Abstract
Chimeric antigen receptor T cells (CAR T Cells) have led to dramatic improvements in the survival of cancer patients, most notably those with hematologic malignancies. Early phase clinical trials in patients with solid tumors have demonstrated them to be feasible, but unfortunately has yielded limited efficacy for various cancer types. In this article we will review the background on CAR T cells for the treatment of solid tumors, focusing on the unique obstacles that solid tumors present for the development of adoptive T cell therapy, and the novel approaches currently under development to overcome these hurdles.
Keywords: CAR T cells, solid tumors, novel approaches
1. Introduction
The treatment of both hematologic and solid malignancies has undergone a large paradigm shift over the past decade with the introduction and adoption of T cell-mediated immunotherapies. The most successful approaches to date have included immune checkpoint inhibitors, bispecific antibodies, and chimeric antigen receptor T cells (CAR T cells). CAR T cells are autologous T lymphocytes that are designed to express the antigen binding region of an antibody directed against tumor-associated antigens (TAAs) [1]. Eshhar was one of the first to develop CAR T cells, repurposing a T cell with new antigen specificity [2]. CAR T cells are composed of three parts: (1) single-chain variable domain of an antibody (scFv), (2) a transmembrane domain, and (3) a signal transduction domain of the T-cell receptor (TCR) [3]. The scFV is created by cloning the variable regions of an antigen specific monoclonal antibody. Gamma retroviral or lentiviral recombinant vectors containing cloned DNA plasmids are then transfected into target cells. This permits the scFv to have antigen specificity [4]. When the CAR engages with a specific antigen, T cell activation occurs via the signal transduction domain of the TCR [5].
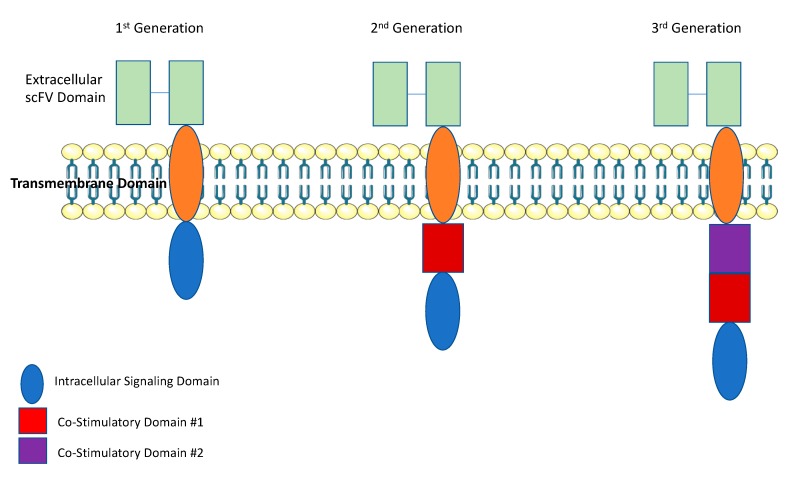
First-generation CAR T cells used a CD3ζ as the signal transduction domain of the TCR. Thus, T-cell activation was solely dependent on interleukin (IL)-2 production (Figure 1) [6]. While this produced excellent tumor-specific killing in vitro, there was poor T-cell expansion and anti- tumor activity in vivo [6]. Inadequate in vivo efficacy for first-generation CAR T cells occurred because under physiologic conditions, T cells require interaction with their TCR and multiple co-stimulatory receptors, such as CD28 and 4-1BB [7]. Thus, first generation CAR T cells were limited by a lack of co-stimulation. To improve upon first-generation CAR T cells, second-generation CAR T cells contained a co-stimulatory domain, either CD28 or 4-1BB. With the addition of a co-stimulatory domain, second- generation CAR T cells demonstrated significantly improved in vivo cytotoxicity, tumor killing, expansion, and persistence [8,9]. Interestingly the choice of co-stimulatory domains leads to a different functional T-cell subset. In CAR T cells with a CD28 co-stimulatory domain, T-cell expansion and activation is characteristic of effector T cells. While in those designed with a 4-1BB co-stimulatory domain, expanded T cells exhibited characteristics of memory T cells [10,11]. Third-generation CAR T cells were designed with two co-stimulatory domains. The first domain was either CD28 or 4-1BB, and the second domain was CD28, 4-1BB, or OXO40 [12]. More recently, a fourth-generation of “armored CAR T cells” has been designed to protect T cells from the immuno-suppressive tumor microenvironment. Armored CAR T cells have been engineered express cytokines, as an independent gene within the CAR vector [13]. This helps promote T-cell expansion and longevity within the tumor microenvironment [14]. In this review we will focus on the most recent advances of CAR T cell therapy for the treatment of solid tumors, the challenges faced thus far and future prospects on how CAR T cell therapy can be effectively used for the treatment of patients with solid tumors.
Figure 1.
CAR T Cell Structure: CAR T cells are composed of 3 parts: (1) single-chain variable domain of an antibody (scFv), (2) a transmembrane domain, and (3) a signal transduction domain of the T-cell receptor (TCR). First-generation CAR T cells used a CD3ζ as the signal transduction domain of the TCR. Second-generation CAR T cells contained a co-stimulatory domain, either CD28 or 4-1BB. Third-generation CAR T cells were designed with two co-stimulatory domains. The first domain was either CD28 or 4-1BB, and the second domain was CD28, 4-1BB, or OXO40. This figure was created with images adapted from Servier Medical Art by Servier. Original images are licensed under a Creative Commons Attribution 3.0 Unported License.
2. CAR T Cell Therapy for Hematologic Malignancies
Thus far, CD19 has been the most extensively studied and successful target of CAR T-cell therapy [15]. The use of second generation anti-CD19 CAR T cells have demonstrated high antitumor efficacy in patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL), chronic lymphocytic leukemia (CLL) and B-cell non-Hodgkin lymphoma (NHL). Response rates differ for each disease subtype but have ranged from about 50–90% [16,17]. This ultimately led to the FDA approval of two second generation anti-CD19 CAR T cell products, tisagenlecleucel and axicabtagene ciloleucel for the treatment of patients with R/R B-ALL and R/R large B cell lymphoma. [18,19]. Specifically, tisagenlecleucel signals through a 4-1BB co-stimulatory domain, while axicabtagene ciloleucel signals through a CD28 co-stimulatory domain [17]. Tisagenlecleucel has been approved both for the treatment of patients with R/R B-ALL who are less then 25 years old and R/R DLBCL, while axicabtagene ciloleucel has only been approved for patients with R/R large B-cell lymphoma [18,19]. The most significant side effects from treatment with anti-CD19 CAR T cells have been the development of cytokine release syndrome (CRS), CAR T Cell encephalopathy syndrome (CRES), and B-cell aplasia [18,19]. Currently, other B-cell specific antigens are being investigated as potential targets. This has included CD20, CD22, CD30, CD79a, and kappa light chain [17]. Two of the most exciting novel targets currently in clinical trials are CAR T cells directed against CD20 and CD22. Early phase clinical trials of CD20 CAR T cells employed the use of third generation CAR T cells with both CD28 and 4-1BB costimulatory domains in patients with R/R indolent B-cell and mantle cell lymphomas [20]. Two out of four patients treated had a clinically significant durable remission. Zhang et al., reported the results of phase IIa clinical trial CD20 CAR T cells in patients with R/R CD20+ lymphomas (8/11 DLBCL). The overall response rate (ORR) was 81.8%, with 6 complete remissions (CRs) and 3 partial remissions (PRs). The median progression-free survival lasted for >6 months [21]. More recently, anti-CD20-CD19 bispecific CARs have demonstrated promising results in preclinical models of ALL [22]. Similarly, CD22 CAR T cells have been investigated. Fry et al., recently reported the results of a phase 1 clinical trial of a second generation CD22 CAR T cell with a 4-1BB co-stimulatory domain in patients with R/R 21 children and 17 adults with B-ALL. In patients who received ≥1 × 106 CD22-CAR T cells/kg the complete response rate (CRR) was 73%, including patients with CD19- leukemias. Relapse was increased in patients with decreased CD22 expression [23].
