Abstract
Lactic acid bacteria (LAB) are involved in several food fermentations and many of them provide strain-specific health benefits. Herein, the probiotic potential of the foodborne strain Lactobacillus fermentum MBC2 was investigated through in vitro and in vivo approaches. Caenorhabditis elegans was used as an in vivo model to analyze pro-longevity and anti-aging effects. L. fermentum MBC2 showed a high gut colonization capability compared to E. coli OP50 (OP50) or L. rhamnosus GG (LGG). Moreover, analysis of pumping rate, lipofuscin accumulation, and body bending showed anti-aging effects in L. fermentum MBC2-fed worms. Studies on PEPT-1 mutants demonstrated that pept-1 gene was involved in the anti-aging processes mediated by this bacterial strain through DAF-16, whereas the oxidative stress protection was PEPT-1 independent. Moreover, analysis of acid tolerance, bile tolerance, and antibiotic susceptibility were evaluated. L. fermentum MBC2 exerted beneficial effects on nematode lifespan, influencing energy metabolism and oxidative stress resistance, resulted in being tolerant to acidic pH and able to adhere to Caco-2 cells. Overall, these findings provide new insight for application of this strain in the food industry as a newly isolated functional starter. Furthermore, these results will also shed light on C. elegans molecular players involved in host-microbe interactions.
Keywords: probiotics, fermented foods, mozzarella di bufala campana, nematode, Caco-2 cells
1. Introduction
Lactic acid bacteria (LAB) are generally recognized as safe (GRAS) microorganisms mainly involved in various food fermentations [1,2].
In recent years, interests in the host health-promoting effects exerted by different LAB strains have been growing. As a result, several probiotic products have been developed to improve health [3]. Probiotics are known to improve gut microbiota balance, confer protection against potential pathogenic bacteria, and prevent and/or cure intestinal diseases [4,5]. In particular, numerous bacterial strains belonging to the Lactobacillus genus are commonly used as probiotics, and accepted as safe by the US Food and Drug Administration and the European Food Safety Authority. Among them, L. fermentum is a heterofermentative species belonging to the Firmicutes phylum [6]. It is a natural inhabitant of the gastrointestinal tract and is often isolated from both human biological samples (i.e., human breast milk and feces) as well as from dairy and non-dairy sources. L. fermentum is one of the most common cultivable and predominant microbes in fermented dairy products and is usually employed as a starter culture during various food fermentation processes, including cheeses [7,8] or sourdough [9,10]. The uniqueness of certain strains is to survive the harsh gastrointestinal (GI) tract conditions, such as low pH and high bile concentration, transiently colonizing the host gut, where they can exert health-promoting activities. Increasing evidence points at different strains belonging to this species as promising probiotic candidates [11,12,13], as also revealed by recent genomic analysis performed on draft genome sequences [14].
Nowadays, growing interest in the pro-longevity effects of probiotics has led to the need for convenient in vivo models to understand the mechanisms of probiotic activity. In recent years, the nematode Caenorhabditis elegans has become a powerful in vivo model to study host-probiotics interactions. Its advantages include ease of handling, transparency of the body, short lifespan, and absence of ethical issues. Another important C. elegans tool is the availability of transgenic animals, allowing the analysis of gene expression patterns or protein localization in live animals [15]. In the context of oxidative stress, the use of GFP-transgenic nematodes allows the investigation of in vivo stress by fluorescence analysis [16]. Moreover, several genes involved in the oxidative stress response are highly conserved between humans and nematodes. To this respect, lmd-3 mutants were employed to demonstrate that LysM domain protein 3 (LMD-3), a homolog of human oxidation resistance 1 (OXR1), protected cells against oxidative stress and aging in C. elegans [17].
Microorganisms represent the only food source for nematodes, which pass through the pharynx to the gut and can influence the nematode physiology through their metabolites [18]. Moreover, the use of nematodes for probiotic screening is favored by the possibility to easily monitor anti-aging markers, as well as body fat storage [19,20]. The availability of a large number of mutants can help to study the mechanism of action of a compound or pathways involved in host-microorganism interaction. Eat-2 mutants, whose mutation reduced nematode pharyngeal pumping rate, were employed to evaluate the effects on nematode longevity promoted by a strain of Lactobacillus salivarius [21]. Furthermore, C. elegans mutants, defective in innate immunity, were successfully used to elucidate the mechanisms involved in lifespan extension mediated by Propionibacterium freudenreichii [22].
Several newly isolated foodborne LAB were reported to induce beneficial effects in nematodes [23,24,25]. Recently, L. pentosus D303.36 and L. coryniformis H307.6 strains, isolated from table olives, were found to increase nematode lifespan and promote anti-aging effects [26].
The L. fermentum strain MBC2 described in the present work was previously isolated from Mozzarella di Bufala Campana (MBC), an example of traditional Italian PDO (Protected Designation of Origin) cheese, containing high titers of live and complex microbiota dominated by Lactobacillus delbrueckii, L. fermentum, and Leuconostoc lactis species [27,28]. Feeding C. elegans with a complex LAB consortium derived from MBC influenced nematode longevity, larval development, fertility, lipid accumulation, and gene expression related to fat metabolism [28]. Moreover, oral administration of the same MBC-derived microbiota to obese mice exerted a protective effect toward high-fat diet induced inflammation [29]. In the present work, we have employed the simplified model organism C. elegans to explore potential health promoting features of L. fermentum MBC2, in light of a possible application of such strain as a starter with probiotic added value.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Bacterial strains used in this study were Lactobacillus fermentum MBC2 (previously isolated from MBC and available within laboratory collection), Lactobacillus rhamnosus GG (LGG, ATCC53103) and Escherichia coli OP50 (OP50), previously obtained from the Caenorhabditis Genetics Center (CGC); probiotic LGG and OP50 were used as control where indicated. LAB strains were routinely grown in De Man Rogosa Sharpe (MRS) medium for 24–48 h at 37 °C under anaerobic conditions. OP50 was grown in Luria–Bertani (LB) broth at 37 °C overnight. All media were provided by Becton Dickinson (Milan, Italy).
2.2. C. elegans Strains and Growth Conditions
The wild-type C. elegans strain N2, CL2166 (dvIs19 [(pAF15)gst-4p::GFP::NLS] III), pept-1(lg601) and CF1038 daf-16(mu86) mutants used in experiments were propagated on nematode growth medium (NGM) modified to be peptone-free (mNGM) [28]. Worms were fed with OP50, LGG or L. fermentum. Afterward, 25 μL of each overnight culture, resuspended in M9 buffer, corresponding to 10 mg of bacterial cells, was spread on 3.5 cm diameter mNGM plates. Heat-killed lactobacilli cells were prepared as follows: bacteria were cultured overnight and resuspended in M9 medium as described above; cells were then incubated at 65 °C for 90 min and deposited onto mNGM agar plates. Heat-killed cells were also plated on MRS agar in parallel to ensure that no viable cells remained. OP50 cells were treated in the same manner.
2.3. Brood Size and Body Size Measurements
Synchronized worms obtained as above were incubated at 16 °C on mNGM plates seeded with bacteria, allowing embryos laying. The number of progeny and body length analysis were recorded as described by Reference [26].
2.4. Bacteria Colonization Assay of C. elegans Gut
For each experiment, 10 animals at L4 stage and at 5 days of adulthood were washed and lysed according to Reference [30]. Whole worm lysates were plated onto MRS-agar plates. The number of colony forming units (CFU) was counted after 48 h of incubation at 37 °C, anaerobically. Instead, OP50-fed worm lysed were plated onto LB-agar plated and incubated at 37 °C.
2.5. Pumping Rate and Body Bending Measurements
About 10 worms of each condition were analysed at the stage of 13 days adult, as described in Reference [26].
2.6. Lipid Droplets Visualization
Nematodes at L4 stage, grown as indicated above, were stained as described by Reference [28].
2.7. Lipofuscin Analysis
The autofluorescence of intestinal lipofuscin was measured as an index of senescence at day 13 of adulthood. Randomly selected worms from the plates with bacterial lawn were washed three times with M9 buffer. Worms were then placed onto a 3% agar pad containing 20 mmol L−1 sodium azide. Lipofuscin autofluorescence was detected by fluorescence microscopy (Zeiss Axiovert 25, Jena, Germany).
2.8. Analysis of C. elegans Strain GST4::GFP Fluorescence
Synchronized transgenic worms were transferred daily on mNGM plates containing different bacteria and incubated at 16 °C, as described above. At the stage of one or 13 days adult the worms were anesthetized with sodium azide (20 mmol L−1) on 3% agarose pad on a glass slide and the fluorescence was viewed under a Zeiss Axiovert 25 microscope. The experiments were repeated three times and 15 worms per group were used in each experiment.
