Abstract
Recent studies have revealed both the promise and challenges of targeting long non-coding RNAs (lncRNAs) to diagnose and treat endometrial cancer (EC). LncRNAs are upregulated or downregulated in ECs compared to normal tissues and their dysregulation has been linked to tumor grade, FIGO stage, the depth of myometrial invasion, lymph node metastasis and patient survival. Tumor suppressive lncRNAs (GAS5, MEG3, FER1L4 and LINC00672) and oncogenic lncRNAs (CCAT2, BANCR, NEAT1, MALAT1, H19 and Linc-RoR) have been identified as upstream modulators or downstream effectors of major signaling pathways influencing EC metastasis, including the PTEN/PI3K/AKT/mTOR, RAS/RAF/MEK/ERK, WNT/β-catenin and p53 signaling pathways. TUG1 and TDRG1 stimulate the VEGF-A pathway. PCGEM1 is implicated in activating the JAK/STAT3 pathway. Here, we present an overview of the expression pattern, prognostic value, biological function of lncRNAs in EC cells and their roles within the tumor microenvironment, focusing on the influence of lncRNAs on established EC-relevant pathways. We also describe the emerging classification of EC subtypes based on their lncRNA signature and discuss the clinical implications of lncRNAs as valuable biomarkers for EC diagnosis and potential targets for EC treatment.
Keywords: long non-coding RNA, microRNA, endometrial cancer, prognostic biomarker, therapeutic target, epigenetics, regulatory mechanism, tumor microenvironment
1. Introduction
Endometrial cancer (EC) arises from the lining of the uterus and is the most common gynecologic cancer in the developed world [1]. Although the majority of patients with endometrial cancer (EC) have good outcomes, advanced or metastatic disease carries a grave prognosis [2]. Thus, this highlights the need to identify biomarkers that would distinguish aggressive from indolent disease and for developing novel therapies to treat EC.
EC has been broadly classified into two groups with distinct clinical and molecular alterations [2,3]. Type I EC is usually low-grade, low-stage endometrioid adenocarcinomas with favorable prognosis [2,3]. The most important altered pathway in type I EC is the PTEN/PI3K/AKT/mTOR pathway [2,3]. The aberrant activation of the RAS/RAF/MEK/ERK and WNT/β-catenin signaling pathway is also common in type I EC, [2,4]. Type II EC is often high-grade endometrioid adenocarcinomas and serous carcinomas with worse prognosis, which harbor TP53 mutations and HER2 overexpression [2,3]. The upregulation of EGFR and VEGF-A is found in both type I and II EC [5,6]. However, this dualistic model has limitations because the significant heterogeneity within and the overlapping features between Type I and II EC have been recognized [7].
The Cancer Genome Atlas (TCGA) has reclassified EC into four genomic subgroups: the ultra-mutated group harboring mutations in the exonuclease domain of POLE gene (POLE ultra-mutated), microsatellite instability, copy number-low as well as copy number-high and serous-like subgroups [8]. However, the copy number-low group (around 40% of all EC patients with no specific molecular profile) still represents a poorly characterized heterogeneous set of EC.
The human genome is pervasively transcribed and most of the human transcriptome is composed of non-coding RNAs (ncRNAs) [9]. Based on transcript size, the long non-coding RNAs (lncRNAs) are defined as transcripts of greater than 200 nucleotides. LncRNAs have been classified as exonic lncRNAs, intronic lncRNAs, intergenic lncRNAs overlapping lncRNAs and anti-sense lncRNAs [10].
A subset of lncRNAs is present in the nucleus, but the majority of lncRNA is enriched in the cytoplasm or is located in both the nucleus and cytoplasm [11]. Although only a few lncRNAs have been fully investigated, it is clear that lncRNAs are expressed in a tissue-specific manner and can act as upstream modulators or downstream effectors in oncogenic pathways through their interactions with other molecules, such as DNA, RNA and protein [12]. In the cytoplasm, lncRNAs interact with target RNAs, such as mRNAs and microRNAs (miRNAs), or with specific signaling proteins. This affects the expression of target genes, thereby regulating the activities of signaling pathways [12]. In the nucleus, lncRNAs act as the signal, guide, decoy or scaffold to modulate the epigenetic and transcriptional processes, affecting chromatin structure and gene transcription [13,14]. By binding with key components within different signaling pathways, lncRNAs participate in in the regulation of multiple signaling pathways at many levels (epigenetic, transcriptional, post-transcriptional and translational), thus facilitating the formation of complex regulatory networks in tumor cells.
In this review, we outline the expression, functional cellular roles and underlying molecular mechanisms of lncRNAs in EC progression, highlighting their significant impact on EC-related signaling pathways. Together, we provide an overview of the emerging opportunities and challenges of targeting lncRNA to diagnose and treat EC.
2. Evidence Acquisition
The PubMed database was searched for articles published up to January 2019 using the following keywords: long non-coding RNA, lncRNA, uterine tumor, endometrial carcinoma and endometrial cancer. All studies recognized were assessed for relevance by two authors by checking the title and abstract. All irrelevant articles, studies without access to the full text of the publication, case reports, letters, expert opinions, meeting records, review articles and articles whose methods do not contain biomedical experimental validation were excluded. After this, the full text of any selected article was reviewed by at least two authors. In addition, we also searched the reference lists of selected studies to identify additional relevant articles.
3. Classification of EC Based on LncRNA Expression
An integrative analysis of lncRNAs using TCGA molecular RNA-sequencing profiles of 191 primary endometrioid ECs has identified 858 lncRNAs that were differentially expressed in EC tissues compared to normal endometrium [15]. By applying the clustering of lncRNA expression, these EC patients were classified into three groups: (i) basal-like, (ii) luminal-like and (iii) β-catenin (CTNNB1)-enriched subgroups. The basal-like subgroup was enriched for more aggressive tumors that had a higher pathological grade and TNM stage, with this group showing a higher incidence of p53 mutation, deletion of PTEN and overexpression of polycomb genes (EZH2 and CBX2). Not surprisingly, this group tends to exhibit poorer survival compared to other groups. The luminal subgroup was associated with the expression of progesterone and estrogen receptor genes, while the β-catenin subgroup was associated with mutations in the β-catenin gene and PTEN mutation [15]. Overall, 33 lncRNAs (such as HOTAIR) were upregulated, whereas 50 lncRNAs (including LINC00488) were downregulated in the basal-like subgroup as compared to the two other subgroups [15]. These results suggest that analyzing the expression pattern of lncRNAs might be very helpful in distinguishing aggressive endometrioid EC from the non-aggressive disease.
4. Differential Expression and Prognostic Value of LncRNAs in EC
LncRNAs are upregulated or downregulated in ECs compared to normal tissues and their dysregulation has been linked to tumor grade, FIGO stage, the depth of myometrial invasion, lymph node metastasis and patient survival [16,17,18] (Table 1).