3. CAR T Cell Therapy for Solid Tumors
While CD19-directed CAR T cells have been very effective for the treatment for B-cell lymphoid malignancies, the use of CAR T cells for solid tumors has not been as successful. The early CAR T cell studies in patients with solid tumors demonstrated feasibility with limited to minimal efficacy. For example, Kershaw et al., administered a first-generation Car T cells directed against the alpha-folate receptor (FR) followed by high dose IL-2 (HD IL-2) in patients with metastatic ovarian cancer. Patients tolerated the infusion well, however the trial was limited by poor persistence of the CAR T cell product, with the majority of patients having absent circulating CAR T cells by three weeks. Unfortunately, no objective responses were seen, and all patients had disease progression [24]. While this first study likely did not demonstrate clinical responses secondary to the use of a first generation CAR T cell product, which were limited by a lack of co-stimulation, clinical outcomes for trials using CAR T cells in solid tumors even using high later generation products have also been largely disappointing (see Table 1 for examples of CAR T cell clinical trials in solid tumors).
Table 1.
Examples of published clinical trials of CAR T cells for the treatment of solid tumors.
| Target | Cancer Subtype | Dosage | Outcome | Persistence | Ref |
|---|---|---|---|---|---|
| CD133 | Carcinomas | Mean: 1.43 × 106/kg CAR T Cells | PR 1 13% (3/23) SD 2 61% (14/23) |
>2 months in 7 patients. | [25] |
| CEA 3 | Colorectal | 1 × 108–1 × 1010/kg | SD 17% (1/6) | N/A | [26] |
| Liver Metastases | CAR T Cells (intrahepatic) | PD 83% (5/6) | |||
| EGFR 4 | NSCLC 5 | Mean 0.97 × 107 cells/kg | PR 18% (2/11) SD 45% (5/11) |
Up to 37 weeks | [27] |
| Biliary | Mean 2.65 × 106 cells/kg | CR 6 6% (1/17) SD 59% (10/17) |
One month | [28] | |
| EGFRvIII 7 | Glioblastoma | 1.75–5 × 108 cells | N/A | One Month | [29] |
| FR-𝛼 8 | Ovarian | 0.3–5 × 1010 Cells | NR | 3 Weeks | [24] |
| GD2 9 | Neuroblastoma | 1.2 × 107–1 × 108 cells/m2 | CR 27% (3/11) | Up to 192 weeks with ATC and 96 weeks with CTLs. | [30] |
| GPC3 10 | Hepatocellular | 0.92 × 107 to 8.72 × 107 cells/kg cohort A | PR 9% (1/11) | N/A | [31] |
| SD 27% (3/11) | |||||
| 0.013 × 107 to 14.68 × 107 cells/kg cohort B | PD 64% (7/11) | ||||
| HER2 11 | Sarcoma | 1 × 104–1 × 108 cells/m2 | SD 24% (4/17) | 9 months | [32] |
| Glioblastoma | 1 × 106–1 × 108 cells/m2 | PR 7% (1/15) | 12 weeks | [33] | |
| SD 27% (4/15) | |||||
| Biliary and Pancreatic Cancer | 1.4–3.8 × 106 cells/kg | PR 9% (1/11) SD 45% (5/11) |
80 days | [34] | |
| IL13-R𝛼3 12 | Glioblastoma | 2 × 106 Cells × 1; 10 × 106 cells × 5; Intracavitary |
CR 7.5 months | 7 Days | [35] |
| Mesothelin | Mesothelioma | 0.1–1 × 109 Cells × 3 1–3 × 108 cell/m2 3 times weekly for 3 weeks |
1/1 PR | Up to 22 days | [36] |
| Pancreatic | 3 × 107–3 × 108 cell/m2 | 2/6 SD | [37] | ||
| Ovarian | 6/6 SD | [38] | |||
| MUC1 13 | Seminal Vesicle | 5 × 105 cells per metastatic site | N/A | N/A | [39] |
| PSMA 14 | Prostate | 1 × 109–1 × 1010 Cells | PR 40% (2/5) | 28 Days | [40] |
| ROR1 15 | Breast | 3.3 × 105–1 × 107 cells/kg | N/A | N/A | [41] |
| NSCLC | |||||
| CAIX 16 | RCC 17 | 10 daily infusions of 2 × 107–2 × 109 CAR T-cells | NR | Up to 4 weeks | [42] |
1 Partial Response. 2 Stable Disease. 3 Carcinoembryonic Antigen. 4 Epidermal Growth Factor Receptor. 5 Nonsmall Cell Lung Cancer. 6 Complete Response. 7 Epidermal Growth Factor Receptor Variant III. 8 Folate Receptor Alpha. 9 Disialoganglioside GD2. 10 Glypican-3. 11 Human Epidermal Growth Factor Receptor 2. 12 Interleukin-13 Receptor Alpha-2. 13 Mucin-1. 14 Prostate-specific membrane antigen. 15 Receptor Tyrosine Kinase Like Orphan Receptor 1. 16 Carbonic anhydrase 9. 17 Renal Cell Carcinoma.
The reason for the lack of clinical efficacy of the early CAR T cell clinical trials is multifactorial. Unlike hematologic malignancies, solid tumors present unique obstacles. (1) There is a lack of specific tumor antigens that are uniformly expressed on solid tumors. (2) Currently administered CAR T cells must traffic from the blood to solid tumor sites, overcoming potential chemokine receptor mismatches, as well a dense and difficult stroma to penetrate. (3) Once in the TME, CAR T cells most overcome the hostile and immunosuppressive elements in order to infiltrate, expand, and elicit TAA-specific cytotoxicity. (4) Lastly, after encountering TAA’s, CAR T cells are at increased risk of developing T cell exhaustion with reduced capacity for long term persistence.
4. Obstacles and Potential Solutions for CAR T Cell Therapy for Solid Tumors
4.1. Absence of Unique TAA/TAA Heterogeneity
One of the more challenging aspects in developing CAR T cells for solid malignancies has been identifying a target antigen. Unlike hematologic malignancies which are defined by cell surface expression markers, solid tumors are defined by anatomic location, specific molecular mutations and markers that may be expressed on the cell surface or intracellularly. Therefore, while an ideal TAA would be expressed on 100% of tumor cells surfaces, without expression on normal healthy tissue; for most solid tumors target antigens typically are expressed both on tumors and normal healthy cells. Furthermore, an ideal antigen would also be primarily expressed on the cell surface, as currently it is more technically challenging to target intracellular antigens than surface antigens. However, in the case in malignancies arising from viral infection such as HPV, where HPV specific oncoproteins, E6 and E7, are primarily expressed intracellularly, and may be better targets then a cell surface marker [43].
Thus, identifying a specific target has been more difficult. In instances where antibodies against specific targets are already being used in clinical practice, the development of a CAR construct for that specific tumor is more straightforward. This has been used in the case of patients with malignancies where there is either overexpression or mutation of epidermal growth factor receptor (EGFR) [44,45]. Thus, CAR T cell products directed against EGFR or EGFR variants have been designed and are currently in clinical trials [29,46]. In other circumstances, targets have been identified to be more highly expressed on tumor tissues compared to healthy tissues. One example of this is mesothelin, a tumor differentiation antigen [47]. Mesothelin is highly overexpressed (>90%) on mesothelioma; while overexpressed to a lower extent on ovarian, pancreatic, and lung cancers. However, it also has low level expression on peritoneal, pleural, and pericardial surfaces [48]. Thus, for constructs directed against mesothelin, there is concern for “on-target off-tumor” toxicity [49]. More recently there has been a focus on using tumor neoantigens. Neoantigens are unique antigens on tumor cells secondary to somatic mutations that may drive tumor growth. Targeting neoantigens can hopefully minimize on-target off-tumor” toxicity, because these antigens should be exclusive to the tumor itself [50]. Currently, the TAAs used most commonly to develop CAR T cells for the treatment of solid malignancies include: carcinoembryonic antigen (CEA), the diganglioside GD2, mesothelin, interleukin 13 receptor α (IL-13Rα), human epidermal growth factor receptor 2 (HER2), fibro-blast activation protein (FAP), and L1 cell adhesion molecule (L1CAM) (Table 2).
Table 2.