2.9. Measurement of Reactive Oxygen Species (ROS)
ROS formation in C. elegans was measured using the fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) according to Reference [31] with some modifications. After larval development on different bacteria, L4 or 13 days adult worms were washed in M9 buffer and then transferred individually into wells of a 96-well microtiter plate, containing 50 μL of M9 buffer. Subsequent to the complete transfer of the worms, 50 μL of 100 μmol L−1 H2DCF-DA (SIGMA-ALDRICH, Milan, Italy) in methanol was added to the wells. H2DCF-DA is a membrane-permeable non-fluorescent probe, which can enter the cells of the worm and it is intracellularly converted to H2DCFs. This probe can be oxidized by ROS to yield the fluorescent dye DCF. The changes of fluorescence in worms indicate the accumulation of ROS at different time points (30, 60, and 120 min). The measurement was performed using a multiplate reader (Promega, GloMax multidetection system, Madison, WI, USA) at excitation/emission wavelengths of 485 and 520 nm.
2.10. Acid and Bile Salt Tolerance Assay
Tolerance to gastrointestinal conditions of L. fermentum MBC2 strain was evaluated as described in Reference [26].
2.11. Antibiotic Susceptibility Tests
The antibiotic discs used in the susceptibility test were purchased from Biolab Zrt. (Budapest, Hungary). Each disc of 6 mm diameter contained the following antibiotic at the amount reported in parenthesis: amikacin (30 µg), aztreonam (30 µg), vancomycin (30 µg), streptomycin (25 µg), erythromycin (15 µg), tetracycline (30 µg), cefalotin (30 µg), gentamicin (10 µg), cefotaxime (30 µg), chloramphenicol (30 µg), clindamycin (30 µg), penicillin G (10 µg), ampicillin (10 µg), tobramycin (10 µg), cefuroxime (30 µg), oxacillin (1 µg), fosfomycin (50 µg), rifampicin (30 µg), carbenicillin (100 µg), and mezlocillin (75 µg). 100 μL of overnight cultures of L. fermentum MBC2 or LGG (OD600 = 1.3) were spread onto MRS agar plates, in which the antibiotic discs were placed. The plates were then incubated under anaerobic conditions for 24 h at 37 °C. The zones of inhibition were measured from the center of the disc and recorded.
2.12. Adhesion of L. fermentum MBC2 to Caco-2 Cells
The human intestinal Caco-2/TC7 cell line was provided by Monique Rousset (Institute National de la Santé et de la Recherche Médicale, INSERM, Paris, France) and routinely maintained as described in Reference [26]. For the adhesion assay, Caco-2 cells were seeded in 12-well plates (Becton Dickinson, Milan, Italy) and, after confluency, were left for 14–17 days to allow differentiation [32]. Medium was changed three times a week. Complete DMEM was replaced with antibiotic- and serum-free DMEM 16 h before the assay. On the day of the assay, overnight bacterial cultures of L. fermentum MBC2 and LGG were diluted 1:100 in MRS broth and grown for 5 h to the exponential growth phase. After monitoring the OD600, appropriate amounts of bacterial cells were harvested by centrifugation at 5000× g for 10 min, resuspended in antibiotic- and serum-free DMEM and added to cell monolayers at a concentration of 1 × 108 or 1 × 109 CFU/well. Co-cultures of bacteria and Caco-2 cells were incubated at 37 °C for 1.5 h. Non-adhering bacteria were then removed by 5 washes with Hanks’ Balanced Salt solution (HBSS: 137 mmol L−1 NaCl, 5.36 mmol L−1 KCl, 1.67 mmol L−1 CaCl2, 1 mmol L−1 MgCl2, 1.03 mmol L−1 MgSO4, 0.44 mmol L−1 KH2PO4, 0.34 mmol L−1 Na2HPO4, 5.6 mmol L−1 glucose) and cell monolayers were lysed with 1% Triton-X-100, according to Reference [33]. Adhering, viable bacterial cells were quantified by plating appropriate serial dilutions of Caco-2 lysates on MRS medium.
2.13. Statistical Analysis
All experiments were performed at least in triplicate. Data are presented as mean ± SD. Prior to analysis, normal distribution and homogeneity of variance of all variables were assumed with Shapiro–Wilk and Levene’s tests, respectively. For in vivo experiments in C. elegans, the statistical significance was determined by Student’s t test or one-way ANOVA analysis coupled with a Bonferroni post test (GraphPad Prism 5.0 software, GraphPad Software Inc., La Jolla, CA, USA). For in vitro experiments, statistical significance was evaluated by one-way ANOVA, followed by post-hoc Tukey honestly significant difference (HSD) test. Statistical univariate analysis was performed with the “Statistica” software package (version 5.0; Stat Soft Inc., Tulsa, OK, USA). Differences with p values < 0.05 were considered significant and were indicated as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. L. fermentum MBC2 Affected Worm Viability and Fertility
L. fermentum MBC2 was previously isolated as a predominant member of the MBC microbiota, which was shown to protect mice from obesity-associated inflammation [29].
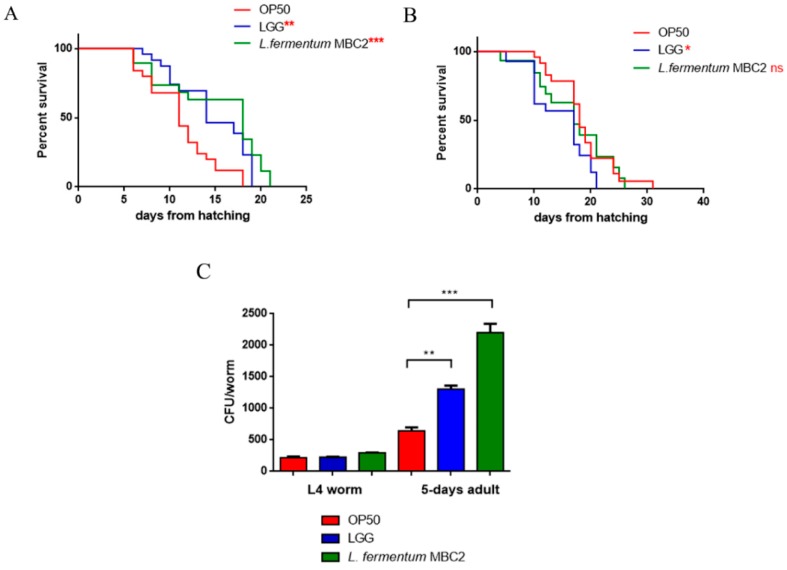
To investigate whether this strain induced beneficial effects on C. elegans physiology, the survival rate was first examined. The median lifespan of wild type nematodes, fed L. fermentum MBC2 from embryo hatching, resulted significantly extended as compared to the LGG and OP50-fed controls (Figure 1A). In particular, 50% of worm viability in L. fermentum MBC2-fed nematodes was recorded at day 18, in comparison with day 11 and 14 recorded in OP50- and LGG-fed animals, respectively. By contrast, the pro-longevity effect was not observed when the dietary administration was based on heat-killed lactobacilli (Figure 1B). Therefore, the positive effect on C. elegans lifespan was dependent on the viability of L. fermentum MBC2 strain. However, it should be noticed that a very small number of worms fed heat killed E. coli OP50 could survive over 30 days. This finding can be attributable to a possible impairment of gut colonization by killed bacteria, leading to less stressed animals, since in aged worms bacterial colonization indeed turns out in the production of harmful molecules acting as virulence factors [34].
Figure 1.
Effect of L. fermentum MBC2 on nematode lifespan and colonization capacity. (A) Kaplan–Meier survival plot of N2 worms fed L. fermentum MBC2. Lifespans of OP50- and LGG-fed animals are reported as controls; n = 60 for each data point of single experiments. (B) Effect of heat-killed strains on C. elegans viability. (C) Bacterial colony forming units (CFU) recovered from nematodes were obtained by plating whole lysates of L4 and 5-day-old adults fed L. fermentum MBC2, LGG or OP50. Bars represent the mean of three independent experiments. Asterisks indicate significant differences (* p < 0.05, ** p < 0.01, *** p < 0.001), ns: not significant.