Table 1.
The expression, function and mechanism of lncRNAs in EC.
| LncRNA | Expression | Function | Mechanism | Ref. |
|---|---|---|---|---|
| CASC2 | Downregulation | — | — | [19] |
| GAS5 | Downregulation | Tumor suppressor | Induced PTEN expression via inhibiting miR-103 | [20] |
| MEG3 | Downregulation | Tumor suppressor | Combined directly with PI3K protein and reduced its expression | [21,22] |
| FER1L4 | Downregulation | Tumor suppressor | Enhanced PTEN expression | [23,24] |
| LINC00672 | Downregulation | Tumor suppressor | Served as a locus-restricted cofactor for p53-mediated gene suppression | [25] |
| CCAT2 | Upregulation | Oncogene | Activated the PI3K/AKT pathway by reducing miR-216b expression | [26] |
| BANCR | Upregulation | Oncogene | Activated the MEK/ERK signaling | [27] |
| NEAT1 | Upregulation | Oncogene | Activated the WNT/β-catenin pathway via inhibiting miR-146b and miR-214 expression | [28] |
| MALAT1 | Upregulation | Oncogene | Inhibited miR-200c expression | [29] |
| H19 | Upregulation | Oncogene | Induced EMT via inhibiting let-7 expression | [30,31,32,33,34,35] |
| HOTAIR | Upregulation | Oncogene | Increased NPM1 expression via suppressing miR-646 expression | [36,37,38,39] |
| UCA1 | Upregulation | Oncogene | — | [40] |
| CARLo-5 | Upregulation | — | — | [41] |
| TDRG1 | Upregulation | Oncogene | Directly interacted with VEGF-A protein and upregulated its expression | [42] |
| TUG1 | Upregulation | Oncogene | Increased VEGF-A expression via suppressing miR-34a and miR-299 | [43] |
| PCGEM1 | Upregulation | Oncogene | Activated the STAT3 pathway via reducing miR-129 expression | [44] |
4.1. Potential Tumor Suppressive lncRNA
CASC2 expression decreased from normal tissues to EC tissues [19].
4.2. Tumor Suppressive lncRNAs
4.2.1. GAS5
GAS5 was downregulated in EC tissues compared with normal tissues and the overexpression of GAS5 in two EC cell lines (HHUA and JEC) resulted in apoptosis in these cells [20].
4.2.2. MEG3
The levels of MEG3 were significantly lower in EC tissues than those in adjacent normal tissues [21]. Consistently, two EC cell lines (HEC-1A and KLE) expressed lower levels of MEG3 compared to the normal endometrial cell line ESC [21]. Another study also demonstrated that the expression of MEG3 was significantly lower in EC samples than in normal endometrial tissues [22]. Stable overexpression of MEG3 significantly induced apoptosis and reduced migration and invasion in HEC-1B and Ishikawa cells [22].
4.2.3. FER1L4
FER1L4 was significantly downregulated in EC tissues compared with adjacent normal tissues [23]. Lower levels of FER1L4 was significantly correlated with higher FIGO stages, lymph node metastasis, distant metastasis and worse patient survival [23]. The gain-of-function studies in EC cell line HEC-50 suggested that FER1L4 could inhibit proliferation and induce apoptosis of HEC-50 cells [24].
4.2.4. LINC00672
Through qRT-PCR analysis in a total of 176 pairs of ECs and adjacent non-tumor tissues of two distinct Chinese populations, the expression of LINC00672 was shown to be significantly lower in EC tissues than in adjacent normal tissues [25]. A further functional investigation revealed that the overexpression of LINC00672 in HEC-1A and Ishikawa cells reduced cell proliferation [25].
4.3. Oncogenic lncRNAs
4.3.1. CCAT2
A recent report illustrated that CCAT2 was aberrantly upregulated in EC tissues compared to the normal endometrial tissues [26]. The silencing of CCAT2 suppressed proliferation, migration and invasion of HEC-1-A and RL95-2 cells [26].
4.3.2. BANCR
BANCR levels were significantly higher in EC tissues than that in normal endometrial tissues [27]. Increased expression of BANCR was positively correlated with FIGO stage, tumor grade, myometrial invasion and lymph node metastasis in patients with EC [27]. In vitro studies showed that knockdown of BANCR significantly suppressed proliferation, migration and invasion of Ishikawa and HEC-1A cells [27].
4.3.3. NEAT1
The levels of NEAT1 were higher in endometrioid EC tissues compared with adjacent normal endometrial tissues [28]. A higher level of expression of NEAT1 was found in those patients with FIGO stage III or IV and with positive lymph node metastasis [28]. Forced overexpression of NEAT1 in HEC-59 cells promoted cell growth and invasion, while knocking down of NEAT1 in HEC-59 cells had the opposite effect [28].
4.3.4. MALAT1
Previous evidence suggested that MALAT1 was upregulated in EC tissues compared to their matched adjacent tissues [29]. Ectopic expression of MALAT1 promoted migration and invasion of RL-952 cells [29].
4.3.5. H19
Gene expression analysis via in situ hybridization showed that H19 was not expressed in the epithelium of normal endometrium, but is overexpressed in 60% of EC [30]. Similarly, ECs expressed higher levels of H19 than normal endometrial tissues as determined by qRT-PCR analysis [31,32]. H19 expression in EC tissues was noted to progressively increase with tumor grade [32]. Another study showed that the levels of H19 were elevated in mixed endometrioid and serous ECs when compared to pure endometrioid ECs as measured by qRT-PCR analysis, which indicates that H19 may serve as a marker for the more aggressive EC subtype [33].
The prognostic value of H19 has been investigated in TCGA EC datasets and those patients with higher H19 expression had a significantly shorter overall survival than those with lower H19 expression [34]. The depletion of H19 in HEC-1B cells and the serous EC cell line ARK2 impaired cell migration and invasion [32,35].
4.3.6. HOTAIR
HOTAIR was upregulated in EC tissues as compared to normal endometrial tissues and a higher level of HOTAIR expression was significantly associated with higher tumor grade, positive lymph node metastasis, the depth of myometrial invasion and the presence of lymphovascular space invasion [36]. The patients with a higher level of HOTAIR expression had a significantly poorer overall survival than those with a lower level of HOTAIR expression [36,37]. The depletion of HOTAIR significantly suppressed the proliferation and invasion of HEC-1A cells in vitro and reduced EC tumorigenesis in vivo [38]. HOTAIR was shown to promote viability, migration and invasion by regulating the miR-646/NPM1 pathway in Ishikawa and HEC-1-A cells [39].
4.3.7. UCA1
The overexpression of UCA1 was detected in EC tissues and was positively associated with tumor grade, FIGO stage, the depth of myometrial invasion and lymph node metastasis [40]. The silencing of UCA1 attenuated the migration and invasion of HTB-111 and Ishikawa cells [40].