Tumor-Associated Antigens Targeted in CAR-T Cell Therapy for Solid Tumors.
| Tumor Type | Target |
|---|---|
| Glioblastoma | Epidermal Growth Factor Receptor (EGFR) |
| Epidermal Growth Factor Receptor vIII (EGFRvIII) | |
| Interleukin-13 Receptor Alpha-2 (IL13Rα2) | |
| CD133 | |
| Neuroblastoma | Disialoganglioside GD2 (GD2) |
| L1 Cell Adhesion Molecule (L1-CAM) | |
| Lung Cancer | EGFR |
| Mesothelin (MSLN) | |
| Human Epidermal Growth Factor 2 (HER2) | |
| Breast Cancer | HER2 |
| MSLN | |
| Tyrosine-Protein Kinase Met (cMET) | |
| Gastric Cancer | Carcinoembryonic antigen (CEA) |
| HER2 | |
| Pancreatic Cancer | CEA |
| MSLN | |
| Renal Cell Carcinoma | Vascular Endothelial Growth Factor Receptor (VEGFR) |
| Carbonic anhydrase 9 (CAIX) | |
| Colon Cancer | HER2 |
| CEA | |
| Prostate Cancer | Prostate Membrane Antigen (PSMA) |
| Prostate Stem Cell Antigen (PSCA) | |
| Ovarian Cancer | Mucin 16(MUC-16)/CA-125 |
| HER2 | |
| MSLN | |
| L1-CAM | |
| Folate Receptor Alpha (FR-α) | |
| Cancer/Testis Antigen 1 (CTAG1B) | |
| Melanoma | GD2 |
| L1-CAM | |
| CTAG1B | |
| Osteosarcoma | GD2 |
| HER2 |
Tumor heterogeneity can also impact response to immunotherapy. There are multiple types of genetic heterogeneity in tumor biology, specifically interpatient and intratumoral. One of the fascinating aspects of the field of oncology is that no two patients behave the same clinically, and this is because each tumor, even if of the same subtype, is genetically different (i.e., interpatient heterogeneity) [51]. This is likely secondary to a combination of somatic mutations that occur during tumorigenesis, as well as unique host factor such as germ line mutations and immune surveillance [51]. Moreover, intratumoral heterogeneity refers to fact that within a tumor there are distinct clonal subpopulations (subclones), with different genetic and epigenetics phenotypes [52]. These differences can be enhanced when comparing primary sites of disease versus metastatic sites, as the clone that is able to escape the primary site may then develop heterogeneity within the metastatic site (i.e. intrametastatic heterogeneity) [53]. Often it is the metastatic site that has in fact the highest degree of genetic instability, contributing to tumor escape [54,55]. Given the significant degree of both intratumoral and intertumoral heterogeneity, response to adoptive T cell therapy can vary significantly between patients and potentially between different tumor sites within a given patient. Furthermore, solid tumors may also express multiple TAAs, each capable of being a potential target. However, the relative expression of each antigen on a specific tumor subclone can vary, affecting response to CAR T Cell therapy.
Under selection pressure from either treatment with conventional chemotherapy, or immunotherapy such as CAR T Cells, subclones can be selected for; promoting resistance [35,56]. Subclonal driver mutations have been found to be important predictors for disease progression and resistant to treatment, with patients with subclonal driver mutations having increased clonal evolution and tumor heterogeneity after treatment [57]. Mechanisms of resistance include the development of new somatic mutations that can affect signal transduction pathways. One particular mechanism that is importance in effecting response to CAR T cell therapy is the principal of immunoediting. Immunoediting is the process by which immunosurveillance selects for subclones lacking a specific immunogenic antigens, or promotes of subclones with reduced sensitivity to immune attack; enhancing clonal evolution, treatment resistance and tumor progression [58]. While tumor heterogeneity may promote resistance, it may also lead to the formation of increased tumor neo-antigens. This increased tumor mutational burden has been demonstrated to be a biomarker for a response with the use of immune checkpoint inhibitors (ICI), with improved responses in tumors with increased mutational burden [59]. How this will ultimately effect responses to CAR T cell therapy is currently being investigated, but it may allow for novel target antigens with the potential for reduced “off-target” toxicity. It also may provide further rationale to combine ICI with adoptive T cell therapy.
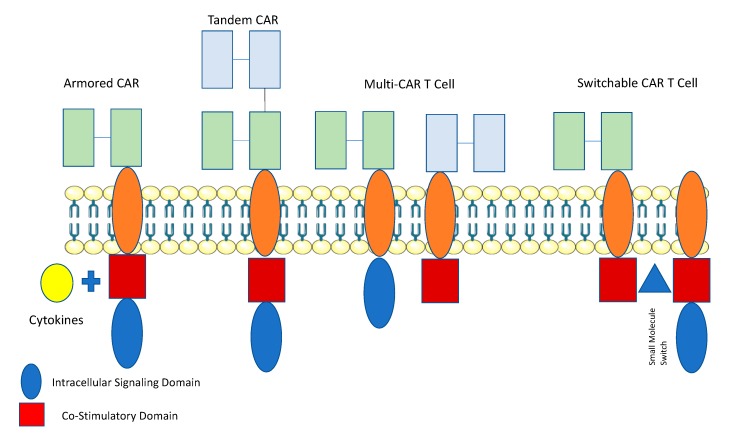
Attempts to overcome the challenge of a lack of ideal TAA in solid tumors, have led to the development of novel types of CAR T cells: specifically “AND”-Gate (Multi CAR and SynNotch) and “OR”-Gate (Tandem CAR T cells) (Figure 2).
Figure 2.
Novel Approaches to Improve CAR T Cell Anti-Tumor Efficacy. Armored CAR T cells have been designed to protect T cells from the immunosuppressive tumor microenvironment by expressing cytokines, as an independent gene within the CAR vector. Tandem CAR T cells have been designed to express two antigen-binding domains arranged in tandem with one intracellular signaling domain. Multi-CAR T Cells consist of one T-cell expressing two CAR structs with different antigen binding domains, with one CAR containing an intracellular and the other a co-stimulatory domain. The presence of both antigens is required to efficiently activate the T cell. Switchable CAR T cells expresses a CAR joined to a co-stimulatory signaling domain. The CD3-ζ intracellular signaling domain can heterodimerize with the CAR co-stimulatory domain only in the presence of a small molecule which acts as an ‘ON’ switch. Thus, both interaction with target antigen and the small molecule are required for activation of the CAR T-cell. This figure was created with images adapted from Servier Medical Art by Servier. Original images are licensed under a Creative Commons Attribution 3.0 Unported License.
Multi or dual CARs have been developed where T cells that are transduced with both a CAR that undergoes suboptimal activation upon binding to one antigen, and requires binding of a chimeric costimulatory receptor (CCR) that recognizes a second antigen in order to undergo activation [60,61]. Roybal et al., employed the use of a Synthetic Notch (SynNotch) system allowing for conditional expression of the CAR. Upon binding to antigen, the intracellular domain of the Notch transcription factor is cleaved, resulting in the activation of the CAR against the target antigen. [62,63,64]. “AND”-gate strategies allow for improved specificity and reduced “off-target” toxicity [65]. Tandem CARs (“OR”-gate) contain two scFv domains against different antigens that are linked together within the same CAR construct. Each of the scFv domains have different specificities against the target antigen, and the CAR can be activated when either of scFvs engages with a specific antigen, however when both are engaged there is functional synergy and enhanced activation [66]. “OR”-gate strategies help increase the number of targetable antigens on the surface of the tumor, potentially enhances potency. Hegde et al. created a tandem CAR by joining an anti-human epidermal growth factor receptor-2 (HER2) scFv and an IL-13 receptor α2 (IL-13Rα2)-binding IL-13 for the treatment of glioblastoma [65]. The tandem CAR was able to bind either HER2 or IL-13Rα2 and to lyse glioblastoma cells. Tandem CARs bound both HER2 and IL-13Rα2 simultaneously by inducing HER2-IL13Rα2 heterodimers, which promoted additive T cell activation when both antigens were encountered concurrently. Tandem CAR T cell activity was more sustained without evidence of T cell exhaustion, compared to that of unispecific CAR T cells. Lastly, tandem CAR T-cells were able to overcome antigen escape, demonstrated, enhanced antitumor efficacy and improved animal survival [65].
