Abstract
Testosterone (17β-Hydroxyandrost-4-en-3-one) is the main sex hormone in males. Maintaining and enhancing testosterone level in men is an incessant target for many researchers. Examples of such research approaches is to utilize specific types of food or dietary supplements as a safe and easily reached means. Here, specifically, since 1967 until now, many research studies have revealed the effect of onion on testosterone; however, this link has yet to be collectively reviewed or summarized. To accomplish this contribution, we searched the Scopus, Web of Science, and PubMed databases for full articles or abstracts (published in English language) from April 1967 through December 2018 using the keywords “onion” versus “testosterone”. In addition, a number of related published articles from the same databases were included to improve the integrity of the discussion, and hence the edge of the future directions. In summary, there is an evidence that onions enhance testosterone level in males. The mechanisms by which this occurs is mainly by increasing the production of luteinizing hormone, enhancing the antioxidant defense mechanism in the tests, neutralizing the damaging effects of the generated free radicals, ameliorating insulin resistance, promoting nitric oxide production, and altering the activity of adenosine 5′-monophosphate -activated protein kinase. However, this effect requires further approval in humans, mainly by conducting clinical trials.
Keywords: onion, testosterone, luteinizing hormone, oxidative stress, antioxidants
1. Introduction
In general, testosterone (17β-Hydroxyandrost-4-en-3-one) is the main sex hormone in humans and other species males [1]. It plays a key role in developing their reproductive organs and other sexual features such as body hair and muscle mass [2]. Decreased levels of testosterone in males are linked with a wide range of diseases/disorders such as diabetes mellitus [3], male infertility [4,5,6], Alzheimer’s [7], osteoporosis [8], depression [9], and cardiovascular disease [10,11]. Therefore, various research studies as well as reports have focused on preserving and enhancing testosterone in males. Examples of this specific research intention included the use of certain types of food or dietary supplements.
The onion (Allium cepa L.), also known as common onion or bulb onion, is a vegetable that has been grown and selectively farmed for more than seven thousand years [12]. At this moment in time, onions become part of humans’ food; for example, in 2016, the world production of onions (dried onions) reached more than 90 million tons, led by China (~26% of the total) and India (~21% of the total).
Naturally, onions can be found in three main subtypes: white, red, and yellow. Regardless of the subtype, in general, raw onion bulbs contain approximately 89% water, 4% sugar, 2% dietary fiber, 1% protein, a negligible amount of fat (~100 mg/100 g), and low amounts of essential nutrients [13]. Accordingly, the energy value of onion is not significant (~40 kcal/100 g) [13]. Most importantly, onions were found to contain several and unique phytochemicals such as quercetin and quercetin glucosides, kaempferol, thiosulfinates, cepaenes, anthocyanins, and sulfur compounds (e.g., S-methyl cysteine sulfoxide, diallyl trisulfide, S-allyl cysteine sulfoxide, dimethyl trisulfide) that have been identified to have an impact on humans’ health [14,15]. Yet, some of these phytochemicals are under basic and translational research to explore their possible biological effects in humans.
It is worth mentioning that Okinawans, the longest-living people out of any state or country in the world according to the World Health Organization reports, were found to consume habitually considerable amounts of certain vegetables including onions [16]. Besides, surprisingly, serum testosterone was found to be higher in Okinawan men compared, for examples, with the age-matched Americans [16]. This could be an evidence that onions may have a potential impact on general health in men.
As per Scopus database, since 1967 until now, there are at least 27 research articles linked onion to testosterone; however, this link has not yet been collectively reviewed and summarized. Here, we searched the Scopus, Web of Science, and PubMed databases for full articles or abstracts (published in the English language) from April 1967 through December 2018 using the keywords “onion” and “testosterone”. In addition, selected indirect published articles from the same databases were included to reach the comprehensive and integral discussion and conclusion.
2. Effect of Onions on Testosterone
Table 1 summarizes the main and direct studies conducted on onions or onion extracts and their reported effects on testosterone level. Almost all of these studies were conducted in vivo utilizing rat models, particularly male rats. Only one study was conducted on healthy men.
Table 1.