4.3.8. Potential Oncogenic lncRNA
Another lncRNA CARLo-5 was significantly upregulated in EC tissues compared to normal tissues and was significantly associated with higher FIGO stages and positive lymph node metastasis [41]. Kaplan-Meier analysis showed that a higher level of CARLo-5 expression was associated with significantly shorter overall survival time in patients with EC [41]. However, the cellular function of CASC2 and CARLo-5 in EC remains to be explored.
4.4. Cancer- or Subtype-Specific lncRNAs in EC
The EC-specific expression pattern observed for H19 supports the notion that H19 may serve as an ideal diagnostic biomarker and interesting potential therapeutic target for EC although further testing will be required.
While most previous studies have focused on the expression of lncRNAs in type I EC, a recent study reported a lower frequency of focal amplification of the lncRNA OVAL in endometrioid ECs but a higher frequency of focal amplification of OVAL locus in serous ECs [45]. This indicates that OVAL amplification is specific to serous EC. The subtype-specific lncRNA expression patterns need to be further investigated.
5. LncRNAs are Key Regulators of Signaling Pathways in EC
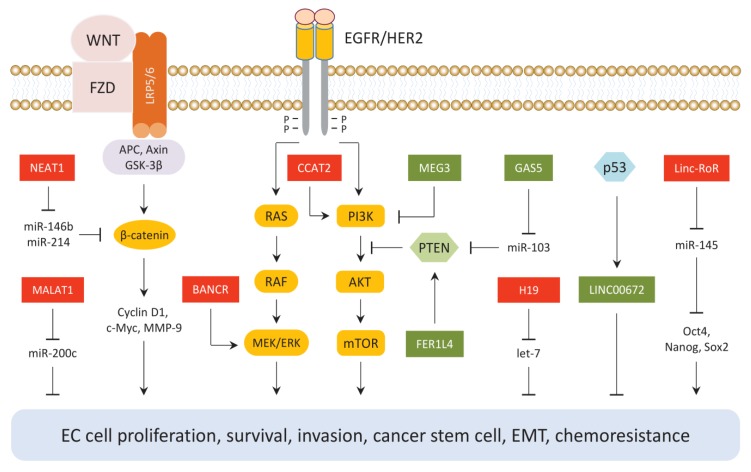
Tumor suppressive lncRNAs (GAS5, MEG3, FER1L4 and LINC00672) and oncogenic lncRNAs (CCAT2, BANCR, NEAT1, MALAT1, H19 and Linc-RoR) have been identified as key regulators of the established tumor suppressor or oncogenic pathways in EC (Figure 1).
Figure 1.
LncRNAs mediate cell signaling in EC cells. Tumor suppressive lncRNAs (GAS5, MEG3, FER1L4 and LINC00672) and oncogenic lncRNAs (CCAT2, BANCR, NEAT1, MALAT1, H19 and Linc-RoR) are upstream modulators or downstream effectors of well-known oncogenic or tumor suppressor pathways in EC, including the PTEN/PI3K/AKT/mTOR, RAS/RAF/MEK/ERK, WNT/β-catenin and p53 signaling pathways. Oncogenic lncRNAs (red) and tumor suppressive lncRNAs (green).
5.1. The PTEN/PI3K/AKT Signaling Pathway
LncRNA CCAT2 promotes EC cell growth and metastasis by reducing the levels of miR-216b, which is a repressor of the PI3K/AKT pathway [26].
Conversely, FER1L4 could enhance PTEN expression and inhibit AKT phosphorylation in EC cells [24]. Another lncRNA GAS5 was also shown to induce PTEN expression by inhibiting miR-103 in EC cells [20]. The results obtained from RNA immunoprecipitation assay showed that MEG3 can combine directly with PI3K protein and reduced its expression, leading to the downregulation of its downstream genes (including mTOR, P70S6K, VEGF-A and BCL-XL) and the attenuation of EC growth in vivo [22].
5.2. The RAS/RAF/MEK/ERK Signaling Pathway
BANCR promotes EC cell proliferation and invasion via activating the MEK/ERK signaling pathway [27].
5.3. The WNT/β-Catenin Signaling Pathway
Wnt proteins, which serve as ligands for the Wnt/β-catenin pathway, bind to the Frizzled (FZD) transmembrane receptor and induce the accumulation of nuclear β-catenin. This leads to the downregulation of target genes, such as cyclin D1, c-Myc and MMP-9 [46]. NEAT1 has been suggested to function as an oncogenic sponge in EC where it sequesters several tumor suppressor miRNAs (miR-146b and miR-214) to activate the WNT/β-catenin pathway [47,48]. Moreover, the ectopic expression of MALAT1, a downstream effector of the Wnt/β-catenin pathway [49], promoted EC cell migration and invasion via inhibiting the expression of miR-200c, which suppressed the migration and invasion of EC cells [29].
5.4. The p53 Signaling Pathway
LINC00672, a direct transcriptional target of p53, was shown to inhibit the development of malignant phenotypes of EC both in vitro and in vivo. This increased the sensitivity of xenograft mice to paclitaxel treatment via serving as a locus-restricted cofactor for p53-mediated gene suppression [25].
5.5. Epithelial to Mesenchymal Transition and Cancer Stem Cells-Associated Signaling Pathways
In tumors, the epithelial to mesenchymal transition (EMT) was shown to play a critical role in promoting cancer invasion, cancer stemness, immune escape and resistance to therapy [50]. Let-7 is frequently silenced in a variety of tumors, with the ectopic expression of let-7 inhibiting proliferation, EMT and invasion of EC cells and abrogating cancer stem cells (CSCs)-like properties [51,52]. H19 directly binds to let-7 and suppresses its levels in EC cells, resulting in de-repression of let-7 targets (c-Myc, Hmga2 and Imp3) and stimulation of EC cell migration and invasion via inducing EMT [35].
Many tumors (including EC) harbor CSCs [53]. CSCs are considered as the primary tumor initiator cells and are resistant to conventional therapies [54]. CSC marker genes, such as Oct4, Sox2, KLF4 and Nanog, are direct targets of miR-145 [55,56]. Interestingly, Linc-RoR, an oncogenic lncRNA, acts as a sponge of miR-145 to induce the expression of Oct4, Sox2 and Nanog in CSC-like EC cells [57], which suggests a mechanism where Linc-RoR could promote CSC properties by negatively regulating the expression of miR-145.
Numerous studies have reported the link between the dysregulation of lncRNAs and the occurrence of chemoresistance in cancers [58]. HOTAIR induces the resistance of EC cells to cisplatin by inducing autophagy [59]. However, the involvement of lncRNAs in EC chemoresistance is still largely unclear.