Microscopy analysis of larval development showed that L. fermentum MBC2 diet significantly affected body size. Body length increased with age in all groups, however, L. fermentum MBC2-fed worms were notably smaller compared to LGG and OP50 starting from 3 days after hatching (Supplementary Figure S1A). Moreover, feeding worms with L. fermentum MBC2 affected the fertility of animals; the reproduction rates of these nematodes indicated a reduction of 40% in the progeny production, as compared to OP50-fed animals. A similar reduction was also noted in the case of LGG-fed animals (Supplementary Figure S1B).
Afterwards, the intestinal colonization capability was explored by plating worm lysates at different time points and evaluating the number of viable bacterial cells, expressed as CFU. Results demonstrated that the intestinal bacterial load of L. fermentum MBC2 diet increased along the lifespan; notably, at 5 days of adulthood the CFU number relative to L. fermentum MBC2 resulted to be about 2-fold and about 4-fold higher than that relative to LGG and OP50, respectively (Figure 1C).
3.2. Worms Fed L. fermentum MBC2 Showed a Delay in Aging
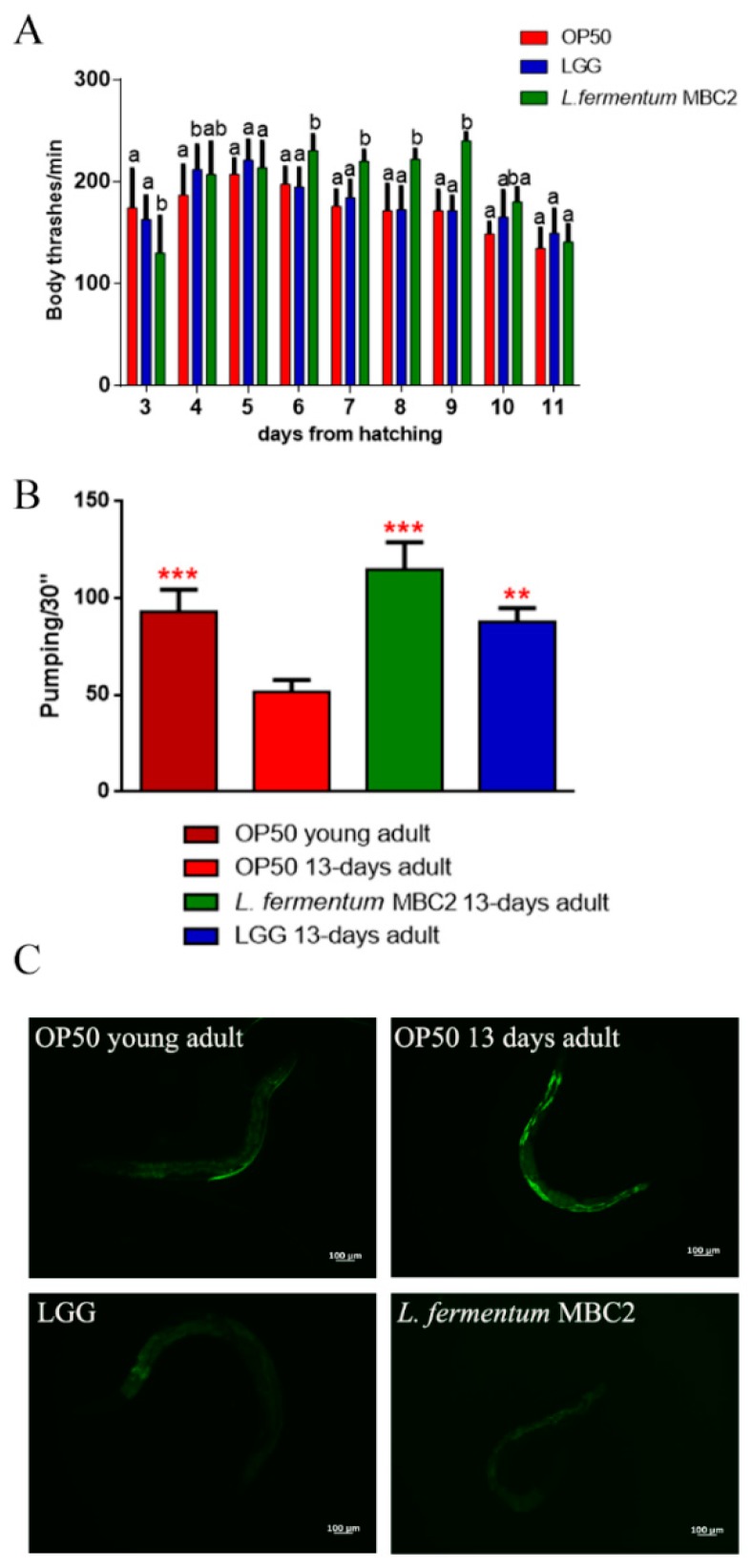
To investigate whether feeding with L. fermentum MBC2 positively impacted on nematode quality of life, changes in age-related biomarkers, such as body movement, pumping, and lipofuscin accumulation were measured.
The locomotory rate of C. elegans was evaluated in the range between 3 and 11 days from hatching. L. fermentum MBC2-fed nematodes displayed, from 6 to 9 days, a higher motility than control worms (Figure 2A).
Figure 2.
Analysis of aging markers in C. elegans fed L. fermentum MBC2. (A) Body bending of C. elegans fed Lactobacillus strains with respect to OP50, measured for 30 s. Bars represent the mean of three independent experiments. Different letters indicate significant differences (p < 0.05) (B) Pumping rate of 13-days-old worms, measured for 30 s and determined from the mean of 10 worms for each bacterial strain. Worms fed OP50 or LGG were used as controls. Statistical analysis was evaluated by one-way ANOVA with the Bonferroni post-test; asterisks indicate significant differences (** p <0.01, *** p <0.001). Bars represent the mean of three independent experiments. (C) Autofluorescence of lipofuscin granules in C. elegans fed L. fermentum MBC2, LGG and OP50 on day 13. Ten worms were used for each measurement. Scale bar = 100 μm.
The pharyngeal pumping rate measures muscle function, and the pumping activity is associated with food intake ability. Although the frequency of pharyngeal pumping generally declines with age, statistical analysis showed a significantly higher pumping rate, at 13 days of adulthood, in L. fermentum MBC2-fed nematodes with respect to OP50-fed worms. In particular, pumping rate measured in both L. fermentum MBC2- and LGG-fed worms was similar to that of young adult OP50-fed worms (Figure 2B). Since intracellular lipofuscin is a marker of cellular damage during aging, the autofluorescence of worms was also analysed (Figure 2C). Old nematodes fed L. fermentum MBC2 showed a reduced fluorescence compared to LGG- and OP50-fed young adults, while OP50-fed old animals displayed a marked accumulation of fluorescent granules along the intestine, typical of aged animals.