A summary of the research studies conducted on onions or onion extracts and their reported effects on testosterone.
| (Onion or Onion Derivative) | Dose (Mode of Treatment) | Duration | Population | Effect on Testosterone | Reference |
|---|---|---|---|---|---|
| Onion juice | 2, 4, and 6 mL day−1 (Orally) | 10 days | Male albino rats | (+) | [20] |
| Onion juice | 0.5 and 1 g rat−1 day−1 (Orally) | 20 days | Wistar male rats | (+) | [21] |
| Onion | 15 mg kg−1 day−1 (Orally) | 4 weeks | Streptozotocin-induced diabetic | (+) | [22] |
| Onion juice | 3 mL day−1 rat−1 (Orally) | 4 weeks | Lamotrigine-induced male rats | (+) | [23,24] |
| Aqueous extract of onion | 1 mL of the extract/100 g of body weight (Orally) |
8 weeks | Male rats with aluminum-induced reproductive toxicity | (±) | [25] |
| Aqueous extract of onion | 1 and 2 mL rat−1 (Orally) | 4 weeks | Rats | (±) | [26] |
| Aqueous extract of onion | 1 mL/100g of body weight (Orally) | 4 weeks | Male rats with cadmium-induced organ toxicity | (±) | [27] |
| Onion juice | 1 and 2 mL day−1 rat−1 (Orally) | 20 days | Adult male abino rats | (+) | [28] |
| Onion juice | 3 mL rat−1 (Orally) | 60 days | Eschericcia coli infected male rats | (+) | [29] |
| Onion juice | 3 mL rat−1 (Orally) | 60 days | Permethrin-induced male rats | (+) | [30] |
| Onion juice | 40 mg kg−1 (Orally) | 14 days | Testicular torsion/detorsion rat models | (+) | [31] |
| Onion extracts containing concentrated cysteine sulfoxides | 30 mg day−1 (Orally) | 14 days | Healthy men | (+) | [17] |
(+) Increase, (−) Decrease, (±) no effect.
In addition, as shown in Table 1, the effect of onions on testosterone level was often measured using onion juice or aqueous onion extracts rather than directly using the onion bulb; only one study has utilized onion bulb. In the animal studies, considering the studies conducted on onion juice, the minimum used dose was approximately 0.5 mL rat−1 day−1 and the maximum used dose was approximately 6 mL rat−1 day−1. Onion juice, extract, or bulb were given orally to all tested in vivo systems over a period ranging from 10 to 60 days.
As a result, in general, the main stream of research (75% of research studies) that are directly linking between onion and testosterone reveal a positive effect of onion on testosterone level in males. The rest of these studies (25%) did not show a significant effect of onion on testosterone level. These blunted studies were mainly conducted on male rats induced by chemical toxicants (cadmium, aluminum), which may induce irreversible damage to the reproductive organs.
Specifically, as shown in Table 1, it is important to point that the only human study that directly link between onion and testosterone reveal a positive effect of onion extracts on testosterone [17]. However, more confirmatory human studies in this specific research context are still very imperative.
The summarized evidence from this study supports the use of onion as an aphrodisiac food, to increase libido and strengthen the reproductive organs, by many cohorts [18]. Testosterone therapy was used to treat hypoactive sexual desire [19]. Traditionally, fried onions in a pure butter mixture was used by many cohorts, particularly Indians, as an aphrodisiac tonic, especially when taken with a spoon of honey [18]. In addition, another Indian traditional mixture called kanji, which is a powder of black gram dipped in onion juice for seven days and then dried, was used as an aphrodisiac food [18].
3. Mechanistic Studies
3.1. Effect of Onion on Luteinizing Hormone
In men, testosterone is mainly synthesized in Leydig cells [32]. The function and the number of Leydig cells in the testis in turn is regulated primarily by luteinizing hormone and secondarily by follicle-stimulating hormone [32]. The amount of the produced testosterone by Leydig cells is under the control of luteinizing hormone [33,34]. Specifically, luteinizing hormone regulates the expression of 17β-hydroxysteroid dehydrogenase, which catalyzes the conversion of androstenedione to testosterone. The formed testosterone is transported to Sertoli cells in the testis to enhance sperm production (i.e., spermatogenesis) [35].
A number of studies have revealed that onions have a positive impact on production of luteinizing hormone; for example, Wistar male rats administered fresh onion juice at 1 g rat−1 day−1, for 20 consecutive days, had higher levels of luteinizing hormone compared with the control [21]. In addition, aqueous onion extract (1 mL of the extract/100 g of body weight, for eight weeks) significantly increased luteinizing hormone in both normal and aluminum chloride-treated male rats [25]. Accordingly, the positive effect of onion on testosterone may be, at least in part, attributable to the increased level of luteninizing hormone.
3.2. Effect of Onion as a Potential Antioxidant on Testosterone
Generally, in cellular systems, accumulation of reactive oxygen species and formation of oxidative stress state has been found to significantly reduce the function of the cell [33,36]. Such reduction may be due to the increased oxidative damage to the functional and structural components (e.g., proteins, lipids, and nucleic acids) of the cell. Therefore, countering this oxidative damage should convalesce the performance of the cell [37].