6. Emerging Role of LncRNA in EC Microenvironment
The tumor microenvironment consists of the extracellular matrix, blood or lymphatic vessels, fibroblasts, immune cells and inflammatory cells [60]. The reciprocal cross-talks between tumor cells and tumor microenvironment can protect tumor cells from immune evasion or nutrient deficiency, both of which are critical for driving tumor progression and resistance to treatment [60].
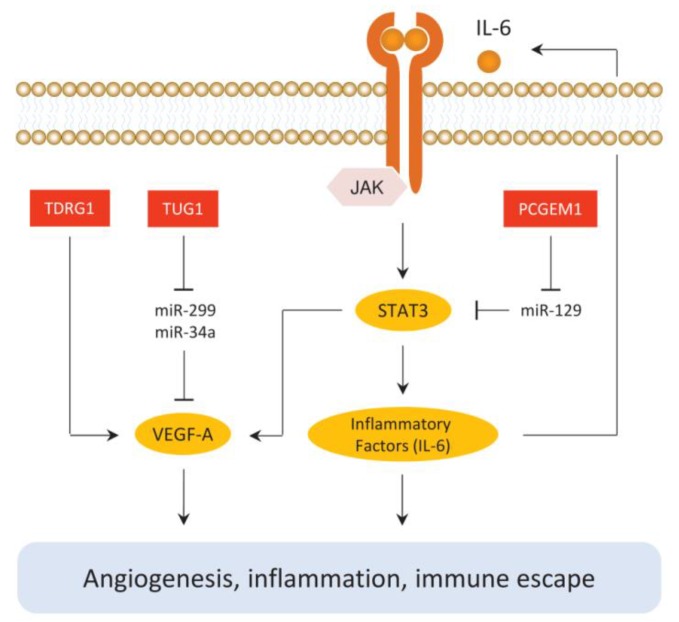
The regulatory roles of lncRNA during EC progression are not limited to tumor cells as they are also implicated in generating a tumor-promoting microenvironment (Figure 2).
Figure 2.
LncRNAs are mediators of angiogenesis and inflammation in EC. TUG1 and TDRG1 stimulate the VEGF-A pathway. PCGEM1 is implicated in activating the JAK/STAT3 pathway.
Angiogenesis, the process of the formation of new blood vessels, is controlled by many angiogenic factors, such as the VEGF family [61]. VEGF-A is one of the most potent and specific endothelial cell growth factors and plays important roles in the growth of EC [6]. In EC cells, TDRG1 was significantly upregulated in ECs compared to normal endometrial tissues. This could directly bind to VEGF-A protein and upregulated its expression, thus promoting EC proliferation, invasion and migratory ability in addition to inhibiting cell apoptosis [42]. In addition, TUG1 expression in EC tissues was significantly higher than that in adjacent normal tissues, which can enhance the progression of EC via enhancing the expression of VEGF-A, possibly through inhibiting miR-34a and miR-299 expression [43].
Tumor-associated inflammation is both a consequence and a driver of tumorigenesis [62]. NF-κB and STAT3 are two major factors controlling tumor inflammation, angiogenesis and invasiveness [63]. The STAT3 pathway can be induced by cytokines, such as IL-6 [64]. The activation of the JAK/STAT3 pathway results in the nuclear translocation of STAT3 and subsequent translation of key downstream target genes, including cyclin D1, Bcl-xL, c-Myc, Mcl-1 and VEGF-A [65]. Importantly, STAT3 induces the expression of many cytokines, chemokines and other mediators, such as interleukin-6 and cyclooxygenase 2. In turn, this activates STAT3, thus forming autocrine and paracrine feed-forward loops that promote tumor inflammation [66]. The levels of PCGEM1 were increased in EC tissues, which was shown to promote the proliferation, migration and invasive ability of EC cells through a STAT3-dependent mechanism. In this mechanism, PCGEM1 indirectly increases the levels of STAT3 through inhibiting miR-129 expression while the elevated STAT3 expression further upregulates the expression of Survivin, VEGF-A and MMP-2 [44].
PD-L1 is a powerful immunosuppressive molecule that inactivates the function of tumor-specific T cells by binding to its receptor PD-1 on T cells [67]. PD-L1 expression was detected in 48% of ECs and a higher level of PD-L1 expression was significantly associated with lymph node metastasis [68]. A recent analysis of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveals that H19 has the potential to induce the expression of PD-L1 in laryngeal squamous cell carcinoma, which possibly occurs by modulating a set of miRNAs (including miR-214) [69]. The involvement of lncRNAs in regulating PD-L1 or other immune inhibitory molecules in EC cells should be explored and the identification of such mechanisms would deepen our understanding of the molecular basis underlying the immune escape mechanisms of EC and facilitate more choices when designing immunotherapy against EC.
7. Future Perspectives: Hope and Challenges
To date, most investigations have centered on the in vitro EC cell line models with less aggressive potential and as a result, very little is known about the role of lncRNA in high-grade endometrioid or serous EC. The lncRNA expression pattern that differentiates the aggressive EC from non-aggressive EC is still unknown.
In addition to aberrant expression, single-nucleotide polymorphisms (SNPs) within the lncRNA transcripts or their promoters or mutations within the non-coding genome may dramatically alter the secondary structure, expression and function of these lncRNAs, thereby exerting important effects in cancer [70]. Genotype–phenotype correlation studies showed that the G allele of rs6983267, a reported oncogenic SNP at 8q24.21 [71], was significantly correlated with a higher level of CARLo-5 expression and poorer overall survival in EC patients [41]. The rapid advance in state-of-the-art next-generation sequencing techniques, re-analysis of the publicly available databases and the progress in bioinformatics computing methods would facilitate large-scale discovery of SNPs and mutations in lncRNAs and help us to identify particularly important EC-associated lncRNAs for future study [72,73,74,75].
Extracellular vesicles (EV; including exosomes and microvesicles) can carry proteins, lipids, miRNA, mRNA, DNA and recently lncRNA from one cell to another [76]. Exosomes released by tumor cells have the potential to induce malignant phenotypes of recipient cells [77,78]. As an example, EVs secreted from invasive breast cancer cell subpopulations were demonstrated to upregulate the proliferative, migratory and angiogenic potential of the parent population [77]. Moreover, EVs released from drug-resistant cancer cells was shown to transmit the anti-cancer drug resistance to other cancer cells [79]. A recent study demonstrated that EC cells could transmit miRNAs to endometrial fibroblasts via exosomes [80]. These results suggest that the transfer of ncRNAs (such as miRNA or lncRNA) via EVs may represent a new mechanism for intercellular communication that promotes the growth and metastasis of EC.
Once released, exosomes enter the circulation and can be isolated from blood, urine, saliva and ascites [81], indicating that tumor cell-derived EVs in circulating bodily fluids are readily accessible and can act as non-invasive specimens for biomarker discovery. The level of EVs bearing a candidate protein was elevated before diagnosis by conventional imaging techniques and the presence of EVs bearing candidate mRNAs was a predictor of progression-free survival in several cancers [82,83]. These findings have suggested that EVs released by tumor cells may be used for early disease detection and disease monitoring. A potential direction for future research is to explore the use of lncRNAs within EC cell-derived EVs for disease diagnosis and monitoring [84].