3.3. L. fermentum MBC2 Affected Lipid Accumulation and Oxidative Stress Responses
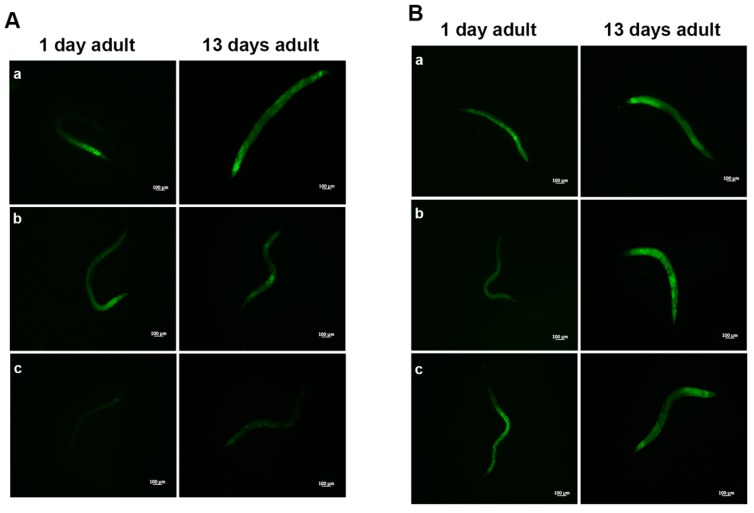
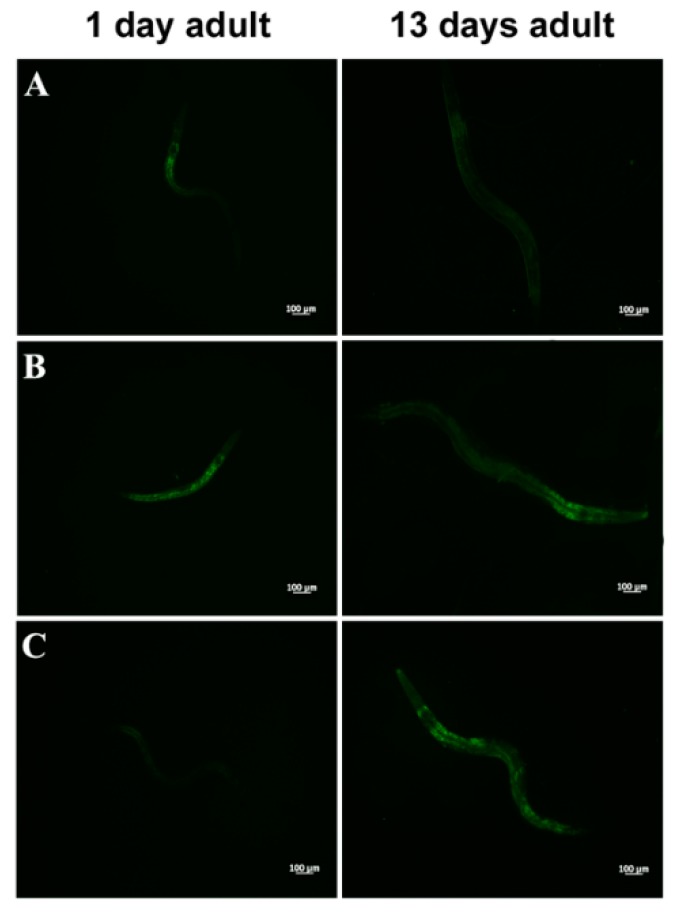
Aging dysregulates intracellular lipid metabolism in several organisms [35,36]. Therefore, we evaluated whether feeding worms with L. fermentum MBC2 affected lipid droplets accumulation during aging. BODIPY staining detected large amounts of intestinal fat in OP50-fed worms, which were not observed in L. fermentum MBC2-fed worms (Figure 3A). Lower amounts of intestinal lipid droplets were also observed when worms were fed probiotic strain LGG, as compared to OP50. By contrast, heat-killed L. fermentum MBC2 diet induced an enhanced fluorescence, comparable to OP50-fed worms, confirming that viability of L. fermentum MBC2 was an essential requirement to exert pro-longevity effects in these animals. On the other hand, animals fed heat-killed LGG maintained a reduced fluorescent signal at the stage of 1-day adult (Figure 3B).
Figure 3.
Visualization of lipid droplets. (A) BODIPY staining of one and 13 days adult worms grown in the presence of OP50 (a), LGG (b) or L. fermentum MBC2 (c) as food sources. (B) BODIPY staining of heat-killed bacteria-fed worms. Nematodes were grown on OP50 (a), LGG (b) or L. fermentum MBC2 (c). Scale bar = 100 μm.
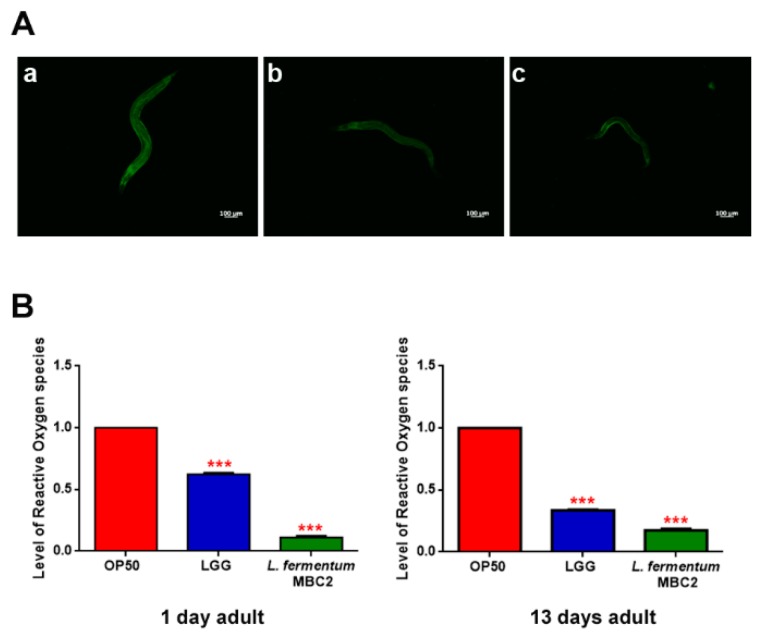
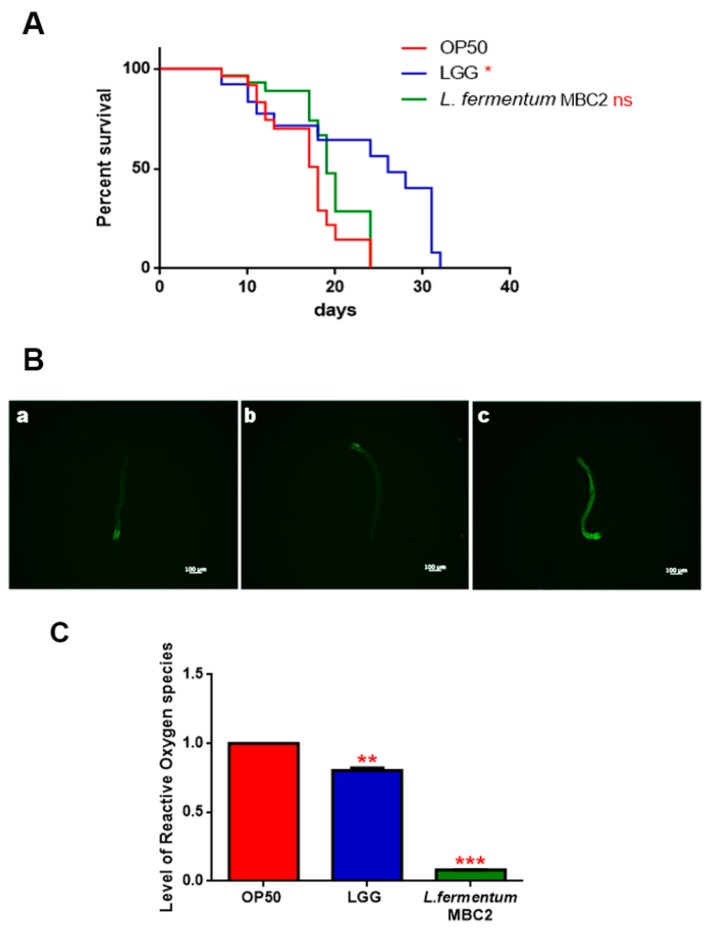
Extended longevity has been shown to correlate with enhanced resistance against oxidative damage and other stresses [37,38]. We therefore analyzed the effect of feeding nematodes with L. fermentum MBC2 in C. elegans transgenic GST4::GFP strain, since GST-4 is a glutathionyl S-transferase with highest activity against lipoperoxides [39]. Microscopy analysis showed that fluorescence intensity of L. fermentum MBC2-fed worms was notably lower than that of OP50- and LGG-fed animals (Figure 4A). To test whether there were differences in ROS accumulation between worms grown on different bacterial lawns, intracellular ROS levels were investigated by using H2DCF-DA, a fluorescent probe. Figure 4B shows that 1-day adult L. fermentum MBC2-fed worms effectively reduced the production of ROS compared to control worms. Similar results were obtained when ROS production was evaluated at 13 days of adulthood.
Figure 4.
Analysis of oxidative stress in GST4::GFP strain and ROS evaluation. (A) Fluorescence microscopy of GST4::GFP worm strain at the stage of 1 day adult fed OP50 (a), LGG (b) or L. fermentum MBC2 (c). Scale bar = 100 μm. (B). Measurement of ROS levels in L. fermentum MBC2-, LGG- and OP50-fed worms at 1 and 13 days of adulthood. Statistical analysis was evaluated by one-way ANOVA with the Bonferroni post-test; asterisks indicate significant differences (*** p < 0.001). Bars represent the mean of three independent experiments.
3.4. PEPT-1 Gene Was Involved in Effects Exerted by L. fermentum MBC2
Further analyses were carried out using C. elegans mutant PEPT-1 strain, since pept-1 gene was found to be one of the major regulators of fat content in C. elegans [40]. PEPT-1 strain carries a deletion in the gene encoding this intestinal peptide transporter, which contributes to lipid storage and metabolism in C. elegans [41].