In particular, increased generation of reactive oxygen species, and hence the oxidative damage in testicular Leydig cells may reduce the synthesis of testosterone [10]. This reduction may be aggregated in the presence of chemical toxicants such as aluminum and cadmium, and in the presence of certain drugs such as permethrin, which is a medication and insecticide used to cure scabies. Indeed, aluminum chloride was found to significantly decrease testosterone synthesis [25], may be by reducing the activity of the testicular antioxidant enzymes such as superoxide dismutase, catalase, and glutathione reductase, and disrupting the gene expression of 3β-hydroxysteroid dehydrogenase and cholesterol side-chain cleavage enzyme in the testis [38]. It has been shown that the enzymatic antioxidants were significantly increased in onion treated-male rats and in onion treated-male rats with aluminum-induced reproductive toxicity [25]. Such enhancement for the antioxidant defense mechanism in Leydig cells by the effect of onion may increase the production of testosterone. In addition, onion was found to decrease the formation of malondialdehyde, a marker of oxidative damage, particularly lipid peroxidation, in normal male rats and male rats with aluminum-induced reproductive toxicity [39]. Moreover, aqueous onion extract was found to protect against cadmium-induced oxidative stress; however, it did not recover cadmium-induced testosterone depletion [27].
Furthermore, in rats, permethrin was found to significantly reduce the number of Leydig cells and the amount of testosterone produced [30]. Via unknown pathway, onion juice (3 mL rat−1, for 60 days) was able to decrease damage to the Leydig cells and improve testosterone production [30]. The mechanism by which this occurs is mainly by reducing the oxidative damage to Leydig cells; given that onion contains potent antioxidant molecules such as quercetin and quercetin glucosides, thiosulfinates, and anthocyanins [30].
Actually, among 28 vegetables, onions were found to rank the highest in quercetin content. In effect, quercetin and quercetin derivatives have been found to have an impact on human’s health. It is important mention that quercetin has been found to protect against wide-range of diseases/disorders such as asthma [40], diabetes [41], gout [42], cancer [43], arthritis [44], osteoporosis [45], and neurodegenerative disorders [46]. Therefore, the high content of the antioxidant quercetin and its derivatives in onion makes it very effective dietary contrivance to enhance humans’ health.
Specifically, quercetin (50 mg kg−1 day−1, for 15 days) significantly increased testosterone level in arsenic-induced reproductive damage in rats [47]. In addition, quercetin at 90 mg−1 kg−1 day−1 for 15 days was found to enhance testosterone production in male rats with di-(2-ethylhexyl) phthalate-induced reproductive toxicity [48]. Moreover, adult male albino rats with atrazine-induced reproductive toxicity administered quercetin at 10 mg−1 kg−1 of body weight for 21 days had higher levels of testosterone compared with the control [49]. Furthermore, quercetin at 20 mg−1 kg−1 of body weight for two weeks restored plasma testosterone induced by sulphasalazine in male rats [50]. Recently, it has been shown that quercetin at 20 mg−1 kg−1 of body weight for 28 days is able to attenuate testosterone depletion induced by cadmium chloride in male Wistar rats [51]. Such protective effects may be attributable to the antioxidant activity of quercetin as it was found to increase the activity of testicular antioxidants (superoxide dismutase, glutathione reductase, and catalase) and significantly reduce the level of malondialdehyde in testicular tissue [47,50,52].
Moreover, it was concluded that vegetables, rich in quercetin-4′-glucoside, such as onion, are likely to serve as valuable antioxidant sources for reducing iron-induced oxidative stress and lipid peroxidation in live cells [53]. In addition, in general, the aqueous extract of onion has been identified as having a potent hydroxyl radical scavenging activity utilizing 2,2-Diphenyl-1-picrylhydrazyl [54]. Furthermore, A. cepa L. polysaccharide fractions were found to have a strong antioxidant actions toward 3-ethylbenzothiazoline-6-sulphonic acid radical cations, superoxide anion radical scavenging, and Fe2+ chelating [55].
Further, the reduction in glutathione may diminish the detoxification process of the produced oxidants in the testes, which delays or reduce the synthesis of testosterone [56]. It was shown that quercetin prevented testicular glutathione depletion, thereby resulting in a decrease in content prooxidants [52]. Such protective effects may contribute to enhance the production of testosterone, and hence the ways of its action.
3.3. Effect of Onion as an Antihyperglycemic Affecter on Testosterone
Several studies have revealed that the generation of free radicals, and hence the level of oxidative damage, in diabetic conditions, most of time, is higher than in normal conditions [57,58,59]. Such hyperglycemic-induced oxidative stress states was found to delay or disrupt the function of the cell [57,60].