However, there are some challenges to be solved before the tumor cell-derived EVs can be used as biomarkers in a clinical setting. The accurate quantification of circulating lncRNA in exosomes faces several challenges, such as the correct procedure for specimen collection and handling, and the proper selection of internal controls for data normalization [85,86].
Given the importance of lncRNAs in tumors, multiple approaches, such as knockdown pathogenic lncRNA using siRNA, prevention of the binding of a lncRNA with its binding partners or creating lncRNA-binding small molecules have been developed [3,87,88]. Hopefully, the in vitro and in vivo studies have shown that RNA interference targeting oncogenic lncRNAs (including BANCR, H19, HOTAIR, UCA1, SNHG8, ASLNC04080 and PVT1) [27,35,38,40,89,90,91] or enforced expression of tumor suppressor lncRNA MEG3 could exert promising anti-tumor effects on EC [21].
Recently, the utilization of genome-editing with CRISPR/Cas9 system represents a promising opportunity for designing effective anti-cancer therapy [92]. Targeting UCA1 via specifically designed gRNAs of CRISPR/Cas9 system effectively inhibited the proliferation, migration and invasion of bladder cancer cells in vitro and suppressed the growth of xenograft tumors from bladder cancer cells in nude mice [93].
However, in a genome-wide analysis, only 38% of 15929 lncRNA loci are safely amenable for CRISPR applications, while the remaining lncRNA loci are at risk to inadvertently deregulate neighboring genes [94], suggesting that targeting some but not all lncRNA regions via the CRISPR/Cas9 system is challenging [95]. Several techniques, such as SpCas9-HF1, a high-fidelity variant of SpCas9 that contains alterations in the amino acid sequence, have been utilized to reduce the off-target effects due to CRISPR/Cas9 [96,97]. Moreover, a tumor-specific promoter may be used to drive Cas9 expression, thus providing a relatively high specificity of CRISPR/Cas9 for cancer cells [98].
8. Conclusions
By employing three basic modules, which are namely RNA–RNA, RNA–protein and RNA–DNA interactions, lncRNAs form both transcriptional and post-transcriptional regulatory networks, which play the pivotal roles in modulating the malignant phenotypes of tumor cells and the remodeling of the tumor microenvironment. Tumor suppressive lncRNAs (GAS5, MEG3, FER1L4 and LINC00672) and oncogenic lncRNAs (CCAT2, BANCR, NEAT1, MALAT1, H19, Linc-RoR, TUG1, TDRG1 and PCGEM1) may be a few such examples. Since lncRNAs can be detected in both cancer samples and various bodily fluids and strongly resist RNases, they have considerable diagnostic value and might be hopeful biomarkers for early detection and disease monitoring of EC. H19 exhibits highly EC-specific expression and might be a potential new target for EC treatment. Further clarification of lncRNA-mediated regulatory networks will shed light into the mechanisms behind EC and new studies continue to suggest novel lncRNAs as possible diagnostic markers and therapeutic targets.
Acknowledgments
We thank Zhujie Xu for her full contribution in preparing the figures.
Author Contributions
Writing—review and editing, P.D., Y.X., J.Y., S.J.B.H., N.K., Y.T. and H.W.
Funding
This work was supported by a grant from JSPS Grant-in-Aid for Scientific Research (C) (16K11123 and 18K09278), the Science and Technology Planning Project of Guangdong Province, China (2014A020212124) and an NIH/NCI grant 1R21CA216585-01A1 to Junming Yue.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA. Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Morice P., Leary A., Creutzberg C., Abu-Rustum N., Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 3.Dong P., Kaneuchi M., Konno Y., Watari H., Sudo S., Sakuragi N. Emerging therapeutic biomarkers in endometrial cancer. Biomed. Res. Int. 2013;2013:130362. doi: 10.1155/2013/130362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., van der Zee M., Fodde R., Blok L.J. Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget. 2010;1:674–684. doi: 10.18632/oncotarget.101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dedes K.J., Wetterskog D., Ashworth A., Kaye S.B., Reis-Filho J.S. Emerging therapeutic targets in endometrial cancer. Nat. Rev. Clin. Oncol. 2011;8:261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 6.Mahecha A.M., Wang H. The influence of vascular endothelial growth factor-A and matrix metalloproteinase-2 and -9 in angiogenesis, metastasis and prognosis of endometrial cancer. Onco. Targets Ther. 2017;10:4617–4624. doi: 10.2147/OTT.S132558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talhouk A., McAlpine J.N. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol. Oncol. Res. Pract. 2016;3:14. doi: 10.1186/s40661-016-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine D.A., Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong P., Xiong Y., Yue J., Hanley S.J.B., Kobayashi N., Todo Y., Watari H. Long Non-coding RNA NEAT1: A Novel Target for Diagnosis and Therapy in Human Tumors. Front. Genet. 2018;9:471. doi: 10.3389/fgene.2018.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Heesch S., van Iterson M., Jacobi J., Boymans S., Essers P.B., de Bruijn E., Hao W., MacInnes A.W., Cuppen E., Simonis M. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 2014;15:R6. doi: 10.1186/gb-2014-15-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik B., Feng F.Y. Long noncoding RNAs in prostate cancer: Overview and clinical implications. Asian J. Androl. 2016;18:568–574. doi: 10.4103/1008-682X.177123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.T. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 14.Hung T., Chang H.Y. Long noncoding RNA in genome regulation: Prospects and mechanisms. RNA Biol. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y., Malouf G.G., Zhang J., Zheng X., Chen Y., Thompson E.J., Weinstein J.N., Yuan Y., Spano J.P., Broaddus R., et al. Long non-coding RNA profiling links subgroup classification of endometrioid endometrial carcinomas with trithorax and polycomb complex aberrations. Oncotarget. 2015;6:39865–39876. doi: 10.18632/oncotarget.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J., Qian Y., Ye M., Fu Z., Jia X., Li W., Xu P., Lv M., Huang L., Wang L., et al. Distinct expression profile of lncRNA in endometrial carcinoma. Oncol. Rep. 2016;36:3405–3412. doi: 10.3892/or.2016.5173. [DOI] [PubMed] [Google Scholar]