Kloss et al., developed a dual CAR T cell using two prostate tumor antigens—prostate- specific membrane antigen (PSMA) and prostate stem cell antigen (PSCA). In a murine prostate cancer model, the investigators demonstrated that PSCA+PSMA+ T Cells were able to eradicate tumors in mice who tumors expressed both PSCA and PSMA [60]. However, mouse tumors that were PSCA+PSMA- did not respond to therapy, demonstrating the potential for immunoediting, resistance, and relapse [60,67]. Roybal et al., demonstrated improved efficacy of the SynNotch CAR T cells in pre-clinical models for hematologic malignancies [64]. In addition to being limited by antigen specificity by only targeting one or two antigens, currently designed CAR T cells are also limited by scalability by only targeting select antigens. To expand antigen recognition as well as scalability, new CARs, Universal CARs have been developed [68,69,70,71]. The principal behind universal adaptor CAR T cells is that they are manufactured such that an intermediate system, i.e. soluble adaptor, splits the antigen targeting domain and the intracellular domain [72]. This allows for a soluble adaptor, to effectively “turn on” the T cells to expand and proliferate against a given TAA. By employing such a system, specificity is enhanced, because a universal CAR T cell allows for multiple TAAs to be targeted simultaneously by applying distinct soluble adaptors; potentially overcoming the difficulty with targeting solid tumors secondary to TAA heterogeneity. This also augments the safety of the product, as removing the soluble adaptor molecule “turns off” the T cells, potentially mitigating any side effects from therapy [72]. One example of the Universal CAR system, is the split, universal, and programmable (SUPRA) CAR system, developed by Cho et al. [68]. The investigators developed a two-component CAR construct consisting of “zipCAR” and “zipFv” fragments. The zipCAR contains intracellular signaling domains connected via a transmembrane segment to an extracellular leucine zipper. The zipFv contains a ligand-binding scFv domain fused to a second leucine zipper. The CAR is only functional when the zipFv fragments bind to the zipCARS by their matching leucine zippers. The investigators found that SUPRA Car T cells could eradicate both solid and hematological malignancies as efficiently as conventional CAR T cells. They also found that they were able to modulate IFN-γ expression and tumor killing by the addition of zipFv fragments, as well changing the binding affinity of the leucine zippers [68].
4.2. Inefficient Trafficking of T Cells to Tumor Sites
Furthermore, effective trafficking is also contingent on the proper match of adhesion receptors on T cells to those of the tumor endothelium. There must also be a match between chemokine receptors on the CAR principally CXCR3 and CCR5, and chemokines expressed by the tumors [73]. Tumor derived CCL2 has also been correlated with greater CCR2-expressing T cell trafficking in several different solid tumors [74]. Unfortunately, ideal matching still rarely occurs clinically, often impairing trafficking. Investigators have thus attempted to design CAR T cells with chemokine receptors that specifically match tumor chemokines. Craddock et al., designed GD2 CAR T cells that coexpressed the chemokine receptor CCR2b, which directs migrations against the chemokine CCL2, expressed by neuroblastoma [75]. The investigators found that CARS coexpressed with the chemokine receptor CCR2b had improved homing and anti-tumor activity to CCL2-secreting neuroblastoma, compared to CCR2-negative CARs [75]. Similarly, Moon et al., coexpressed mesothelin CAR T cells with CCR2b is a malignant pleural mesothelioma preclinical model demonstrating enhanced T-cell infiltration and anti-tumor activity [76]. Newick et al., designed CAR T cells that expressed a small peptide called the regulatory subunit I anchoring disruptor (RIAD) that prevents the association of PGE2 and adenosine activate protein kinase A (PKA), which is known to inhibit TCR activation. The investigators found CAR T cells coexpressed with RIAD had improved anti-tumor efficacy and T-cell migration within the TME in mesothelioma murine preclinical models [77].
Currently, standard CAR T cell therapy is delivered through intravenous infusion. Thus, T cells must migrate to the site(s) of where the solid tumor is present. One method to improve upon trafficking that is currently being explored is the local administration of CAR T cells to the tumor site. One advantage to regional/local delivery of CAR T cells is that fewer number of cells are required for adoptive transfer, which results in decreased systemic toxicity as compared to systemic administration [78,79]. Adusumillii et al., studied a preclinical model of mesothelin expressing pleural based malignancies, and performed a comparative analysis of systemic versus regional delivery of mesothelin-targeted T cells using the M28z CAR. The investigators found that that intrapleurally administered CAR T cells was superior to systemically infused T cells, requiring the administration of 30-fold fewer T cells to induce long-term complete remissions. Interestingly, they found that resulted in enhanced antitumor efficacy and functional T cell persistence for up 200 days. Lastly, regionally administered T cells were able to traffic to extrathoracic tumor sites and promote tumor elimination [78]. This has led to the development of a phase I trial of regionally delivered anti-mesothelin CAR T cells for patients with malignant pleural disease (NCT02414269). One particular attractive malignancy to the regional deployment of CAR T cells are central nervous system (CNS) tumors, specifically glioblastoma multiforme (GBM) and brain metastases. The CNS is an immune privileged site secondary to the blood brain barrier (BBB), thus regional delivery of T cells into the CNS would allow for circumventing the BBB and potentially enhance immunotherapy [80]. Preclinical models of both CAR T and CAR NK have been found to be efficacious against both primary brain tumors as well as metastatic CNS disease [81,82,83]. Brown et al., performed an early phase clinical trial of intracranially delivered IL13(E13Y)-zetakine CD8(+) CARs targeting IL13Rα2 in patients with recurrent GBM. The investigators found the infusions to be safe, with manageable brain inflammation, with responses seen in two out of three patients [84]. In a follow-up to this study the investigators modified the CAR construct to by incorporating 4-1BB costimulatory domain and a mutated IgG4-Fc linker to reduce off-target Fc-receptor interactions [85]. The investigators reported one case of a patient with recurrent GBM who received multiple infusions of the modified IL13Rα2 CAR T cell into the resected tumor bed, and was found to have regression of all intracranial and spinal tumors that lasted for 7.5 months [85]. CAR T cells were found to persist in the cerebrospinal fluid (CSF) for 7 days post-infusion, with a subsequent increase in cytokines included interferon-γ, tumor necrosis factor α, interleukins 2, 10, 5, 6, and 8; chemokines C-X-C motif chemokine ligand 9 (CXCL9), C-X-C motif chemokine ligand 10 (CXCL10), CCR2; and soluble receptor interleukin-1 receptor α within the CSF [85]. Current clinical trials investigating the administration of regionally delivered CAR T cells include intratumoral (NCT02587689), intracranial (NCT00730613), via hepatic artery (NCT01373047), and pleural (NCT02414269).
Recently, Smith et al., explored the use of an implantable biopolymer device to efficiently allow for the delivery of CAR T cell directly to solid tumors [79,85]. The investigators demonstrated that multiple preclinical solid tumor models that the use of a biopolymer effectively support CAR T cells within the resection beds and nearby lymph nodes, reducing tumor relapse compared to systemic administration [79,86]. In a preclinical murine pancreatic model, they were also able to demonstrate that implants designed to co-deliver STING agonists along with CAR T cells were able to limit tumor escape, enhance survival, and elicit both local and distant anti-tumor immunity [79].
As tumors enlarge, they outgrow the normal blood supply, and thus require neovascularization in order to maintain an adequate supply of nutrients for further growth. Vasculogenesis and angiogenesis are typically dependent on release of cytokines such as vascular endothelial growth factor-A (VEGF), basic fibroblast growth factor (bFGF), platelet-derived endothelial growth factor (PDGF), transforming growth factor (TGF)-α, fibroblast growth factor (FGF), and placental growth factor (PGF) [87]. However, compared to normal vasculature, these new tumor associated blood vessels are characterized by being tortuous, with irregular branch points, having increased permeability and overall irregular blood flow. This results in hypoxia to the tumor, but also impairs trafficking of immune cells, and CAR T cells, to the tumor beds [87]. Furthermore, tumor associated endothelial cells (ECs) themselves can also impair trafficking and promote an immunosuppressive microenvironment. They can downregulate adhesion molecules, such as intracellular adhesion molecule 1 (ICAM1) and vasculature cell adhesion molecule 1 (VCAM1), which are required for leukocyte extravasation into the TME [88]. ECs can also upregulate co-inhibitory receptors within the TME, impairing anti-tumor immunity. Lastly, they can also express TNF-related apoptosis-inducing ligand and FAS ligand which can result in apoptosis of immune cells [89,90].
Given the significant impact that the abnormal tumor vasculature has on impairing both trafficking and activation of T cells, CAR T cells the specifically target the vasculature have been developed. Santoro et al., generated anti-PSMA CAR T cells that recognized and eliminated PSMA+ ECs. In preclinical murine models of ovarian cancer anti-PSMA CAR T cells were destroyed PSMA+ vessels, resulting in a reduction of tumor burden [91]. Chinnasamy et al., developed a CAR T cell against both VEGFR2 and melanoma specific antigens: gp100, TRP-1 or TRP-2. They demonstrated in a preclinical murine melanoma model that combination of targeting both the tumor vasculature and melanoma was synergistic resulting in improved tumor growth and improved mouse survival [92].