It was demonstrated that that onion has hypoglycemic effects and can be used as a dietary supplement to manage the progression in patients with type 1 and type 2 diabetes [61]. In addition, onion peel extracts, rich with quercetin, was found to reduce insulin resistance in male diabetic rats on high fat diet [62]. Moreover, streptozotocin-induced diabetic rats administered onion and fenugreek had lower oxidative stress levels compared to those administered only fenugreek [63]. In addition, onion juice was found to have an insulin-like action as it decreased blood glucose level in hyperglycemic animal models [20]. Moreover, one of the major bioactive compounds in onion are sulfur compounds such as S-methyl cysteine sulfoxide, diallyl trisulfide, S-allyl cysteine sulfoxide, dimethyl trisulfide, dipropyl disulfide, propenyl propyl disulfide, propenyl methyl disulfide, and methyl propyl trisulfide [15]. It has been shown that many of these bioactive compounds have insulinotropic as well as antidiabetic activity in animal models, which may explain an improvement in beta-cell function and increase in insulin sensitivity [15]. Actually, these sulfur compounds in onions were found to enhance the acticity of hepatic enzymes (e.g., glucose-6-phosphatase and glucokinase), which could be, at least in part, behind the antihyperglycemic effect of onion in the experimental animals.
Collectively, according to these evidences, it can be suggested that the hypoglycemic effect of onion has a positive effect on testosterone production, particularly in diabetic conditions. Indeed, streptozotocin-induced diabetic rats orally administered onion at 15 mg kg−1 day−1 for four weeks had higher testosterone levels compared with the control [22].
3.4. Effect of Onion as a Nitric Oxide Stimulator on Testosterone
It is well-known that the decrease in blood flow to the testis reduces the synthesis of testosterone, which negatively affect spermatogenesis [64,65]. Enhancing nitric oxide, a free radical gas generated by nitric oxide synthase and acts as a vasodilator, may increase blood flow in the testis and promote testosterone synthesis [33]. It has been suggested that aqueous onion extract has nitric oxide-releasing properties [66]. Therefore, enhancing nitric oxide production could be a contributing factor behind the reported positive effects of onions on testosterone level.
3.5. Effect of Onion on 5′ AMP-Activated Protein Kinase
5′ AMP-activated protein kinase is an enzyme (heterotrimeric protein-complex) plays a vital role in energy homeostasis in cellular systems; specifically, by enhancing fatty acids oxidation and glucose uptake [67]. It has been shown that 5′ AMP-activated protein kinase activates the production of testosterone in Leydig cells in the testes [68]. In general, studies have shown that, quercetin, a functional molecule in onion, activates 5′ AMP-activated protein kinase [69,70]. Therefore, it can be suggested that onion may induce testosterone production by enhancing 5′ AMP-activated protein kinase. However, this suggestion requires approval by further research studies.
4. Conclusions and Future Perspectives
Collectively, there is an evidence that onion or onion extracts (e.g., aqueous extract ~30 mg day−1) enhances testosterone production in males. However, this effect requires further approval in humans, mainly by conducting clinical trials.
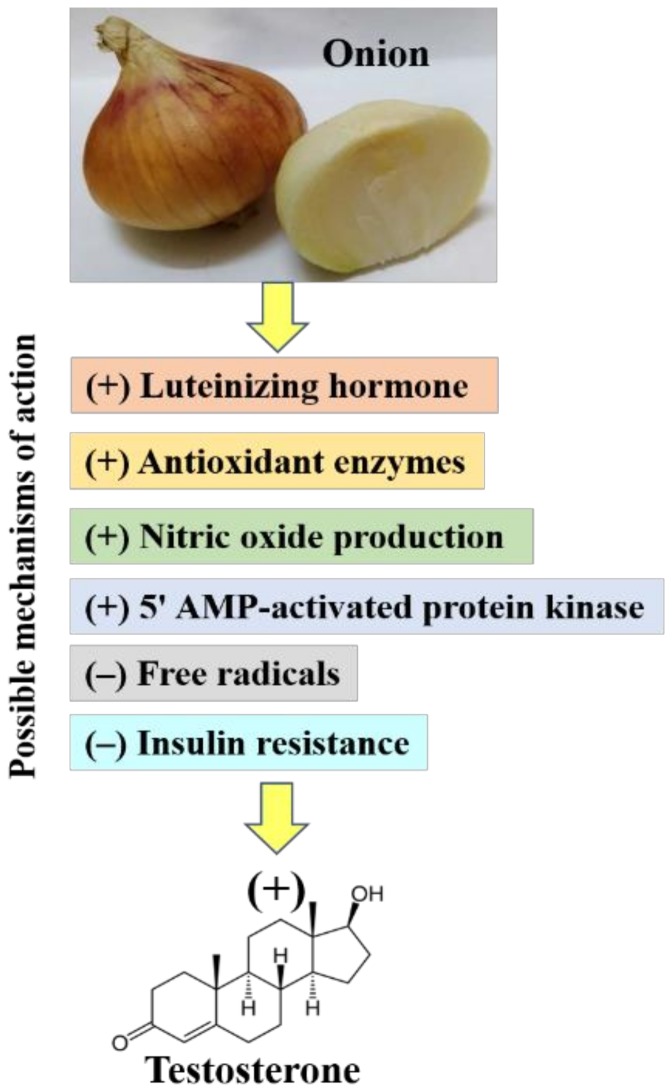
The mechanisms by which onion enhances testosterone production in males is mainly by enhancing the production of luteinizing hormone, neutralizing the damaging effects of the formed free radicals, mainly in the testes, enhancing the antioxidant defense mechanism (e.g., antioxidant enzymes, glutathione) in the testis, ameliorating insulin resistance, promoting nitric oxide production in Leydig cells, and altering the activity of 5′ AMP-activated protein kinase (Figure 1).