- 17.Yang L., Zhang J., Jiang A., Liu Q., Li C., Yang C., Xiu J. Expression profile of long non-coding RNAs is altered in endometrial cancer. Int. J. Clin. Exp. Med. 2015;8:5010–5021. [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y., Zou X., He J., Mao Y. Identification of long non-coding RNAs biomarkers associated with progression of endometrial carcinoma and patient outcomes. Oncotarget. 2017;8:52604–52613. doi: 10.18632/oncotarget.17537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldinu P., Cossu A., Manca A., Satta M.P., Sini M.C., Rozzo C., Dessole S., Cherchi P., Gianfrancesco F., Pintus A., et al. Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum. Mutat. 2004;23:318–326. doi: 10.1002/humu.20015. [DOI] [PubMed] [Google Scholar]
- 20.Guo C., Song W.Q., Sun P., Jin L., Dai H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015;22:100. doi: 10.1186/s12929-015-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Q., Qian Z., Yan D., Li L., Huang L. LncRNA-MEG3 inhibits cell proliferation of endometrial carcinoma by repressing Notch signaling. Biomed. Pharmacother. 2016;82:589–594. doi: 10.1016/j.biopha.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 22.Sun K.X., Wu D.D., Chen S., Zhao Y., Zong Z.H. LncRNA MEG3 inhibit endometrial carcinoma tumorigenesis and progression through PI3K pathway. Apoptosis. 2017;22:1543–1552. doi: 10.1007/s10495-017-1426-7. [DOI] [PubMed] [Google Scholar]
- 23.Kong Y., Ren Z. Overexpression of LncRNA FER1L4 in endometrial carcinoma is associated with favorable survival outcome. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8113–8118. doi: 10.26355/eurrev_201812_16502. [DOI] [PubMed] [Google Scholar]
- 24.Qiao Q., Li H. LncRNA FER1L4 suppresses cancer cell proliferation and cycle by regulating PTEN expression in endometrial carcinoma. Biochem Biophys. Res. Commun. 2016;78:507–512. doi: 10.1016/j.bbrc.2016.06.160. [DOI] [PubMed] [Google Scholar]
- 25.Li W., Li H., Zhang L., Hu M., Li F., Deng J., An M., Wu S., Ma R., Lu J., et al. Long non-coding RNA LINC00672 contributes to p53 protein-mediated gene suppression and promotes endometrial cancer chemosensitivity. J. Biol. Chem. 2017;292:5801–5813. doi: 10.1074/jbc.M116.758508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie P., Cao H., Li Y., Wang J., Cui Z. Knockdown of lncRNA CCAT2 inhibits endometrial cancer cells growth and metastasis via sponging miR-216b. Cancer Biomark. 2017;21:123–133. doi: 10.3233/CBM-170388. [DOI] [PubMed] [Google Scholar]
- 27.Wang D., Wang D., Wang N., Long Z., Ren X. Long Non-Coding RNA BANCR Promotes Endometrial Cancer Cell Proliferation and Invasion by Regulating MMP2 and MMP1 via ERK/MAPK Signaling Pathway. Cell Physiol. Biochem. 2016;40:644–656. doi: 10.1159/000452577. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Wei D., Yang C., Sun H., Lu T., Kuang D. Overexpression of long noncoding RNA, NEAT1 promotes cell proliferation, invasion and migration in endometrial endometrioid adenocarcinoma. Biomed. Pharmacother. 2016;84:244–251. doi: 10.1016/j.biopha.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Li Q., Zhang C., Chen R., Xiong H., Qiu F., Liu S., Zhang M., Wang F., Wang Y., Zhou X., et al. Disrupting MALAT1/miR-200c sponge decreases invasion and migration in endometrioid endometrial carcinoma. Cancer Lett. 2016;383:28–40. doi: 10.1016/j.canlet.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Tanos V., Ariel I., Prus D., De-Groot N., Hochberg A. H19 and IGF2 gene expression in human normal, hyperplastic and malignant endometrium. Int J. Gynecol. Cancer. 2004;14:521–525. doi: 10.1111/j.1048-891x.2004.014314.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Wang D.L., Yu P. LncRNA H19 regulates the expression of its target gene HOXA10 in endometrial carcinoma through competing with miR-612. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4820–4827. doi: 10.26355/eurrev_201808_15617. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L., Li Z., Chen W., Zhai W., Pan J., Pang H., Li X. H19 promotes endometrial cancer progression by modulating epithelial-mesenchymal transition. Oncol. Lett. 2017;13:363–369. doi: 10.3892/ol.2016.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrenson K., Pakzamir E., Liu B., Lee J.M., Delgado M.K., Duncan K., Gayther S.A., Liu S., Roman L., Mhawech-Fauceglia P. Molecular Analysis of Mixed Endometrioid and Serous Adenocarcinoma of the Endometrium. PLoS ONE. 2015;10:e0130909. doi: 10.1371/journal.pone.0130909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng L., Yuan X.Q., Liu Z.Y., Li W.L., Zhang C.Y., Zhang Y.Q., Pan X., Chen J., Li Y.H., Li G.C. High lncRNA H19 expression as prognostic indicator: Data mining in female cancers and polling analysis in non-female cancers. Oncotarget. 2017;8:1655–1667. doi: 10.18632/oncotarget.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan L., Zhou J., Gao Y., Ghazal S., Lu L., Bellone S., Yang Y., Liu N., Zhao X., Santin A.D., et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015;34:3076–3084. doi: 10.1038/onc.2014.236. [DOI] [PubMed] [Google Scholar]
- 36.Łuczak A., Supernat A., Łapińska-Szumczyk S., Jachimowicz D., Majewska H., Gulczyński J., Żaczek A.J. HOTAIR in relation to epithelial-mesenchymal transition and cancer stem cells in molecular subtypes of endometrial cancer. Int. J. Biol. Markers. 2016;31:e245–e251. doi: 10.5301/jbm.5000187. [DOI] [PubMed] [Google Scholar]
- 37.He X., Bao W., Li X., Chen Z., Che Q., Wang H., Wan X.P. The long non-coding RNA HOTAIR is upregulated in endometrial carcinoma and correlates with poor prognosis. Int. J. Mol. Med. 2014;33:325–332. doi: 10.3892/ijmm.2013.1570. [DOI] [PubMed] [Google Scholar]
- 38.Huang J., Ke P., Guo L., Wang W., Tan H., Liang Y., Yao S. Lentivirus-mediated RNA interference targeting the long noncoding RNA HOTAIR inhibits proliferation and invasion of endometrial carcinoma cells in vitro and in vivo. Int. J. Gynecol. Cancer. 2014;24:635–642. doi: 10.1097/IGC.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y.X., Wang C., Mao L.W., Wang Y.L., Xia L.Q., Zhao W., Shen J., Chen J. Long noncoding RNA HOTAIR mediates the estrogen-induced metastasis of endometrial cancer cells via the miR-646/NPM1 axis. Am. J. Physiol. Cell. Physiol. 2018;314:C690–C701. doi: 10.1152/ajpcell.00222.2017. [DOI] [PubMed] [Google Scholar]
- 40.Lu L., Shen Y., Tseng K.F., Liu W., Duan H., Meng W. Silencing of UCA1, a poor prognostic factor, inhibited the migration of endometrial cancer cell. Cancer Biomark. 2016;17:171–177. doi: 10.3233/CBM-160628. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X., Wei X., Zhao L., Shi L., Cheng J., Kang S., Zhang H., Zhang J., Li L., Zhang H., et al. The rs6983267 SNP and long non-coding RNA CARLo-5 are associated with endometrial carcinoma. Environ. Mol. Mutagen. 2016;57:508–515. doi: 10.1002/em.22031. [DOI] [PubMed] [Google Scholar]