4.3. Immunosuppressive Microenvironment
Even if adoptively delivered T cells are able to migrate to the tumor site, they unfortunately face a hostile and immunosuppressive TME. The tumor microenvironment (TME) is a complex system of cells, signaling molecules, soluble factors, a fibrotic extracellular matrix, stromal elements and other immunoregulatory cells that all play a crucial role in tumor pathogenesis, metastasis and treatment resistance [93]. All of these conditions within the TME make delivery of adoptive T cell therapy challenging, impeding the ability of CAR T cells from engaging with a target antigen [93,94]. Furthermore, the TME is characterized by oxidative stress, nutritional depletion, acidic pH, and hypoxia [95]. Nutrient starvation leads to low levels of glucose and essential amino acids, resulting in acidosis which can impair T cell proliferation. Specifically, tumor derived lactic acid has been found to suppress the proliferation and production of cytokines by cytotoxic T cells cytotoxic T lymphocytes by up to 95% with a subsequent 50% decrease in cytotoxicity [96]. One of the most essential amino acids for proper T cell function is tryptophan [97]. Indoleamine 2,3-dioxygenase (IDO) catabolized tryptophan to kynurenine, leading to GCN2 activation and mTOR inhibition, resulting in anergy of effector T cells and Treg accumulation [97]. Within the TME IDO is produced both by the tumor as well other immunoregulatory cells, which can result in failure of CAR T cells to control IDO-expressing tumors [98].
Furthermore, within the TME various immunoregulatory cells are present including: regulatory T cell (Tregs), Tumor Associated Macrophages (TAMS), and Tumor Associated Neutrophils (TANS) [94]. Frequently TAMS and TANS are polarized towards a pro-tumor phenotype, M2 and N2, and in combination with Tregs produce immunosuppressive cytokines/ligands including transforming growth factor β (TGF-β), IL-4, arginase, reactive oxygen species, and programmed death ligand-1 (PD-L1) [94]. All of these have the potential to decrease T cell mediated tumor immunity and enhancing tumor escape. TGF-β has specifically been found to inhibit cytotoxic T cell anti-tumor immunity, subsequently enhancing tumor proliferation [99]. In an attempt to try and overcome the immunosuppressive TME, investigators are now developing strategies to specifically target the TME as well as the primary tumor. Recently, Kloss et al., engineered a PSMA CAR T cell that co-expressed a dominant-negative TGF-βRII, which allows for the blocking of TGFβ signaling within the T cell. Using a murine prostate cancer model the investigators were able to demonstrate that PSMA CAR T cell with co-expression of TGF-βRII had increased proliferation of these lymphocytes, enhanced cytokine secretion, resistance to exhaustion, long-term in vivo persistence, and the induction of tumor eradication. This has subsequently led to the development of a phase I clinical trial of PSMA CAR T cell with co-expression of TGF-βRII in patients with R/R metastatic prostate cancer (NCT03089203) [100]. Selective depletion of Tregs in combination with systemic treatment with CAR T cells has also been demonstrating to enhance anti-tumor efficacy in both hematologic and solid tumor preclinical models. This is likely secondary to decreased production of immunosuppressive cytokines such as TGF-β, PD-1, and IL-10 by Tregs [101,102].
While cells of the TME produce and express cytokines/ligands that can augment T cell function, T cells have also been demonstrated to express co-inhibitory receptors such as PD-1, Lag3, and Tim-3 that reduce efficacy and are markers of T cell exhaustion [103]. PD-1 has been studied the most as a potential target that could enhance CAR T cell efficacy [104]. Strategies to manipulate PD-1 expression on CAR T cells include co-administration of PD-1/PD-L1 blocking antibody, genetic removal or PD-1 from CAR T cell product, or in vivo production of PD-1/PD-L1 blockade by CAR T cell product. Cherkassky et al., demonstrated in an orthotopic pleural mesothelioma mouse model that a CD28 compared to 4-1BB mesothelin CAR T cells had decreased persistence and cytotoxicity within the TME secondary to enhanced PD-1/PD-L1 signaling. PD-1/PD-L1 blockade restored CD28 CAR T cell effector function demonstrating mechanistically the role of the PD-1/PD-L1 axis in CAR T cell exhaustion [105]. Furthermore, Ren et al., employed the use of a CRISPR/CAS9 system to genetically delete PD-1 from PSCA-CAR T cells and demonstrated enhanced anti-tumor efficacy both in vitro and in vivo in a murine prostate cancer model [106,107].
Suarez et al., engineered CAR T cells that were able to secrete antibodies targeting PD-L1 [108]. The investigators employed the use of a lentiviral vector encoding both an anti-carbonic anhydrase (CAIX) CAR and secreting anti-PD-L1 scFv [108]. They found using the antibody producing CAR T cells were able to decrease tumor growth 5-fold greater compared to anti-CAIX CAR T cells alone, in a humanized mouse model of renal cell carcinoma. Furthermore, the anti-CAIX-PD-L1 CAR T cell lead to increased levels of granzyme B, with reduced expression of PD-L1 on the tumors themselves [108]. Similarly, Li et al., engineered CAR T cells that were able to secrete antibodies targeting PD-1 (CARαPD1-T) [109]. The investigators employed the use of a retroviral vector encoding both an anti-CD19 CAR and secreting anti-PD-1 scFv was designated as CAR19.αPD1, for the treatment of hematologic malignancies, and found that local delivery of antibody was more efficacious compared to systemic delivery [109].
This has led to the development of several clinical trials of PD-1 expressing antibody CAR T cells for the treatment solid tumors. [(NCT03030001), (NCT02873390), (NCT03179007), (NCT03182816), (NCT03182803), (NCT03615313)]. Preliminary clinical trial experience of CD19 CAR T cells in combination with PD-L1 blockade in patients with R/R NHL have been encouraging with 100% ORR, and 1 CR [110]. Interestingly, all patients had a least a 2-fold greater expansion of CAR T cells when compared to patients who received CD19 CAR T cells on comparative clinical trials [110]. However, while disruption of the PD-1/PD-L1 axis appears to improve CAR T cell efficacy and potentially contribute to the reversal of T cell exhaustion within the TME, it alone is not entirely responsible. Odorizzie et al., have demonstrated that in the setting of chronic lymphocytic choriomeningitis virus, PD-1 knockout (PD-1 KO) in CD8+ T cells leads to the accumulation of more cytotoxic, but terminally differentiated, CD8+ T cells, with decreased survival [111]. Thus, continued exploration of other targets will likely be required to fine tune the interactions that occur within the TME to optimize CAR T cell anti-tumor function.
Similarly, targeting the stroma cells within the TME also has the potential to help overcome its immunosuppressive effects. Fibroblast activation protein (FAP) is predominately expressed on tumor associated fibroblasts but is also on a number of solid tumors. Wang et al., demonstrated that using anti-FAP CAR T cell selectively reduced FAP+ stromal cells and inhibited the growth of multiple types in preclinical murine models. Interestingly, the administration of the CAR T cell lead to enhanced endogenous CD8+ T-cell antitumor responses. Similarly, Lo et al., demonstrated that the administrated of anti-FAP CAR T cells reduced extracellular matrix proteins and glycosaminoglycans; with a concurrent decrease in tumor vascular density and growth in preclinical desmoplastic human lung cancer xenografts and syngeneic murine pancreatic cancers [112]. Oncolytic viruses also have the potential to transform the TME reducing its immunosuppressive effects, potentially synergizing with CAR T cell therapy. Specifically, oncolytic viruses have been found to be antiangiogenic, decreasing levels VEGF within infected tumor cells [113]. They have also been found to be able to modify the TME to enhance trafficking of anti-tumor immune cells [114].
4.4. Lack of Persistence of CAR T Cell Products
Both the length of T cell persistence and rate of in vivo expansion of T cells have been found to be prognostic in patients being treated with CAR T cell products [30,115,116]. While, CAR T cells have been found to persist for many months to years in patients with hematologic malignancies, this has not been the case for patients with solid tumors, with most clinical trials demonstrating a minority of patients with detectable CAR T cells months after administration [25,27,28,29,117]. Poor persistence of T cells likely contributes to the poor clinical responses that have been reported thus far. One method to improve in vivo expansion and persistence has been the use of lymphodepleting chemotherapy prior to the administration of CAR T cells. Administration of lymphodepleting chemotherapy with cyclophosphamide and fludarabine, results in the reduction of host lymphocytes, specifically Tregs, which have been found to negatively impact response to adoptive T cell therapy [118]. Unfortunately, this has not provided the impressive clinical benefit in solid tumors as has been achieved for hematological malignancies [119].
