. Cyclin D1 is a NF-κB corepressor. Biochim. Biophys. Acta. 2012;1823:1119–1131. doi: 10.1016/j.bbamcr.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponti G., Bertazzoni G., Pastorino L., Monari E., Cuoghi A., Bergamini S., Bellei E., Benassi L., Azzoni P., Petrachi T., et al. Proteomic analysis of PTCH1 +/− fibroblast lysate and conditioned culture media isolated from the skin of healthy subjects and Nevoid Basal Cell Carcinoma Syndrome patients. Biomed. Res. Int. 2013;2013:1–8. doi: 10.1155/2013/794028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruvost-Balland C., Gorry P., Boutet N., Magnaldo T., Mamelle G., Margulis A., Kolb F., Duvillard P., Spatz A., Brugières L., et al. Étude clinique et recherche de mutations germinales du gène PTCH 1 dans le syndrome des hamartomes basocellulaires. Ann. Dermatol. Venereol. 2006;133:117–123. doi: 10.1016/S0151-9638(06)70861-4. [DOI] [PubMed] [Google Scholar]
- 25.Pastorino L., Cusano R., Nasti S., Faravelli F., Forzano F., Baldo C., Barile M., Gliori S., Muggianu M., Ghigliotti G., et al. Molecular characterization of Italian nevoid basal cell carcinoma syndrome patients. Hum. Mutat. 2005;25:322–323. doi: 10.1002/humu.9317. [DOI] [PubMed] [Google Scholar]
- 26.Hahn H., Wicking C., Zaphiropoulous P.G., Gailani M.R., Shanley S., Chidambaram A., Vorechovsky I., Holmberg E., Unden A.B., Gillies S., et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/S0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 27.Knudson A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini C., Maturo M.G., Di Nardo L., Ciciarelli V., Gutiérrez García-Rodrigo C., Fargnoli M.C. Understanding the molecular genetics of basal cell carcinoma. Int. J. Mol. Sci. 2017;18:2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Z., Li J., Du J., Zhang H., Shen Y., Wang C.-Y., Wang S. A missense mutation in PTCH2 underlies dominantly inherited NBCCS in a Chinese family. J. Med. Genet. 2008;45:303–308. doi: 10.1136/jmg.2007.055343. [DOI] [PubMed] [Google Scholar]
- 30.Pastorino L., Ghiorzo P., Nasti S., Battistuzzi L., Cusano R., Marzocchi C., Garrè M.L., Clementi M., Scarrà G.B. Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am. J. Med. Genet. A. 2009;149:1539–1543. doi: 10.1002/ajmg.a.32944. [DOI] [PubMed] [Google Scholar]
- 31.Unden A.B., Holmberg E., Lundh-Rozell B., Stähle-Bäckdahl M., Zaphiropoulos P.G., Toftgård R., Vorechovsky I. Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: Different in vivo mechanisms of PTCH inactivation. Cancer Res. 1996;56:4562–4565. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 32.Gu X.-M., Zhao H.-S., Sun L.-S., Li T.-J. PTCH mutations in sporadic and Gorlin-syndrome-related odontogenic keratocysts. J. Dent. Res. 2006;85:859–863. doi: 10.1177/154405910608500916. [DOI] [PubMed] [Google Scholar]
- 33.Pastorino L., Pollio A., Pellacani G., Guarneri C., Ghiorzo P., Longo C., Bruno W., Giusti F., Bassoli S., Bianchi-Scarrà G., et al. Novel PTCH1 mutations in patients with keratocystic odontogenic tumors screened for nevoid basal cell carcinoma (NBCC) syndrome. PLoS ONE. 2012;7:e43827. doi: 10.1371/journal.pone.0043827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y.-L., Zhang W.-F., Peng B., Wang C.-N., Wang Q., Bian Z. Germline mutations of the PTCH gene in families with odontogenic keratocysts and nevoid basal cell carcinoma syndrome. Tumor Biol. 2006;27:175–180. doi: 10.1159/000093054. [DOI] [PubMed] [Google Scholar]
- 35.Levanat S., Gorlin R.J., Fallet S., Johnson D.R., Fantasia J.E., Bale A.E. A two-hit model for developmental defects in Gorlin syndrome. Nat. Genet. 1996;12:85–87. doi: 10.1038/ng0196-85. [DOI] [PubMed] [Google Scholar]
- 36.Reifenberger J., Wolter M., Weber R.G., Megahed M., Ruzicka T., Lichter P., Reifenberger G. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–1803. [PubMed] [Google Scholar]
- 37.Zurawel R.H., Allen C., Wechsler-Reya R., Scott M.P., Raffel C. Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer. 2000;28:77–81. doi: 10.1002/(SICI)1098-2264(200005)28:1<77::AID-GCC9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]