In PEPT-1 mutants, L. fermentum MBC2 diet did not induce a pro-longevity effect as observed in wild type animals. Indeed, mutant nematodes fed L. fermentum MBC2 displayed a lifespan almost identical to that of animals fed OP50, while LGG diet still prolonged mutant lifespan (Figure 5A). In particular, the median survival of worms was recorded at days 18, 19, and 26 for OP50-, L. fermentum MBC2-, and LGG-based diets, respectively. Furthermore, pumping contractions were about 20% higher with respect to OP50 (Supplementary Figure S2A), unlike N2 worms. Body bending in L. fermentum MBC2-fed worms was slightly higher as compared to OP50 and reduced as compared to LGG (Supplementary Figure S2B). Microscopy analysis of 13 days adult nematodes showed that accumulation of lipofuscin was higher in L. fermentum MBC2-fed animals as compared to those grown on LGG and similar to OP50-fed worms. These results notably differ from that obtained in the case of N2 strain, highlighting the absence of anti-aging effects on mutant worms (Figure 5B). Then, ROS production was evaluated in PEPT-1 mutants: surprisingly, in L. fermentum MBC2-fed worms ROS levels were reduced with respect to OP50 control, similarly to results observed in N2 animals (Figure 5C). Moreover, correlation between lipid accumulation and pro-longevity effects was confirmed also in PEPT-1 mutants: microscopy analysis of BODIPY at the stage of one and 13 days adult showed an increased fluorescence in L. fermentum MBC2-fed worms with respect to controls (Figure 6).
Figure 5.
Effect of L. fermentum MBC2 on PEPT-1 mutant strain and analysis of aging biomarkers. (A) Kaplan-Meier survival plot of PEPT-1 worms fed L. fermentum MBC2. Lifespans of OP50- and LGG-fed animals are reported as controls; n = 60 for each data point of single experiments. (B) Analysis of autofluorescence of lipofuscin in PEPT-1 worms fed OP50 (a), LGG (b) and L. fermentum MBC2 (c) at day 13. Ten worms were used for each measurement. Scale bar = 100 μm. (C) Measurement of ROS levels in L. fermentum MBC2-, LGG- and OP50-fed PEPT-1 mutants at 1 day of adulthood. Statistical analysis was evaluated by one-way ANOVA with the Bonferroni post-test; asterisks indicate significant differences (** p <0.01; *** p <0.001). Bars represent the mean of three independent experiments.
Figure 6.
Visualization of lipid droplets in PEPT-1 mutants. BODIPY staining of one and 13 days adult worms grown on OP50 (A), LGG (B) or L. fermentum MBC2 (C) lawns.
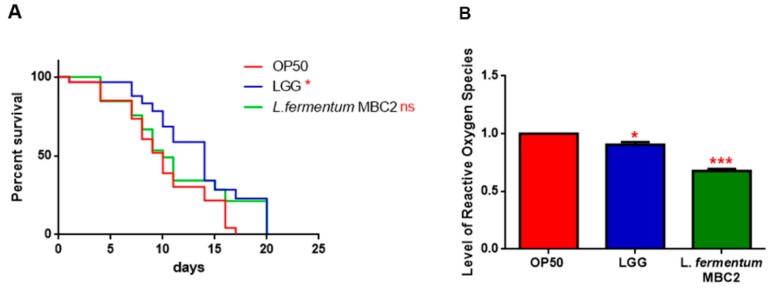
Since the transcriptional factor DAF-16 is reported to be involved in the signalling cascade mediating the longevity of PEPT-1 mutants [42], the effects of L. fermentum MBC2 on DAF-16 mutants’ lifespan were analyzed. As shown in PEPT-1 mutants, diet based on L. fermentum MBC2 led to an effect similar to that based on OP50, while LGG diet still prolonged lifespan (Figure 7A). In particular, the median survival of worms was recorded at day 9 for OP50 and L. fermentum MBC2 and at day 12 for LGG-based diets, respectively. Furthermore, ROS levels in L. fermentum MBC2-fed mutants were 40% lower than those detected in OP50-fed animals (Figure 7B).
Figure 7.
Effect of L. fermentum MBC2 on DAF-16 mutant animals and evaluation of ROS levels (A) Kaplan-Meier survival plot of DAF-16 worms fed L. fermentum MBC2. Lifespans of OP50- and LGG-fed animals are reported as controls; n = 60 for each data point of single experiments. (B) Measurement of ROS levels in L. fermentum MBC2-, LGG- and OP50-fed DAF-16 mutants at 1 day of adulthood. Statistical analysis was evaluated by one-way ANOVA with the Bonferroni post-test; asterisks indicate significant differences (* p < 0.05; *** p <0.001). Bars represent the mean of three independent experiments.
3.5. In Vitro Evaluation of Probiotic Features
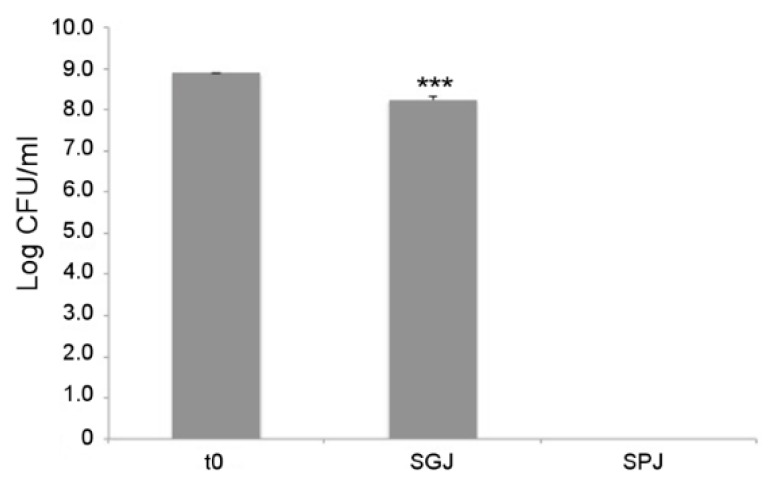
As a first probiotic trait, tolerance to gastrointestinal conditions was assayed for L. fermentum MBC2 using a series of sequential treatments that simulate bacterial transit along the mammalian GI tract. As shown in Figure 8, each treatment differentially affected the survival of L. fermentum MBC2. In particular, 1 h incubation in simulated gastric juice (SGJ), characterised by low pH (2.5), exerted a mild reduction of bacterial counts with respect to the initial time point, suggesting that this strain was able to endure acidic environments. However, subsequent treatment of the surviving bacterial cells in simulated pancreatic juice (SPJ) containing bile salts and pancreatin, severely affected bacterial survival, as revealed by the absence of L. fermentum MBC2 colonies recovered after 2 h of incubation (Figure 8).
Figure 8.
In vitro tolerance to simulated gastro-intestinal conditions. Cell counts of viable L. fermentum MBC2 recovered at the initial time point (t0), following 1h incubation in Simulated Gastric Juice (SGJ) and at the end of 2h incubation in Simulated Pancreatic Juice (SPJ). Data are reported as log of bacterial CFU recovered after plating. Columns represent the mean ± SD of two independent experiments, each performed in triplicate. Statistical analysis was performed by one-way ANOVA, followed by post-hoc Tukey honestly significant difference (HSD) test. Asterisks indicate significant differences (*** p < 0.001).
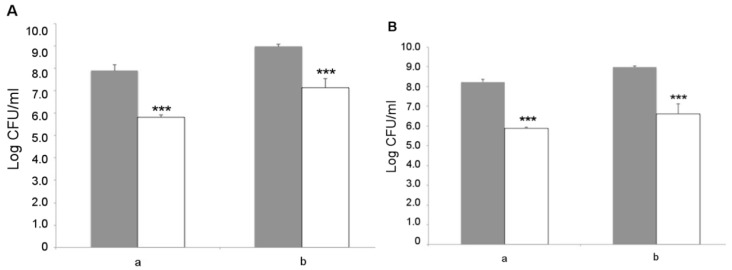
L. fermentum MBC2 was next analyzed for its capacity to adhere to Caco-2 cells, which represent a valuable in vitro model of human intestinal epithelium. Two different initial amounts of bacterial cells, namely 1 × 108 and 1 × 109 CFU, were co-cultured with intestinal cells and the resulting adhering bacteria were counted on MRS agar medium. The results showed that L. fermentum MBC2 was able to adhere to Caco-2 cells with an efficiency of about 1%, irrespective of the initial bacterial titer (Figure 9A). Similar results were obtained with the LGG probiotic control (Figure 9B), suggesting a good adhesion capacity displayed by L. fermentum MBC2. As an important trait to be verified for safety purposes, antibiotic susceptibility profiling was analyzed for a panel of 20 antibiotics, including inhibitors of cell wall synthesis, protein synthesis, nucleic acid synthesis, and cytoplasmic membrane function. Antibiotic susceptibility was determined semi-quantitatively by disc diffusion, and the results are reported in Table 1, in comparison with the LGG control strain. Overall, antibiotic susceptibility pattern displayed by L. fermentum MBC2 was almost completely overlapping with that of the probiotic control strain. Differences in the size of inhibition zone were observed for some antibiotics, but in all cases, they were higher for L. fermentum MBC2 than for LGG (Table 1).