Currently, most CAR T cell products are manufactured via leukapheresis of unselected T cells from the peripheral blood patients. However, by using unselected T cells there may be greater variability of anti-tumor activity of T cells between different patients, which may contribute to disparities in vivo persistence and response. Sommermeyer et al. produced CAR T cells distinct subsets of CD4+ and CD8+ T cells: Naïve (TN), Effector (TE), Central Memory (TCM) and Effector Memory (TEM). The investigators found that CD4+ and CD8+ CAR-T cells derived from TN and TCM were more effective than those from TEM, and combining the two subsets resulted in synergistic antitumor effects in vivo [120]. Similarly, Zheng et al., demonstrated CAR-T cells produced with a higher percentage of effector subsets versus naïve and stem memory subsets were associated with reduced in vivo persistence [121]. Mechanistically, decreased persistence was secondary to the activation of the PI3K pathways which resulted in persistent signaling of the CAR through the CAR CD3ζ immunoreceptor tyrosine-based activation motif. Inhibition of the PI3K pathway resulted in improved in vivo persistence and anti-tumor efficacy [121]. Similarly, co-administration of ibrutinib with CD-19 CAR T cells has been found to enhance CAR T cell function, expansion, engraftment, and clinical response in patients with CLL. Mechanistically, this is likely secondary to inhibition of interleukin-2-inducible T-cell kinase (ITK) on T cells, with a potential skewing towards a Th1 response [122].
Cytokines are well known to support the function and help promote expansion of T cells, including those that are adoptively transferred. When CAR T cells engage the hostile TME, the potential to add cytokines within the microenvironment that support T cell function, could potentially enhance anti-tumor efficacy. As systemic administration of cytokines can lead to increase side effects, that has been a focus of developing CAR T cells with mechanisms to allow for local delivery of cytokines to enhance T cell activity while mitigating potential toxicity. This has led to the development of fourth generation CAR T cell, also known as: T-cells redirected for universal cytokine-mediated killing (TRUCKs) [123]. Chmielewski et al., first showed that the addition CAR T cells engineered to release IL-12 upon engagement with tumor, resulted in recruitment of activated macrophages, enhanced inflammatory response, and destruction of tumor cells with antigen loss [14]. The same group has also shown that CAR T cells engineered with inducible IL-18 release exhibited enhanced activity against pancreatic and lung tumors refractory to CAR T cells without cytokines, with augmentation of the TME to become less immunosuppressive [124]. Koneru et al., demonstrated that the use of IL-12 secreting MUC-16(ecto) CAR T cells enhanced cytotoxicity, persistence, and modulated the tumor microenvironment in an ovarian cancer model [125]. This led to the development of a phase 1 clinical trial of administering intraperitoneally IL-12 secreting MUC-16(ecto) directed CAR T cells for patients with recurrent ovarian cancer [126]. Likewise, IL-7 and IL-15 engineered CAR T cells have also been developed, which have demonstrated improved persistence and anti-tumor function in solid tumor models [127,128]. Mohammed et al., cleverly designed CAR T cells to take advantage of immunosuppressive cytokines produced within the TME. The investigators generated a PSCA-directed CAR T cell with an inverted IL-4 receptor exodomain fused to an IL-7 receptor endodomain (4/7 ICR). Thus, binding by the immunosuppressive cytokine IL-4 paradoxically leads to an activating signaling cascade within the CAR T cell. The investigators demonstrated in a preclinical pancreatic model, known for high IL-4 expression within the TME, that ICR CAR T cells have enhanced anti-tumor activity, increased expansion, and persistence [129].
Currently, CAR T cells are generated using retroviruses or lentiviruses that are randomly integrated into the T cell. However, if specific sites of integration or disruption of the genome are found that enhance CAR T cell expansion and persistence, site directed insertion of viral vectors or transposons could be employed to improve their clinical utility. Presently, there has been significant interest and investigation site directed knock out of specific genes in T cells to enhance persistence. Eyquem et al., demonstrated that employing the use of a CRISPR/CAS9 system to direct a CD19-specific CAR to the T-cell receptor α constant (TRAC) locus results in uniform CAR expression in T cells, enhances T-cell potency, with improved anti-tumor activity compared to conventional CAR T cells [130]. Mechanistically, the investigators found that specific targeting to the TRAC locus prevents tonic CAR signaling, improving persistence, delaying effector T-cell differentiation and exhaustion [130]. Recently, Fraietta et al., reported on a case of a patient with CLL who received CD19 CAR T cell, and developed a CR. Upon analysis of the CAR T cell expansion, the investigators found that 94% of the CAR T cells originated from a single clone, in which a lentiviral vector insertion disrupted the TET2 gene, and coincidentally the patient was found also to have a hypomorphic mutation in the second TET2 allele. Further analysis of the TET2 disrupted CAR T cells revealed that they exhibited altered differentiation, skewed towards a TCM, with enhanced cytotoxic and cytolytic function, potentially explaining the clinical response in the patient [131]. Qasim et al., generated a universal CAR T cells by employing transcription activator-like effector nucleases (TALENS) mediated gene editing of TRAC and CD52 gene loci, demonstrating enhanced persistence and anti-tumor activity [132]. Other investigators have used site directed gene editing to modify PD-1 and CTLA-4 [106].
5. Conclusions
With the advent of checkpoint inhibitors, the fourth pillar for the treatment of cancer, immunotherapy, has become a paramount for the treatment of solid tumors. The first generation of clinical trials for CAR T cells in solid tumors has been completed and has demonstrated that these products can feasibly administered. However, while CAR T cells have made yielded dramatic improvements for patients with hematologic malignancies, much work is to be done in improving outcomes for patients with solid tumors. Compared to traditional therapies such as chemotherapy for patients with advances solid tumors that rarely yield durable responses with considerable side effects, CAR T cells offer the prospect of improved targeting, with the potential for durable outcomes. In the future focus will be on identifying ideal targets for select tumor types that will minimize “on-target off-tumor” effects. A robust focus on isolating tumor neo-antigens as well as continued development of Tandem CAR T cells against multiple tumor-associated antigens will be required. Lastly, identifying methods to improve the fitness of CAR T cells so that they can survive, expand, and persist within the TME is required. Novel approaches including the regional delivery of CAR T cells, along co-delivery of cytokines to improve expansion and persistence will continue to be explored in the next phase of clinical trials to assess if this improves efficacy. Co-administration strategies with tumor vaccines or oncolytic virus may be also required, and potentially could play a major role in the near future. Finally, further research in identifying exactly which genes enhance persistence and expansion of T cells is needed, such that targeted gene editing of CAR T cells can be employed for further optimization. During the months and years ahead, many of these exciting answers should be determined to help in further development of immunotherapy for the treatment of solid malignancies.