- 42.Chen S., Wang L.L., Sun K.X., Liu Y., Guan X., Zong Z.H., Zhao Y. LncRNA TDRG1 enhances tumorigenicity in endometrial carcinoma by binding and targeting VEGF-A protein. Biochim. Biophys. Acta Mol. Basis. Dis. 2018;1864:3013–3021. doi: 10.1016/j.bbadis.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Liu L., Chen X., Zhang Y., Hu Y., Shen X., Zhu W. Long non-coding RNA TUG1 promotes endometrial cancer development via inhibiting miR-299 and miR-34a-5p. Oncotarget. 2017;8:31386–31394. doi: 10.18632/oncotarget.15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q., Shen F., Zhao L. The relationship between lncRNA PCGEM1 and STAT3 during the occurrence and development of endometrial carcinoma. Biomed. Pharmacother. 2018;107:918–928. doi: 10.1016/j.biopha.2018.08.091. [DOI] [PubMed] [Google Scholar]
- 45.Akrami R., Jacobsen A., Hoell J., Schultz N., Sander C., Larsson E. Comprehensive analysis of long non-coding RNAs in ovarian cancer reveals global patterns and targeted DNA amplification. PLoS ONE. 2013;8:e80306. doi: 10.1371/journal.pone.0080306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eritja N., Yeramian A., Chen B.J., Llobet-Navas D., Ortega E., Colas E., Abal M., Dolcet X., Reventos J., Matias-Guiu X. Endometrial Carcinoma: Specific Targeted Pathways. Adv. Exp. Med. Biol. 2017;943:149–207. doi: 10.1007/978-3-319-43139-0_6. [DOI] [PubMed] [Google Scholar]
- 47.Huang X., Zhong R., He X., Deng Q., Peng X., Li J., Luo X. Investigations on the mechanism of progesterone in inhibiting endometrial cancer cell cycle and viability via regulation of long noncoding RNA NEAT1/microRNA-146b-5p mediated Wnt/β-catenin signaling. IUBMB Life. 2018 doi: 10.1002/iub.1959. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Zhao X., Guo Z., Ma X., Song Y., Guo Y. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch. Gynecol. Obstet. 2017;295:1469–1475. doi: 10.1007/s00404-017-4365-1. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y., Yang Y., Trovik J., Sun K., Zhou L., Jiang P., Lau T.S., Hoivik E.A., Salvesen H.B., et al. A novel wnt regulatory axis in endometrioid endometrial cancer. Cancer Res. 2014;74:5103–5117. doi: 10.1158/0008-5472.CAN-14-0427. [DOI] [PubMed] [Google Scholar]
- 50.Sistigu A., Di Modugno F., Manic G., Nisticò P. Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine Growth Factor Rev. 2017;36:67–77. doi: 10.1016/j.cytogfr.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J., Ma L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012;31:653–662. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ihira K., Dong P., Xiong Y., Watari H., Konno Y., Hanley S.J., Noguchi M., Hirata N., Suizu F., Yamada T., et al. EZH2 inhibition suppresses endometrial cancer progression via miR-361/Twist axis. Oncotarget. 2017;8:13509–13520. doi: 10.18632/oncotarget.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato K. Endometrial cancer stem cells: A new target for cancer therapy. Anticancer Res. 2012;32:2283–2293. [PubMed] [Google Scholar]
- 54.Batlle E., Clevers H. Cancer stem cells revisited. Nat. Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 55.Xu N., Papagiannakopoulos T., Pan G., Thomson J.A., Kosik K.S. MicroRNA-145 regulates OCT4, SOX2 and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 56.Gao S., Wang P., Hua Y., Xi H., Meng Z., Liu T., Chen Z., Liu L. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget. 2016;7:1608–1618. doi: 10.18632/oncotarget.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X., Gao Q., Wang J., Zhang X., Liu K., Duan Z. Linc-RNA-RoR acts as a “sponge” against mediation of the differentiation of endometrial cancer stem cells by microRNA-145. Gynecol. Oncol. 2014;133:333–3339. doi: 10.1016/j.ygyno.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 58.Chen Q.N., Wei C.C., Wang Z.X., Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8:1925–1936. doi: 10.18632/oncotarget.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun M.Y., Zhu J.Y., Zhang C.Y., Zhang M., Song Y.N., Rahman K., Zhang L.J., Zhang H. Autophagy regulated by lncRNA HOTAIR contributes to the cisplatin-induced resistance in endometrial cancer cells. Biotechnol. Lett. 2017;39:1477–1484. doi: 10.1007/s10529-017-2392-4. [DOI] [PubMed] [Google Scholar]
- 60.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Żyła M.M., Kostrzewa M., Litwińska E., Szpakowski A., Wilczyński J.R., Stetkiewicz T. The role of angiogenic factors in endometrial cancer. Prz Menopauzalny. 2014;13:122–126. doi: 10.5114/pm.2014.42714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li N., Grivennikov S.I., Karin M. The unholy trinity: Inflammation, cytokines and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan Y., Mao R., Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C.L., Hsieh F.C., Lieblein J.C., Brown J., Chan C., Wallace J.A., Cheng G., Hall B.M., Lin J. Stat3 activation in human endometrial and cervical cancers. Br. J. Cancer. 2007;96:591–599. doi: 10.1038/sj.bjc.6603597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S.W., Sun Y.M. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review) Int. J. Oncol. 2014;44:1032–1040. doi: 10.3892/ijo.2014.2259. [DOI] [PubMed] [Google Scholar]
- 66.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Escors D., Gato-Cañas M., Zuazo M., Arasanz H., García-Granda M.J., Vera R., Kochan G. The intracellular signalosome of PD-L1 in cancer cells. Signal. Transduct. Target. Ther. 2018;3:26. doi: 10.1038/s41392-018-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tawadros A.I.F., Khalafalla M.M.M. Expression of programmed death-ligand 1 and hypoxia-inducible factor-1α proteins in endometrial carcinoma. J. Cancer Res. Ther. 2018;14:S1063–S1069. doi: 10.4103/0973-1482.202891. [DOI] [PubMed] [Google Scholar]
- 69.Sun J., Lian M., Ma H., Wang R., Ma Z., Wang H., Zhai J., Meng L., Feng L., Bai Y., et al. Competing endogenous RNA network analysis of CD274, IL-10 and FOXP3 co-expression in laryngeal squamous cell carcinoma. Mol. Med. Rep. 2018;17:3859–3869. doi: 10.3892/mmr.2017.8307. [DOI] [PubMed] [Google Scholar]