Figure 9.
Adhesion to Caco-2 cells. Cell counts of viable L. fermentum MBC2 (A) and LGG (B) adhering on differentiated Caco-2 cells co-cultured with 1 × 108 (a) or 1 × 109 (b) bacterial colony forming units (CFU)/well. Grey columns refer to the initial bacterial load, while white columns refer to adhering bacteria recovered at the end of co-incubation. Data are reported as log of bacterial CFU recovered after plating. Columns represent the mean ± SD of two independent experiments, each performed in triplicate. Statistical analysis was performed by one-way ANOVA, followed by post-hoc Tukey honestly significant difference (HSD) test. Asterisks indicate significant differences (*** p < 0.001).
Table 1.
Antibiotic susceptibility of L. fermentum MBC2 and LGG strains.
| Antibiotic | Amount on Disc (µg) | Zone of Inhibition (mm) a | |
|---|---|---|---|
| L. fermentum MBC2 | L. rhamnosus GG (LGG) | ||
| Vancomycin | 30 | - b | - b |
| Clindamycin | 30 | 15 | 15 |
| Cefalotin | 30 | 10 | - b |
| Cefuroxime | 30 | 9 | 8 |
| Tobramycin | 10 | 4 | 4 |
| Ampicillin | 10 | 20 | 7 |
| Cefotaxime | 30 | 8 | 4 |
| Chloramphenicol | 30 | 13 | 11 |
| Tetracycline | 30 | 11 | 14 |
| Erythromycin | 15 | 15 | 9 |
| Amikacin | 30 | 4 | 4 |
| Oxacillin | 1 | 9 | - b |
| Fosfomycin | 50 | 5 | - b |
| Rifampicin | 30 | 16 | 15 |
| Gentamicin | 10 | 5 | 4 |
| Penicillin | 10 | 20 | 16 |
| Aztreonam | 30 | -b | -b |
| Carbenicillin | 100 | 9 | 8 |
| Mezlocillin | 75 | 18 | 15 |
| Streptomycin | 25 | 4 | 4 |
a The zones of inhibition were measured from the center of the disc and recorded. b Absence of inhibition halo.
4. Discussion
In the present work, we took advantage of the simplified model organism C. elegans to highlight health promoting features of L. fermentum MBC2, a strain previously isolated from MBC [28], with the aim of a possible application as a starter with probiotic added value. Our hypothesis was based on the evidence that supplementation of obese mice with an MBC-derived microbial community, which included this strain as one of the principal members, exerted protective effect toward obesity-associated inflammation [29]. Positive impact of L. fermentum MBC2 on wild-type nematodes was dependent on bacterial viability, suggesting the involvement of bacterial metabolism. It is important to consider that fermented dairy products are often characterized by high titers of live microbes, which could reach human gut through ingestion. L. fermentum MBC2 also determined alterations in the reproductive process and in the larval development of nematodes, causing a reduction of progeny and body length, similarly to what was observed for the commercial probiotic LGG. Recently, different L. fermentum strains were tested on C. elegans to investigate the relationship between feeding foodborne bacteria and aging, reporting that L. fermentum LA12 could contribute to enhance immune response and prolong the lifespan of animals [43]. Similarly, LAB strains belonging to L. delbrueckii, Bifidobacterium longum, B. infantis, L. helveticus, L. plantarum, and L. rhamnosus influenced C. elegans growth [25,28,44]. The effects exerted by L. fermentum MBC2 on C. elegans physiology could be due to the high gut colonization capacity of the bacterial strain. In fact, L. fermentum MBC2-fed animals showed a higher gut-associated bacterial titer than that of nematodes fed OP50 or LGG. The pro-longevity effects observed in lifespan experiments were correlated to a delay in aging processes and a reduction of oxidative stress. When worms were fed L. fermentum MBC2 an anti-aging effect was highlighted by monitoring different markers, accompanied by a reduced ROS production. Furthermore, low level of fluorescence was observed when the transgenic GST4::GFP C. elegans strain was fed L. fermentum MBC2. These data were in agreement with studies demonstrating that resistance to oxidative stress is important to maintain a healthy status and to counteract the adverse effects of aging [38,45].
Moreover, in order to shed light on the possible effectors of L. fermentum MBC2-mediated longevity, C. elegans PEPT-1 and DAF-16 mutants were utilized. PEPT-1 mutants lack intestinal di- and tripeptide transporter that is involved in worm development and growth. Nematode mutants show a retard in postembryonic development, an increase of body fat and resistance to oxidative stress, and a reduction in body size and progeny compared to N2 [41,46,47]. Insulin/DAF-2 and TOR signaling pathways, with a strict dependence of the transcription factor DAF-16, regulate amino acid uptake through PEPT-1 [48]. L. fermentum MBC2 diet was not able to prolong DAF-16 and PEPT-1 mutant lifespan, unlike wild type C. elegans strain, suggesting that amino acid metabolism of L. fermentum MBC2 could be responsible for the observed anti-aging effect; however, this aspect will deserve further investigations.
One of the most important processes closely related to the vitality and aging in C. elegans is fat metabolism; indeed, the accumulation rate, as well as the size of lipid droplets, are reported to increase with aging [35]. In agreement with these observations, BODIPY staining of N2 animals fed L. fermentum MBC2 showed a decreased lipid droplet accumulation; on the other hand, this was not observed for PEPT-1 mutants. Our data thus indicated that pept-1 gene is involved in the impact exerted by L. fermentum MBC2 on C. elegans viability and fat metabolism, further supporting the strong correlation between reduction of fat storage and extension of lifespan in nematodes.
The observed PEPT-1-dependent pro-longevity effect is mediated by the transcriptional factor DAF-16. This has been already involved in the signalling cascade mediating the longevity of PEPT-1 mutants [42]. Indeed, L. fermentum MBC2 diet was not able to induce an increase of nematode lifespan, and ROS levels were higher than those measured in N2 and PEPT-1 animals. This is in agreement with the fact that among the DAF-16 target genes, there are enzymes involved in the defence against oxidative stress [49].
In light of a possible use as a starter with probiotic added value, guidelines established by the FAO and WHO affirm the need to evaluate the functional as well as the safety features of candidate microorganisms before proposing their use within a food matrix [1]. We therefore evaluated the survival capacity of L. fermentum MBC2 to gastric and pancreatic juice treatments, as resistance to the harsh conditions of the upper GI tract is a key pre-requisite for efficient colonization by a probiotic strain [50]. Indeed, the gastrointestinal environment can be hostile for several bacteria, since different stressors such as acidity, digestive enzymes, and bile salts may affect their survival during transit through this district [51]. Moreover, acid tolerance is not only important in gastrointestinal conditions but also in acidic food matrices where lactobacilli may be added as adjuncts. Overall, L. fermentum MBC2 showed good tolerance to low pH, suggesting potential capacity to survive in gastric environment as well as to be employed as a starter in food fermentations. However, this strain did not survive in simulated pancreatic juice, containing bile salts and pancreatin. A possible solution to overcome this issue could be represented by the use of protective methods, such as microencapsulation, that protect bacteria during their transit along the GI tract [52], or by delivering the probiotic strain as part of a fermented product, in which the food matrix could play a key protective role [53]. To this respect, dairy products are usually employed as an adequate matrix to vehicle probiotic strains through ingestion [54,55].
The L. fermentum MBC2 strain was also able to adhere to Caco-2 cells, a well characterized enterocyte-like cell line, representing a useful in vitro model of human intestinal epithelium [32]. Adhesion capacity could promote the maintenance of intestinal integrity, a particularly relevant feature in the case of intestinal injury induced by pathogens [56]. An important additional trait to be verified for safety purposes concerns antibiotic susceptibility [57]. Overall, the antibiotic susceptibility profiling displayed by L. fermentum MBC2 to a panel of 20 antibiotics, including inhibitors of cell wall synthesis, protein synthesis, nucleic acid synthesis, and cytoplasmic membrane function was almost completely overlapping with that of the probiotic control strain LGG, supporting the possibility of its safe use as a starter and/or probiotic adjunct. However, further studies are needed to more deeply evaluate phenotypic antibiotic resistance (i.e. by using standardized methods to evaluate minimum inhibitory concentrations for relevant antibiotics) and to detect the presence of the corresponding resistance genes. It must be pointed out that the possibility of horizontal gene transfer to pathogens within the host gut should be carefully verified, to exclude associated risks for health. On the other hand, the presence of intrinsic antibiotic resistance could not represent a safety issue in itself, in light of the possible application of resistant probiotic strains for restoring the normal gut microbiota balance following antibiotic treatment [58].