Figure 1.
Possible mechanisms by which onion enhances testosterone production in males.
Acknowledgments
This study was supported by the deanship of research at Jordan University of Science and Technology.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Kelly D.M., Jones T.H. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. 2013;217:R25–R45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 2.Mooradian A.D., Morley J.E., Korenman S.G. Biological actions of androgens. Endocr. Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Karakas M., Schafer S., Appelbaum S., Ojeda F., Kuulasmaa K., Bruckmann B., Berisha F., Schulte-Steinberg B., Jousilahti P., Blankenberg S., et al. Testosterone levels and type 2 diabetes-no correlation with age, differential predictive value in men and women. Biomolecules. 2018;8:76. doi: 10.3390/biom8030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Migdadi F., Banihani I., Banihani S.A. Clinico-hormonal correlation of oligospermic patients in the below sea level environment (Jordan Valley) Neuroendocrinol. Endocrinol. Lett. 2005;26:13–18. [PubMed] [Google Scholar]
- 5.Sonmez M., Turk G., Yuce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male wistar rats. Theriogenology. 2005;63:2063–2072. doi: 10.1016/j.theriogenology.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Du Plessis S.S., Cabler S., McAlister D.A., Sabanegh E., Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010;7:153–161. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 7.Lv W., Du N., Liu Y., Fan X., Wang Y., Jia X., Hou X., Wang B. Low testosterone level and risk of Alzheimer’s disease in the elderly men: A systematic review and meta-analysis. Mol. Neurobiol. 2016;53:2679–2684. doi: 10.1007/s12035-015-9315-y. [DOI] [PubMed] [Google Scholar]
- 8.Tuck S.P., Francis R.M. Testosterone, bone and osteoporosis. Front. Horm. Res. 2009;37:123–132. doi: 10.1159/000176049. [DOI] [PubMed] [Google Scholar]
- 9.McHenry J., Carrier N., Hull E., Kabbaj M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocr. 2014;35:42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banihani S.A. Effect of coenzyme q10 supplementation on testosterone. Biomolecules. 2018;8:172. doi: 10.3390/biom8040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloner R.A., Carson C., 3rd., Dobs A., Kopecky S., Mohler E.R., 3rd. Testosterone and cardiovascular disease. J. Am. Coll. Cardiol. 2016;67:545–557. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Brewster J. Onions and Other Vegetable Alliums. CABI; Wallingford, UK: 2008. [Google Scholar]
- 13.US National Onion Association . History of Onion. US National Onion Association; Greeley, CO, USA: 2017. [Google Scholar]
- 14.Tedesco I., Carbone V., Spagnuolo C., Minasi P., Russo G.L. Identification and quantification of flavonoids from two southern Italian cultivars of Allium cepa L., tropea (red onion) and montoro (copper onion), and their capacity to protect human erythrocytes from oxidative stress. J. Agric. Food Chem. 2015;63:5229–5238. doi: 10.1021/acs.jafc.5b01206. [DOI] [PubMed] [Google Scholar]
- 15.Islam M.S., Choi H., Loots du T. Effects of dietary onion (Allium cepa L.) in a high-fat diet streptozotocin-induced diabetes rodent model. Ann. Nutr. Metab. 2008;53:6–12. doi: 10.1159/000152868. [DOI] [PubMed] [Google Scholar]
- 16.Willcox D.C., Willcox B.J., Todoriki H., Suzuki M. The Okinawan diet: Health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J. Am. Coll. Nutr. 2009;28:500S–516S. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama Y., Tanaka K., Hiramoto S., Yahata N., Inagawa Y., Nukui K., Hori S. Alleviation of the aging males’ symptoms by the intake of onion-extracts containing concentrated cysteine sulfoxides for 4 weeks—Randomized, double-blind, placebo-controlled, parallel-group comparative study. Jpn. Pharmacol. Ther. 2017;45:595–608. [Google Scholar]
- 18.Kumar K.S., Debjit B., Chiranjib B., Pankaj T. Allium cepa: A traditional medicinal herb and its health benefits. J. Chem. Pharm. Res. 2010;2:283–291. [Google Scholar]
- 19.Petering R.C., Brooks N.A. Testosterone therapy: Review of clinical applications. Am. Fam. Physician. 2017;96:441–449. [PubMed] [Google Scholar]