Author Contributions
B.H. conceived the idea for work and wrote the manuscript. Y.Y. provided and conceived the idea for work, helped write the manuscript, provided critical feedback, edited, and contributed to the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Batlevi C.L., Matsuki E., Brentjens R.J., Younes A. Novel immunotherapies in lymphoid malignancies. Nat. Rev. Clin. Oncol. 2016;13:25–40. doi: 10.1038/nrclinonc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and t-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor t-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 4.Hollyman D., Stefanski J., Przybylowski M., Bartido S., Borquez-Ojeda O., Taylor C., Yeh R., Capacio V., Olszewska M., Hosey J., et al. Manufacturing validation of biologically functional t cells targeted to cd19 antigen for autologous adoptive cell therapy. J. Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentjens R.J., Latouche J.B., Santos E., Marti F., Gong M.C., Lyddane C., King P.D., Larson S., Weiss M., Riviere I., et al. Eradication of systemic b-cell tumors by genetically targeted human t lymphocytes co-stimulated by cd80 and interleukin-15. Nat. Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 6.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral t cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 7.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savoldo B., Ramos C.A., Liu E., Mims M.P., Keating M.J., Carrum G., Kamble R.T., Bollard C.M., Gee A.P., Mei Z., et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawalekar O.U., O’Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey A.D., Jr., Patel P.R., Guedan S., Scholler J., Keith B., et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G., Plotkin J., Sadelain M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong X.S., Matsushita M., Plotkin J., Riviere I., Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeku O.O., Brentjens R.J. Armored car t-cells: Utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem. Soc. Trans. 2016;44:412–418. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chmielewski M., Kopecky C., Hombach A.A., Abken H. Il-12 release by engineered t cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 15.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey N. The what, when and how of CAR T cell therapy for all. Best Pract. Res. Clin. Haematol. 2017;30:275–281. doi: 10.1016/j.beha.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Heyman B., Yang Y. New developments in immunotherapy for lymphoma. Cancer Biol. Med. 2018;15:189–209. doi: 10.20892/j.issn.2095-3941.2018.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havard R., Stephens D.M. Anti-cd19 chimeric antigen receptor t cell therapies: Harnessing the power of the immune system to fight diffuse large b cell lymphoma. Curr. Hematol. Malig. Rep. 2018;13:534–542. doi: 10.1007/s11899-018-0482-6. [DOI] [PubMed] [Google Scholar]
- 19.Maude S.L. Tisagenlecleucel in pediatric patients with acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 2018;16:664–666. [PubMed] [Google Scholar]
- 20.Till B.G., Jensen M.C., Wang J., Qian X., Gopal A.K., Maloney D.G., Lindgren C.G., Lin Y., Pagel J.M., Budde L.E., et al. Cd20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: Pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W.Y., Wang Y., Guo Y.L., Dai H.R., Yang Q.M., Zhang Y.J., Zhang Y., Chen M.X., Wang C.M., Feng K.C., et al. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-hodgkin lymphoma: An early phase IIa trial report. Signal. Transduct. Target. Ther. 2016;1:16002. doi: 10.1038/sigtrans.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martyniszyn A., Krahl A.C., Andre M.C., Hombach A.A., Abken H. Cd20-cd19 bispecific CAR T cells for the treatment of B-cell malignancies. Hum. Gene Ther. 2017;28:1147–1157. doi: 10.1089/hum.2017.126. [DOI] [PubMed] [Google Scholar]
- 23.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B., et al. CD22-targeted car t cells induce remission in B-all that is naive or resistant to CD19-targeted car immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kershaw M.H., Westwood J.A., Parker L.L., Wang G., Eshhar Z., Mavroukakis S.A., White D.E., Wunderlich J.R., Canevari S., Rogers-Freezer L., et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Chen M., Wu Z., Tong C., Dai H., Guo Y., Liu Y., Huang J., Lv H., Luo C., et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology. 2018;7:e1440169. doi: 10.1080/2162402X.2018.1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz S.C., Burga R.A., McCormack E., Wang L.J., Mooring W., Point G.R., Khare P.D., Thorn M., Ma Q., Stainken B.F., et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin. Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng K., Guo Y., Dai H., Wang Y., Li X., Jia H., Han W. Chimeric antigen receptor-modified t cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 2016;59:468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y., Feng K., Liu Y., Wu Z., Dai H., Yang Q., Wang Y., Jia H., Han W. Phase I study of chimeric antigen receptor-modified t cells in patients with EGFR-positive advanced biliary tract cancers. Clin. Cancer Res. 2018;24:1277–1286. doi: 10.1158/1078-0432.CCR-17-0432. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A., et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive t cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai B., Shi D., Gao H., Qi X., Jiang H., Zhang Y., Chi J., Ruan H., Wang H., Ru Q.C., et al. A phase I study of anti-GPC3 chimeric antigen receptor modified t cells (GPC3 CAR-T) in chinese patients with refractory or relapsed GPC3+ hepatocellular carcinoma. J. Clin. Oncol. 2017;35:3049. doi: 10.1200/JCO.2017.35.15_suppl.3049. [DOI] [Google Scholar]
- 32.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A., et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed N.M., Brawley V.S., Diouf O., Ghazi A., Yi J., Liu H., Rooney C.M., Dotti G., Gee A.P., Grossman R., et al. Autologous HER2 cmv bispecific CAR T cells for progressive glioblastoma: Results from a phase I clinical trial. J. Clin. Oncol. 2015;33:3008. doi: 10.1016/S1525-0016(16)33623-1. [DOI] [Google Scholar]
- 34.Feng K., Liu Y., Guo Y., Qiu J., Wu Z., Dai H., Yang Q., Wang Y., Han W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018;9:838–847. doi: 10.1007/s13238-017-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosaka T., Yatabe Y., Endoh H., Yoshida K., Hida T., Tsuboi M., Tada H., Kuwano H., Mitsudomi T. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin. Cancer Res. 2006;12:5764–5769. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 36.Beatty G.L., Haas A.R., Maus M.V., Torigian D.A., Soulen M.C., Plesa G., Chew A., Zhao Y., Levine B.L., Albelda S.M., et al. Mesothelin-specific chimeric antigen receptor mrna-engineered t cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanyi J., Haas A.R., Beatty G.L., Stashwick C.J., O’Hara M.H., Morgan M.A., Porter D.L., Melenhors J.J., Plesa G., Lacey S.F., et al. Anti-mesothelin chimeric antigen receptor T cells in patients with epithelial ovarian cancer. J. Clin. Oncol. 2016;34:5511. doi: 10.1200/JCO.2016.34.15_suppl.5511. [DOI] [Google Scholar]
- 38.Beatty G.L., O’Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M., et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You F., Jiang L., Zhang B., Lu Q., Zhou Q., Liao X., Wu H., Du K., Zhu Y., Meng H., et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci. China Life Sci. 2016;59:386–397. doi: 10.1007/s11427-016-5024-7. [DOI] [PubMed] [Google Scholar]
- 40.Junghans R.P., Ma Q., Rathore R., Gomes E.M., Bais A.J., Lo A.S., Abedi M., Davies R.A., Cabral H.J., Al-Homsi A.S., et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: Possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257–1270. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- 41.Specht J., Lee S., Turtle C.J., Baladrishnan A., Srivastava S., Voillet V., Voillet V., Veatch J., Gooley T., Mullane E., et al. A phase I study of adoptive immunotherapy for advanced ror1+ malignancies with defined subsets of autologous t cells expressing a ror1-specific chimeric antigen receptor (ror1-car) Cancer Res. 2018;78 doi: 10.1158/1538-7445.AM2018-CT131. [DOI] [Google Scholar]
- 42.Lamers C.H., Klaver Y., Gratama J.W., Sleijfer S., Debets R. Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells-a completed study overview. Biochem. Soc. Trans. 2016;44:951–959. doi: 10.1042/BST20160037. [DOI] [PubMed] [Google Scholar]
- 43.Tomaic V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers (Basel) 2016;8:95. doi: 10.3390/cancers8100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson L.A., Scholler J., Ohkuri T., Kosaka A., Patel P.R., McGettigan S.E., Nace A.K., Dentchev T., Thekkat P., Loew A., et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 2015;7:275ra222. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frederick L., Wang X.Y., Eley G., James C.D. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 46.Morgan R.A., Johnson L.A., Davis J.L., Zheng Z., Woolard K.D., Reap E.A., Feldman S.A., Chinnasamy N., Kuan C.T., Song H., et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan R., Ho M. Mesothelin targeted cancer immunotherapy. Eur J. Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morello A., Sadelain M., Adusumilli P.S. Mesothelin-targeted cars: Driving T cells to solid tumors. Cancer Discov. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamers C.H., Sleijfer S., van Steenbergen S., van Elzakker P., van Krimpen B., Groot C., Vulto A., den Bakker M., Oosterwijk E., Debets R., et al. Treatment of metastatic renal cell carcinoma with CAIX car-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol. Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klebanoff C.A., Rosenberg S.A., Restifo N.P. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat. Med. 2016;22:26–36. doi: 10.1038/nm.