5. Conclusions
In the present work, administration of L. fermentum MBC2 isolated from a foodborne microbiota influenced nematode physiology. In particular, the strain positively impacted on C. elegans longevity and influenced energy metabolism as well as oxidative stress resistance. The molecular players involved resulted in being dependent on the PEPT-1 transporter and on the transcriptional factor DAF-16, highlighting their possible involvement in host-microbe interactions. Moreover, L. fermentum MBC2 resulted in being tolerant to acidic pH and able to adhere to intestinal Caco-2 cells. Taken together, these findings provide new insight for a possible application of this strain in the food industry as a novel functional starter.
Acknowledgments
The authors acknowledge the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis) for the nematode and E. coli OP50 strains. The authors wish to thank Kariklia Pascucci for her kind support in daily lab work.
Supplementary Materials
The following are available online at http://www.mdpi.com/2076-2607/7/2/45/s1, Figure S1: Effect of L. fermentum MBC2 on body size and nematode fertility; Figure S2: Effect of L. fermentum MBC2 on pumping rate and body bending in PEPT-1 mutants.
Author Contributions
C.D. and D.U. designed the experiments and wrote the paper. E.S. and S.M. performed animal experiments and treatments. P.Z. and B.G. performed microbiological analyses. M.R. and B.G. performed cell culture experiments.
Funding
This research was partially funded by Ateneo Avvio alla Ricerca 2018, grant number AR1181641CCF43BD to E.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.FAO/WHO Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation. [(accessed on 7 February 2019)]; Available online: http://www.fao.org/3/a-a0512e.pdf.
- 2.Sornplang P., Piyadeatsoontorn S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016;58:26. doi: 10.1186/s40781-016-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller K.J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 2001;73:374S–379S. doi: 10.1093/ajcn/73.2.374s. [DOI] [PubMed] [Google Scholar]
- 4.Schiffrin E.J., Brassart D., Servin A.L., Rochat F., Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: Criteria for strain selection. Am. J. Clin. Nutr. 1997;66:515S–520S. doi: 10.1093/ajcn/66.2.515S. [DOI] [PubMed] [Google Scholar]
- 5.Gionchetti P., Rizzello F., Venturi A., Campieri M. Probiotics in infective diarrhoea and inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2000;15:489–493. doi: 10.1046/j.1440-1746.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- 6.Dellaglio F., Torriani S., Felis G.E. Reclassification of Lactobacillus cellobiosus Rogosa et al. 1953 as a later synonym of Lactobacillus fermentum Beijerinck 1901. Int. J. Syst. Evol. Microbiol. 2004;54:809–812. doi: 10.1099/ijs.0.02947-0. [DOI] [PubMed] [Google Scholar]
- 7.Baruzzi F., Quintieri L., Caputo L., Cocconcelli P., Borcakli M., Owczarek L., Jasinska U.T., Skapska S., Morea M. Improvement of Ayran quality by the selection of autochthonous microbial cultures. Food Microbiol. 2016;60:92–103. doi: 10.1016/j.fm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Mangia N.P., Fancello F., Deiana P. Microbiological characterization using combined culture dependent and independent approaches of Casizolu pasta filata cheese. J. Appl. Microbiol. 2016;120:329–345. doi: 10.1111/jam.13001. [DOI] [PubMed] [Google Scholar]
- 9.Denkova R., Ilieva S., Denkova Z., Georgieva L., Yordanova M., Nikolova D., Evstatieva Y. Production of wheat bread without preservatives using sourdough starters. Biotechnol. Biotechnol. Equip. 2014;28:889–898. doi: 10.1080/13102818.2014.965057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vuyst L., Van Kerrebroeck S., Harth H., Huys G., Daniel H.M., Weckx S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014;37:11–29. doi: 10.1016/j.fm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Tulumoglu S., Kaya H.I., Simsek O. Probiotic characteristics of Lactobacillus fermentum strains isolated from tulum cheese. Anaerobe. 2014;30:120–125. doi: 10.1016/j.anaerobe.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Archer A.C., Halami P.M. Probiotic attributes of Lactobacillus fermentum isolated from human feces and dairy products. Appl. Microbiol. Biotechnol. 2015;99:8113–8123. doi: 10.1007/s00253-015-6679-x. [DOI] [PubMed] [Google Scholar]
- 13.Owusu-Kwarteng J., Tano-Debrah K., Akabanda F., Jespersen L. Technological properties and probiotic potential of Lactobacillus fermentum strains isolated from West African fermented millet dough. BMC Microbiol. 2015;15:261. doi: 10.1186/s12866-015-0602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azmal Ali S., Kumar S., Mohanty A.K., Behare P. Draft Genome Sequence of Lactobacillus fermentum NCDC 400, Isolated from a Traditional Indian Dairy Product. Genome Announc. 2018;6 doi: 10.1128/genomeA.01492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalfie M., Tu Y., Euskirchen G., Ward W.W., Prasher D.C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 16.Jeon H., Cha D.S. Anti-aging properties of Ribes fasciculatum in Caenorhabditis elegans. Chin. J. Nat. Med. 2016;14:335–342. doi: 10.3724/SP.J.1009.2016.00335. [DOI] [PubMed] [Google Scholar]
- 17.Sanada Y., Asai S., Ikemoto A., Moriwaki T., Nakamura N., Miyaji M., Zhang-Akiyama Q.M. Oxidation resistance 1 is essential for protection against oxidative stress and participates in the regulation of aging in Caenorhabditis elegans. Free Radic. Res. 2014;48:919–928. doi: 10.3109/10715762.2014.927063. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H., Wang D. The Microbial Zoo in the C. elegans Intestine: Bacteria, Fungi and Viruses. Viruses. 2018;10:85. doi: 10.3390/v10020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park M.R., Yun H.S., Son S.J., Oh S., Kim Y. Short communication: Development of a direct in vivo screening model to identify potential probiotic bacteria using Caenorhabditis elegans. J. Dairy Sci. 2014;97:6828–6834. doi: 10.3168/jds.2014-8561. [DOI] [PubMed] [Google Scholar]
- 20.Lee H.K., Choi S.H., Lee C.R., Lee S.H., Park M.R., Kim Y., Lee M.K., Kim G.B. Screening and Characterization of Lactic Acid Bacteria Strains with Anti-inflammatory Activities through in vitro and Caenorhabditis elegans Model Testing. Korean J. Food Sci. Anim. Resour. 2015;35:91–100. doi: 10.5851/kosfa.2015.35.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y., Zhao L., Zheng X., Fu T., Guo H., Ren F. Lactobacillus salivarius strain FDB89 induced longevity in Caenorhabditis elegans by dietary restriction. J. Microbiol. 2013;51:183–188. doi: 10.1007/s12275-013-2076-2. [DOI] [PubMed] [Google Scholar]
- 22.Kwon G., Lee J., Lim Y.H. Dairy Propionibacterium extends the mean lifespan of Caenorhabditis elegans via activation of the innate immune system. Sci. Rep. 2016;6:31713. doi: 10.1038/srep31713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azat R., Liu Y., Li W., Kayir A., Lin D.B., Zhou W.W., Zheng X.D. Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J. Zhejiang Univ. Sci. B. 2016;17:597–609. doi: 10.1631/jzus.B1500250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerbaba T.K., Green-Harrison L., Buret A.G. Modeling Host-Microbiome Interactions in Caenorhabditis elegans. J. Nematol. 2017;49:348–356. doi: 10.21307/jofnem-2017-082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanni E., Schifano E., Motta S., Sciubba F., Palleschi C., Mauri P., Perozzi G., Uccelletti D., Devirgiliis C., Miccheli A. Combination of Metabolomic and Proteomic Analysis Revealed Different Features among Lactobacillus delbrueckii Subspecies bulgaricus and lactis Strains While In Vivo Testing in the Model Organism Caenorhabditis elegans Highlighted Probiotic Properties. Front. Microbiol. 2017;8:1206. doi: 10.3389/fmicb.2017.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guantario B., Zinno P., Schifano E., Roselli M., Perozzi G., Palleschi C., Uccelletti D., Devirgiliis C. In Vitro and in Vivo Selection of Potentially Probiotic Lactobacilli From Nocellara del Belice Table Olives. Front. Microbiol. 