- 20.Sharaf A. Some hormone-like properties of Allium cepa L. (onion) Qual. Plant. Mater. Veg. 1967;14:267–275. doi: 10.1007/BF02419929. [DOI] [Google Scholar]
- 21.Khaki A., Fathiazad F., Nouri M., Khaki A.A., Khamenehi H.J., Hamadeh M. Evaluation of androgenic activity of Allium cepa on spermatogenesis in the rat. Folia Morphol. 2009;68:45–51. [PubMed] [Google Scholar]
- 22.Gholamhosini B., Khaki A., Ahmadi-Ashtiani H.R., Rezazadeh S., Rastegar H., Fathiazad F., Ghanbari M. Treatment effects of onion on spermatogenesis in streptozotocin-induced diabetic rat. J. Med. Plants. 2009;8:153–161. [Google Scholar]
- 23.Khaki A., Farnam A., Ahmadi-Ashtiani H.R., Rezazadeh S., Rastgar H., Eftekharzadeh S., Aghamohamadi R., Abiri N. Treatment effect of onion on sexual behavior after induces an antiepileptic drug (lamotrigine) in male rat. J. Med. Plants. 2010;9:49–57. [Google Scholar]
- 24.Khaki A., Farnam A., Badie A.D., Nikniaz H. Treatment effects of onion (Allium cepa) and ginger (Zingiber officinale) on sexual behavior of rat after inducing an antiepileptic drug (lamotrigine) Balk. Med. J. 2012;29:236–242. doi: 10.5152/balkanmedj.2012.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhigbe R.E., Ige S.F. The role of Allium cepa on aluminum-induced reproductive dysfunction in experimental male rat models. J. Hum. Reprod. Sci. 2012;5:200–205. doi: 10.4103/0974-1208.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghalehkandi J.G., Asghari A., Beheshti R., Valilu M., Yeghaneh A. Effect of onion (Allium cepa. Linn) aqueous extract on serum concentration of lh, fsh and testosterone compared with zinc sulfate supplementation in the rats. J. Anim. Vet. Adv. 2012;11:3346–3349. [Google Scholar]
- 27.Ige S.F., Akhigbe R.E. Common onion (Allium cepa) extract reverses cadmium-induced organ toxicity and dyslipidaemia via redox alteration in rats. Pathophysiology. 2013;20:269–274. doi: 10.1016/j.pathophys.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Allouh M.Z., Daradka H.M., Barbarawi M.M., Mustafa A.G. Fresh onion juice enhanced copulatory behavior in male rats with and without paroxetine-induced sexual dysfunction. Exp. Boil. Med. 2014;239:177–182. doi: 10.1177/1535370213508360. [DOI] [PubMed] [Google Scholar]
- 29.Shahverdi S., Khaki A., Jafari B. Investigating the antioxidant effect of Allium cepa after exposure to escherichia coli on biochemical factors, the blood antioxidants, and testis tissue in rats. Crescent J. Med. Boil. Sci. 2017;4:32–38. [Google Scholar]
- 30.Khaki A., Khaki A.A., Rajabzadeh A. The effects of Permethrin and antioxidant properties of Allium cepa (onion) on testicles parameters of male rats. Toxin Rev. 2017;36:1–6. doi: 10.1080/15569543.2016.1235582. [DOI] [Google Scholar]
- 31.Shokoohi M., Madarek E.O.S., Khaki A., Shoorei H., Khaki A.A., Soltani M., Ainehchi N. Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/detorsion by intrauterine insemination method. Int. J. Womens Health Reprod. Sci. 2018;6:499–505. doi: 10.15296/ijwhr.2018.82. [DOI] [Google Scholar]
- 32.Khaki A., Khaki A.A., Hajhosseini L., Golzar F.S., Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr. J. Tradit. Complement. Altern. Med. 2014;11:1–8. doi: 10.4314/ajtcam.v11i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banihani S.A. Ginger and testosterone. Biomolecules. 2018;8:119. doi: 10.3390/biom8040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Chen F., Ye L., Zirkin B., Chen H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction. 2017;154:R111–R122. doi: 10.1530/REP-17-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griswold M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 36.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 37.Banihani S.A. Role of uric acid in semen. Biomolecules. 2018;8:65. doi: 10.3390/biom8030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammad N.S., Arafa M.H., Atteia H.H. Coenzyme q10 and fish oil synergistically alleviate aluminum chloride-induced suppression of testicular steroidogenesis and antioxidant defense. Free Radic. Res. 2015;49:1319–1334. doi: 10.3109/10715762.2015.1069290. [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal N., Rizvi S.I. Onion extract (Allium cepa L.), quercetin and catechin up-regulate paraoxonase 1 activity with concomitant protection against low-density lipoprotein oxidation in male wistar rats subjected to oxidative stress. J. Sci. Food Agric. 2014;94:2752–2757. doi: 10.1002/jsfa.6620. [DOI] [PubMed] [Google Scholar]