- 70.Minotti L., Agnoletto C., Baldassari F., Corrà F., Volinia S. SNPs and Somatic Mutation on Long Non-Coding RNA: New Frontier in the Cancer Studies? High. Throughput. 2018;7 doi: 10.3390/ht7040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haiman C.A., Le Marchand L., Yamamato J., Stram D.O., Sheng X., Kolonel L.N., Wu A.H., Reich D., Henderson B.E. A common genetic risk factor for colorectal and prostate cancer. Nat. Genet. 2007;39:954–956. doi: 10.1038/ng2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hrdlickova B., de Almeida R.C., Borek Z., Withoff S. Genetic variation in the non-coding genome: Involvement of micro-RNAs and long non-coding RNAs in disease. Biochim. Biophys. Acta. 2014;1842:1910–1922. doi: 10.1016/j.bbadis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Miao Y.R., Liu W., Zhang Q., Guo A.Y. lncRNASNP2: an updated database of functional SNPs and mutations in human and mouse lncRNAs. Nucleic Acids Res. 2018;46:D276–D280. doi: 10.1093/nar/gkx1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.You Q., Yang X., Peng Z., Xu L., Wang J. Development and Applications of a High Throughput Genotyping Tool for Polyploid Crops: Single Nucleotide Polymorphism (SNP) Array. Front. Plant Sci. 2018;9:104. doi: 10.3389/fpls.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jalali S., Kapoor S., Sivadas A., Bhartiya D., Scaria V. Computational approaches towards understanding human long non-coding RNA biology. Bioinformatics. 2015;31:2241–2251. doi: 10.1093/bioinformatics/btv148. [DOI] [PubMed] [Google Scholar]
- 76.Lane R.E., Korbie D., Hill M.M., Trau M. Extracellular vesicles as circulating cancer biomarkers: Opportunities and challenges. Clin. Transl. Med. 2018;7:14. doi: 10.1186/s40169-018-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Brien K., Rani S., Corcoran C., Wallace R., Hughes L., Friel A.M., McDonnell S., Crown J., Radomski M.W., O’Driscoll L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur. J. Cancer. 2013;49:1845–1859. doi: 10.1016/j.ejca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Namee N.M., O’Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim. Biophys. Acta Rev. Cancer. 2018;1870:123–136. doi: 10.1016/j.bbcan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Xu C.G., Yang M.F., Ren Y.Q., Wu C.H., Wang L.Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4362–4368. [PubMed] [Google Scholar]
- 80.Maida Y., Takakura M., Nishiuchi T., Yoshimoto T., Kyo S. Exosomal transfer of functional small RNAs mediates cancer-stroma communication in human endometrium. Cancer Med. 2016;5:304–3414. doi: 10.1002/cam4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Provencio M., Rodríguez M., Cantos B., Sabín P., Quero C., García-Arroyo F.R., Rueda A., Maximiano C., Rodríguez-Abreu D., Sánchez A., et al. mRNA in exosomas as a liquid biopsy in non-Hodgkin Lymphoma: A multicentric study by the Spanish Lymphoma Oncology Group. Oncotarget. 2017;8:50949–50957. doi: 10.18632/oncotarget.16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N., et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–1782. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muinelo-Romay L., Casas-Arozamena C., Abal M. Liquid Biopsy in Endometrial Cancer: New Opportunities for Personalized Oncology. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whiteside T.L. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert. Rev. Mol. Diagn. 2015;15:1293–1310. doi: 10.1586/14737159.2015.1071666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qi P., Zhou X.Y., Du X. Circulating long non-coding RNAs in cancer: Current status and future perspectives. Mol Cancer. 2016;15:39. doi: 10.1186/s12943-016-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arun G., Diermeier S.D., Spector D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018;24:257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parasramka M.A., Maji S., Matsuda A., Yan I.K., Patel T. Long non-coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol. Ther. 2016;161:67–78. doi: 10.1016/j.pharmthera.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang C.H., Zhang X.Y., Zhou L.N., Wan Y., Song L.L., Gu W.L., Liu R., Ma Y.N., Meng H.R., Tian Y.L., Zhang Y. LncRNA SNHG8 participates in the development of endometrial carcinoma through regulating c-MET expression by miR-152. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1629–1637. doi: 10.26355/eurrev_201803_14698. [DOI] [PubMed] [Google Scholar]
- 90.Zhai W., Li X., Wu S., Zhang Y., Pang H., Chen W. Microarray expression profile of lncRNAs and the upregulated ASLNC04080 lncRNA in human endometrial carcinoma. Int. J. Oncol. 2015;46:2125–2137. doi: 10.3892/ijo.2015.2897. [DOI] [PubMed] [Google Scholar]
- 91.Kong F., Ma J., Yang H., Yang D., Wang C., Ma X. Long non-coding RNA PVT1 promotes malignancy in human endometrial carcinoma cells through negative regulation of miR-195-5p. Biochim. Biophys Acta Mol. Cell Res. 2018 doi: 10.1016/j.bbamcr.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Lage M., Puig-Serra P., Menendez P., Torres-Ruiz R., Rodriguez-Perales S. CRISPR/Cas9 for Cancer Therapy: Hopes and Challenges. Biomedicines. 2018;6:105. doi: 10.3390/biomedicines6040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhen S., Hua L., Liu Y.H., Sun X.M., Jiang M.M., Chen W., Zhao L., Li X. Inhibition of long non-coding RNA UCA1 by CRISPR/Cas9 attenuated malignant phenotypes of bladder cancer. Oncotarget. 2017;8:9634–9646. doi: 10.18632/oncotarget.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goyal A., Myacheva K., Groß M., Klingenberg M., Duran Arqué B., Diederichs S. Challenges of CRISPR/Cas9 applications for long non-coding RNA genes. Nucleic Acids Res. 2017;45:e12. doi: 10.1093/nar/gkw883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Worku T., Bhattarai D., Ayers D., Wang K., Wang C., Rehman Z.U., Talpur H.S., Yang L. Long Non-Coding RNAs: The New Horizon of Gene Regulation in Ovarian Cancer. Cell Physiol. Biochem. 2017;44:948–966. doi: 10.1159/000485395. [DOI] [PubMed] [Google Scholar]
- 96.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., Joung J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang J., Meng X., Pan J., Jiang N., Zhou C., Wu Z., Gong Z. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 2018;15:35–43. doi: 10.1080/15476286.2017.1391443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kia A., Przystal J.M., Nianiaris N., Mazarakis N.D., Mintz P.J., Hajitou A. Dual systemic tumor targeting with ligand-directed phage and Grp78 promoter induces tumor regression. Mol. Cancer Ther. 2012;1:2566–2577. doi: 10.1158/1535-7163.MCT-12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]