2018;9:595. doi: 10.3389/fmicb.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devirgiliis C., Caravelli A., Coppola D., Barile S., Perozzi G. Antibiotic resistance and microbial composition along the manufacturing process of Mozzarella di Bufala Campana. Int. J. Food Microbiol. 2008;128:378–384. doi: 10.1016/j.ijfoodmicro.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Zanni E., Laudenzi C., Schifano E., Palleschi C., Perozzi G., Uccelletti D., Devirgiliis C. Impact of a Complex Food Microbiota on Energy Metabolism in the Model Organism Caenorhabditis elegans. Biomed. Res. Int. 2015;2015:621709. doi: 10.1155/2015/621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roselli M., Devirgiliis C., Zinno P., Guantario B., Finamore A., Rami R., Perozzi G. Impact of supplementation with a food-derived microbial community on obesity-associated inflammation and gut microbiota composition. Genes Nutr. 2017;12:25. doi: 10.1186/s12263-017-0583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uccelletti D., Zanni E., Marcellini L., Palleschi C., Barra D., Mangoni M.L. Anti-Pseudomonas activity of frog skin antimicrobial peptides in a Caenorhabditis elegans infection model: A plausible mode of action in vitro and in vivo. Antimicrob. Agents Chemother. 2010;54:3853–3860. doi: 10.1128/AAC.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kampkotter A., Pielarski T., Rohrig R., Timpel C., Chovolou Y., Watjen W., Kahl R. The Ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol. Res. 2007;55:139–147. doi: 10.1016/j.phrs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Sambuy Y., De Angelis I., Ranaldi G., Scarino M.L., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 33.Roselli M., Finamore A., Britti M.S., Mengheri E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2006;95:1177–1184. doi: 10.1079/BJN20051681. [DOI] [PubMed] [Google Scholar]
- 34.Gomez F., Monsalve G.C., Tse V., Saiki R., Weng E., Lee L., Srinivasan C., Frand A.R., Clarke C.F. Delayed accumulation of intestinal coliform bacteria enhances life span and stress resistance in Caenorhabditis elegans fed respiratory deficient E. coli. BMC Microbiol. 2012;12:300. doi: 10.1186/1471-2180-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palikaras K., Mari M., Petanidou B., Pasparaki A., Filippidis G., Tavernarakis N. Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans. J. Lipid Res. 2017;58:72–80. doi: 10.1194/jlr.M069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg A.A., Bourque S.D., Kyryakov P., Boukh-Viner T., Gregg C., Beach A., Burstein M.T., Machkalyan G., Richard V., Rampersad S., et al. A novel function of lipid droplets in regulating longevity. Biochem. Soc. Trans. 2009;37:1050–1055. doi: 10.1042/BST0371050. [DOI] [PubMed] [Google Scholar]
- 37.Fontana L., Partridge L., Longo V.D. Extending healthy life span—From yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ristow M., Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Ayyadevara S., Dandapat A., Singh S.P., Siegel E.R., Shmookler Reis R.J., Zimniak L., Zimniak P. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007;128:196–205. doi: 10.1016/j.mad.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashrafi K., Chang F.Y., Watts J.L., Fraser A.G., Kamath R.S., Ahringer J., Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 41.Meissner B., Boll M., Daniel H., Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J. Biol. Chem. 2004;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- 42.Zhao X., Lu L., Qi Y., Li M., Zhou L. Emodin extends lifespan of Caenorhabditis elegans through insulin/IGF-1 signaling pathway depending on DAF-16 and SIR-2.1. Biosci. Biotechnol. Biochem. 2017;81:1908–1916. doi: 10.1080/09168451.2017.1365592. [DOI] [PubMed] [Google Scholar]
- 43.Lee J., Yun H.S., Cho K.W., Oh S., Kim S.H., Chun T., Kim B., Whang K.Y. Evaluation of probiotic characteristics of newly isolated Lactobacillus spp.: Immune modulation and longevity. Int. J. Food Microbiol. 2011;148:80–86. doi: 10.1016/j.ijfoodmicro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda T., Yasui C., Hoshino K., Arikawa K., Nishikawa Y. Influence of lactic acid bacteria on longevity of Caenorhabditis elegans and host defense against Salmonella enterica serovar enteritidis. Appl. Environ. Microbiol. 2007;73:6404–6409. doi: 10.1128/AEM.00704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grompone G., Martorell P., Llopis S., Gonzalez N., Genoves S., Mulet A.P., Fernandez-Calero T., Tiscornia I., Bollati-Fogolin M., Chambaud I., et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS ONE. 2012;7:e52493. doi: 10.1371/journal.pone.0052493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spanier B., Lasch K., Marsch S., Benner J., Liao W., Hu H., Kienberger H., Eisenreich W., Daniel H. How the intestinal peptide transporter PEPT-1 contributes to an obesity phenotype in Caenorhabditits elegans. PLoS ONE. 2009;4:e6279. doi: 10.1371/journal.pone.0006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luersen K., Faust U., Gottschling D.C., Doring F. Gait-specific adaptation of locomotor activity in response to dietary restriction in Caenorhabditis elegans. J. Exp. Biol. 2014;217:2480–2488. doi: 10.1242/jeb.099382. [DOI] [PubMed] [Google Scholar]
- 48.Martin F.P., Spanier B., Collino S., Montoliu I., Kolmeder C., Giesbertz P., Affolter M., Kussmann M., Daniel H., Kochhar S., et al. Metabotyping of Caenorhabditis elegans and their culture media revealed unique metabolic phenotypes associated to amino acid deficiency and insulin-like signaling. J. Proteome Res. 2011;10:990–1003. doi: 10.1021/pr100703a. [DOI] [PubMed] [Google Scholar]
- 49.Spanier B., Rubio-Aliaga I., Hu H., Daniel H. Altered signalling from germline to intestine pushes daf-2;pept-1 Caenorhabditis elegans into extreme longevity. Aging Cell. 2010;9:636–646. doi: 10.1111/j.1474-9726.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- 50.Dicks L.M., Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: Health benefits, safety and mode of action. Benef. Microbes. 2010;1:11–29. doi: 10.3920/BM2009.0012. [DOI] [PubMed] [Google Scholar]
- 51.Kleerebezem M., Hols P., Bernard E., Rolain T., Zhou M., Siezen R.J., Bron P.A. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 2010;34:199–230. doi: 10.1111/j.1574-6976.2009.00208.x. [DOI] [PubMed] [Google Scholar]
- 52.Heidebach T., Forst P., Kulozik U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012;52:291–311. doi: 10.1080/10408398.2010.499801. [DOI] [PubMed] [Google Scholar]
- 53.Bergamini C., Hynes E., Meinardi C., Suarez V., Quiberoni A., Zalazar C. Pategras cheese as a suitable carrier for six probiotic cultures. J. Dairy Res. 2010;77:265–272. doi: 10.1017/S0022029910000051. [DOI] [PubMed] [Google Scholar]
- 54.Gardiner G., Stanton C., Lynch P.B., Collins J.K., Fitzgerald G., Ross R.P. Evaluation of cheddar cheese as a food carrier for delivery of a probiotic strain to the gastrointestinal tract. J. Dairy Sci. 1999;82:1379–1387. doi: 10.3168/jds.S0022-0302(99)75363-4. [DOI] [PubMed] [Google Scholar]
- 55.Vinderola C.G., Prosello W., Ghiberto T.D., Reinheimer J.A. Viability of probiotic (Bifidobacterium, Lactobacillus acidophilus and Lactobacillus casei) and nonprobiotic microflora in Argentinian Fresco cheese. J. Dairy Sci. 2000;83:1905–1911. doi: 10.3168/jds.S0022-0302(00)75065-X. [DOI] [PubMed] [Google Scholar]
- 56.Resta-Lenert S., Barrett K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imperial I.C., Ibana J.A. Addressing the Antibiotic Resistance Problem with Probiotics: Reducing the Risk of Its Double-Edged Sword Effect. Front. Microbiol. 2016;7:1983. doi: 10.3389/fmicb.2016.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gueimonde M., Sanchez B., de los Reyes-Gavilán C.G., Margolles A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013;4:202. doi: 10.3389/fmicb.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.