- 40.Mlcek J., Jurikova T., Skrovankova S., Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016;21:623. doi: 10.3390/molecules21050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eitah H.E., Maklad Y.A., Abdelkader N.F., Gamal El Din A.A., Badawi M.A., Kenawy S.A. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol. Appl. Pharm. 2019;36:30–40. doi: 10.1016/j.taap.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Miyazawa K.W., Staurengo-Ferrari L., Mizokami S.S., Domiciano T.P., Vicentini F., Camilios-Neto D., Pavanelli W.R., Pinge-Filho P., Amaral F.A., Teixeira M.M., et al. Quercetin inhibits gout arthritis in mice: Induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology. 2017;25:555–570. doi: 10.1007/s10787-017-0356-x. [DOI] [PubMed] [Google Scholar]
- 43.Brito A.F., Ribeiro M., Abrantes A.M., Pires A.S., Teixo R.J., Tralhao J.G., Botelho M.F. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr. Med. Chem. 2015;22:3025–3039. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- 44.Khanna D., Sethi G., Ahn K.S., Pandey M.K., Kunnumakkara A.B., Sung B., Aggarwal A., Aggarwal B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharm. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Law Y.Y., Chiu H.F., Lee H.H., Shen Y.C., Venkatakrishnan K., Wang C.K. Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-aged and post-menopausal healthy subjects. Food Funct. 2016;7:902–912. doi: 10.1039/C5FO01251A. [DOI] [PubMed] [Google Scholar]
- 46.Ren F., Reilly K., Kerry J.P., Gaffney M., Hossain M., Rai D.K. Higher antioxidant activity, total flavonols, and specific quercetin glucosides in two different onion (Allium cepa L.) varieties grown under organic production: Results from a 6-year field study. J. Agric. Food Chem. 2017;65:5122–5132. doi: 10.1021/acs.jafc.7b01352. [DOI] [PubMed] [Google Scholar]
- 47.Baltaci B.B., Uygur R., Caglar V., Aktas C., Aydin M., Ozen O.A. Protective effects of quercetin against arsenic-induced testicular damage in rats. Andrologia. 2016;48:1202–1213. doi: 10.1111/and.12561. [DOI] [PubMed] [Google Scholar]
- 48.Abd-Ellah M.F., Aly H.A., Mokhlis H.A., Abdel-Aziz A.H. Quercetin attenuates di-(2-ethylhexyl) phthalate-induced testicular toxicity in adult rats. Hum. Exp. Toxicol. 2016;35:232–243. doi: 10.1177/0960327115580602. [DOI] [PubMed] [Google Scholar]
- 49.Abdel Aziz R.L., Abdel-Wahab A., Abo El-Ela F.I., Hassan N.E.Y., El-Nahass E.S., Ibrahim M.A., Khalil A.A.Y. Dose- dependent ameliorative effects of quercetin and l-carnitine against atrazine- induced reproductive toxicity in adult male albino rats. Biomed. Pharm. 2018;102:855–864. doi: 10.1016/j.biopha.2018.03.136. [DOI] [PubMed] [Google Scholar]
- 50.Osawe S.O., Farombi E.O. Quercetin and rutin ameliorates sulphasalazine-induced spermiotoxicity, alterations in reproductive hormones and steroidogenic enzyme imbalance in rats. Andrologia. 2018;50:e12981. doi: 10.1111/and.12981. [DOI] [PubMed] [Google Scholar]
- 51.Ujah G.A., Nna V.U., Agah M.I., Omue L.O., Leku C.B., Osim E.E. Effect of quercetin on cadmium chloride-induced impairments in sexual behaviour and steroidogenesis in male wistar rats. Andrologia. 2018;50:e12866. doi: 10.1111/and.12866. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P., Aslam Khan I., Singh R. Curcumin and quercetin ameliorated cypermethrin and deltamethrin-induced reproductive system impairment in male wistar rats by upregulating the activity of pituitary-gonadal hormones and steroidogenic enzymes. Int. J. Fertil. Steril. 2018;12:72–80. doi: 10.22074/ijfs.2018.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murota K., Mitsukuni Y., Ichikawa M., Tsushida T., Miyamoto S., Terao J. Quercetin-4′-glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J. Agric. Food Chem. 2004;52:1907–1912. doi: 10.1021/jf035151a. [DOI] [PubMed] [Google Scholar]
- 54.Kim I.S., Yang M., Goo T.H., Jo C., Ahn D.U., Park J.H., Lee O.H., Kang S.N. Radical scavenging-linked antioxidant activities of commonly used herbs and spices in Korea. Int. J. Food Sci. Nutr. 2012;63:603–609. doi: 10.3109/09637486.2011.641942. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y.L., Zhu D.Y., Thakur K., Wang C.H., Wang H., Ren Y.F., Zhang J.G., Wei Z.J. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.) Int. J. Biol. Macromol. 2018;111:92–101. doi: 10.1016/j.ijbiomac.2017.12.154. [DOI] [PubMed] [Google Scholar]
- 56.Akinyemi A.J., Adedara I.A., Thome G.R., Morsch V.M., Rovani M.T., Mujica L.K.S., Duarte T., Duarte M., Oboh G., Schetinger M.R.C. Dietary supplementation of ginger and turmeric improves reproductive function in hypertensive male rats. Toxicol. Rep. 2015;2:1357–1366. doi: 10.1016/j.toxrep.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banihani S.A. Radish (Raphanus sativus) and diabetes. Nutrients. 2017;9:1014. doi: 10.3390/nu9091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maiese K. New insights for oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2015;2015:875961. doi: 10.1155/2015/875961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thakur P., Kumar A., Kumar A. Targeting oxidative stress through antioxidants in diabetes mellitus. J. Drug Target. 2018;26:766–776. doi: 10.1080/1061186X.2017.1419478. [DOI] [PubMed] [Google Scholar]
- 60.Denu R.A., Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid. Med. Cell. Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taj-Eldin I.M., Ahmed E.M., Elwahab H.M.A. Preliminary study of the clinical hypoglycemic effects of Allium cepa (red onion) in type 1 and type 2 diabetic patients. Environ. Health Insights. 2010;4:71–77. doi: 10.4137/EHI.S5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung J.Y., Lim Y., Moon M.S., Kim J.Y., Kwon O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr. Metab. 2011;8:18. doi: 10.1186/1743-7075-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pradeep S.R., Srinivasan K. Amelioration of oxidative stress by dietary fenugreek (Trigonella foenum-graecum L.) seeds is potentiated by onion (Allium cepa L.) in streptozotocin-induced diabetic rats. Appl. Physiol. Nutr. Metab. 2017;42:816–828. doi: 10.1139/apnm-2016-0592. [DOI] [PubMed] [Google Scholar]
- 64.Wang J.M., Gu C.H., Tao L., Wu X.L. Effect of surgery and efferent duct ligation on testicular blood flow and testicular steroidogenesis in the rat. J. Reprod. Fertil. 1985;73:191–196. doi: 10.1530/jrf.0.0730191. [DOI] [PubMed] [Google Scholar]
- 65.Clavijo R.I., Carrasquillo R., Ramasamy R. Varicoceles: Prevalence and pathogenesis in adult men. Fertil. Steril. 2017;108:364–369. doi: 10.1016/j.fertnstert.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 66.Grman M., Misak A., Cacanyiova S., Kristek F., Tomaskova Z., Bertova A., Ondrias K. The aqueous garlic, onion and leek extracts release nitric oxide from S-nitrosoglutathione and prolong relaxation of aortic rings. Gen. Physiol. Biophys. 2011;30:396–402. doi: 10.4149/gpb_2011_04_396. [DOI] [PubMed] [Google Scholar]
- 67.Merrill G.F., Kurth E.J., Hardie D.G., Winder W.W. Aica riboside increases amp-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 68.Taibi N., Dupont J., Bouguermouh Z., Froment P., Rame C., Anane A., Amirat Z., Khammar F. Expression of adenosine 5′-monophosphate-activated protein kinase (AMPK) in ovine testis (ovis aries): In vivo regulation by nutritional state. Anim. Reprod. Sci. 2017;178:9–22. doi: 10.1016/j.anireprosci.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Kim S.G., Kim J.R., Choi H.C. Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. J. Med. Food. 2018;21:146–153. doi: 10.1089/jmf.2017.4052. [DOI] [PubMed] [Google Scholar]
- 70.Rivera Rivera A., Castillo-Pichardo L., Gerena Y., Dharmawardhane S. Anti-breast cancer potential of quercetin via the Akt/AMPK/mammalian target of rapamycin (mTOR) signaling cascade. PLoS ONE. 2016;11:e0157251. doi: 10.1371/journal.pone.0157251. [DOI] [PMC free article] [PubMed] [Google Scholar]