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., Fujimoto J., Zhang J., Wedge D.C., Song X., Zhang J., Seth S., Chow C.W., Cao Y., Gumbs C., et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell P.J., Yachida S., Mudie L.J., Stephens P.J., Pleasance E.D., Stebbings L.A., Morsberger L.A., Latimer C., McLaren S., Lin M.L., et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerlinger M., Rowan A.J., Horswell S., Math M., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katayama R., Shaw A.T., Khan T.M., Mino-Kenudson M., Solomon B.J., Halmos B., Jessop N.A., Wain J.C., Yeo A.T., Benes C., et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl. Med. 2012;4:120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Misale S., Yaeger R., Hobor S., Scala E., Janakiraman M., Liska D., Valtorta E., Schiavo R., Buscarino M., Siravegna G., et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landau D.A., Carter S.L., Stojanov P., McKenna A., Stevenson K., Lawrence M.S., Sougnez C., Stewart C., Sivachenko A., Wang L., et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samstein R.M., Lee C.H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y., Barron D.A., Zehir A., Jordan E.J., Omuro A., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019 doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanitis E., Poussin M., Klattenhoff A.W., Song D., Sandaltzopoulos R., June C.H., Powell D.J., Jr. Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol. Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morsut L., Roybal K.T., Xiong X., Gordley R.M., Coyle S.M., Thomson M., Lim W.A. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164:780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roybal K.T., Williams J.Z., Morsut L., Rupp L.J., Kolinko I., Choe J.H., Walker W.J., McNally K.A., Lim W.A. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell. 2016;167:419–432.e16. doi: 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roybal K.T., Rupp L.J., Morsut L., Walker W.J., McNally K.A., Park J.S., Lim W.A. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K., et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grada Z., Hegde M., Byrd T., Shaffer D.R., Ghazi A., Brawley V.S., Corder A., Schonfeld K., Koch J., Dotti G., et al. Tancar: A novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol. Ther. Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Themeli M., Sadelain M. Combinatorial antigen targeting: Ideal T-cell sensing and anti-tumor response. Trends Mol. Med. 2016;22:271–273. doi: 10.1016/j.molmed.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho J.H., Collins J.J., Wong W.W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1426–1438.e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urbanska K., Powell D.J. Development of a novel universal immune receptor for antigen targeting: To infinity and beyond. Oncoimmunology. 2012;1:777–779. doi: 10.4161/onci.19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lohmueller J.J., Ham J.D., Kvorjak M., Finn O.J. Msa2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology. 2017;7:e1368604. doi: 10.1080/2162402X.2017.1368604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodgers D.T., Mazagova M., Hampton E.N., Cao Y., Ramadoss N.S., Hardy I.R., Schulman A., Du J., Wang F., Singer O., et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl. Acad. Sci. USA. 2016;113:E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao J., Lin Q., Song Y., Liu D. Universal cars, universal T cells, and universal CAR T cells. J. Hematol. Oncol. 2018;11:132. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harlin H., Meng Y., Peterson A.C., Zha Y., Tretiakova M., Slingluff C., McKee M., Gajewski T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown C.E., Vishwanath R.P., Aguilar B., Starr R., Najbauer J., Aboody K.S., Jensen M.C. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred t cells. J. Immunol. 2007;179:3332–3341. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 75.Craddock J.A., Lu A., Bear A., Pule M., Brenner M.K., Rooney C.M., Foster A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2B. J. Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moon E.K., Carpenito C., Sun J., Wang L.C., Kapoor V., Predina J., Powell D.J., Jr., Riley J.L., June C.H., Albelda S.M. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human t cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newick K., O’Brien S., Sun J., Kapoor V., Maceyko S., Lo A., Pure E., Moon E., Albelda S.M. Augmentation of CAR T-cell trafficking and antitumor efficacy by blocking protein kinase a localization. Cancer Immunol. Res. 2016;4:541–551. doi: 10.1158/2326-6066.CIR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adusumilli P.S., Cherkassky L., Villena-Vargas J., Colovos C., Servais E., Plotkin J., Jones D.R., Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting cd4-dependent tumor immunity. Sci. Transl. Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith T.T., Moffett H.F., Stephan S.B., Opel C.F., Dumigan A.G., Jiang X., Pillarisetty V.G., Pillai S.P.S., Wittrup K.D., Stephan M.T. Biopolymers codelivering engineered T cells and sting agonists can eliminate heterogeneous tumors. J. Clin. Invest. 2017;127:2176–2191. doi: 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Louveau A., Harris T.H., Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi B.D., Suryadevara C.M., Gedeon P.C., Herndon J.E., 2nd, Sanchez-Perez L., Bigner D.D., Sampson J.H. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J. Clin. Neurosci. 2014;21:189–190. doi: 10.1016/j.jocn.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han J., Chu J., Keung Chan W., Zhang J., Wang Y., Cohen J.B., Victor A., Meisen W.H., Kim S.H., Grandi P., et al. Car-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Priceman S.J., Tilakawardane D., Jeang B., Aguilar B., Murad J.P., Park A.K., Chang W.C., Ostberg J.R., Neman J., Jandial R., et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin. Cancer Res. 2018;24:95–105. doi: 10.1158/1078-0432.CCR-17-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.C., Naranjo A., Starr R., Wagner J., Wright C., et al. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stephan S.B., Taber A.M., Jileaeva I., Pegues E.P., Sentman C.L., Stephan M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dvorak H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 88.Lanitis E., Irving M., Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 2015;33:55–63. doi: 10.1016/j.coi.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Motz G.T., Santoro S.P., Wang L.P., Garrabrant T., Lastra R.R., Hagemann I.S., Lal P., Feldman M.D., Benencia F., Coukos G. Tumor endothelium fasl establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Secchiero P., Zauli G. The puzzling role of trail in endothelial cell biology. Arterioscler. Thromb. Vasc. Biol. 2008;28:e4–e6. doi: 10.1161/ATVBAHA.107.158451. [DOI] [PubMed] [Google Scholar]
- 91.Santoro S.P., Kim S., Motz G.T., Alatzoglou D., Li C., Irving M., Powell D.J., Jr., Coukos G. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol. Res. 2015;3:68–84. doi: 10.1158/2326-6066.CIR-14-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chinnasamy D., Tran E., Yu Z., Morgan R.A., Restifo N.P., Rosenberg S.A. Simultaneous targeting of tumor antigens and the tumor vasculature using t lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. 2013;73:3371–3380. doi: 10.1158/0008-5472.CAN-12-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swartz M.A., Iida N., Roberts E.W., Sangaletti S., Wong M.H., Yull F.E., Coussens L.M., DeClerck Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Newick K., Moon E., Albelda S.M. Chimeric antigen receptor T-cell therapy for solid tumors. Mol. Ther. Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J., Li W., Huang K., Zhang Y., Kupfer G., Zhao Q. Chimeric antigen receptor t cell (car-t) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018;11:22. doi: 10.1186/s13045-018-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human t cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 97.Brochez L., Chevolet I., Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer. 2017;76:167–182. doi: 10.1016/j.ejca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Ninomiya S., Narala N., Huye L., Yagyu S., Savoldo B., Dotti G., Heslop H.E., Brenner M.K., Rooney C.M., Ramos C.A. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang C.Y., Wang H., Liao W., Han F., Li Y.Q., Chen S.W., Lao X.M. Transforming growth factor beta is a poor prognostic factor and inhibits the favorable prognostic value of CD8+ CTL in human hepatocellular carcinoma. J. Immunother. 2017;40:175–186. doi: 10.1097/CJI.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 100.Kloss C.C., Lee J., Zhang A., Chen F., Melenhorst J.J., Lacey S.F., Maus M.V., Fraietta J.A., Zhao Y., June C.H. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018;26:1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y., Wang L., Predina J., Han R., Beier U.H., Wang L.C., Kapoor V., Bhatti T.R., Akimova T., Singhal S., et al. Inhibition of p300 impairs Foxp3(+) T regulatory cell function and promotes antitumor immunity. Nat. Med. 2013;19:1173–1177. doi: 10.1038/nm.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Q., Munger M.E., Highfill S.L., Tolar J., Weigel B.J., Riddle M., Sharpe A.H., Vallera D.A., Azuma M., Levine B.L., et al. Program death-1 signaling and regulatory t cells collaborate to resist the function of adoptively transferred cytotoxic t lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moon E.K., Wang L.C., Dolfi D.V., Wilson C.B., Ranganathan R., Sun J., Kapoor V., Scholler J., Pure E., Milone M.C., et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human t cells in solid tumors. Clin. Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chong E.A., Melenhorst J.J., Lacey S.F., Ambrose D.E., Gonzalez V., Levine B.L., June C.H., Schuster S.J. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: Refueling the car. Blood. 2017;129:1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cherkassky L., Morello A., Villena-Vargas J., Feng Y., Dimitrov D.S., Jones D.R., Sadelain M., Adusumilli P.S. Human car T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren J., Liu X., Fang C., Jiang S., June C.H., Zhao Y. Multiplex genome editing to generate universal car T cells resistant to pd1 inhibition. Clin. Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren J., Zhang X., Liu X., Fang C., Jiang S., June C.H., Zhao Y. A versatile system for rapid multiplex genome-edited car T cell generation. Oncotarget. 2017;8:17002–17011. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suarez E.R., Chang de K., Sun J., Sui J., Freeman G.J., Signoretti S., Zhu Q., Marasco W.A. Chimeric antigen receptor T cells secreting anti-PD-l1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S., Siriwon N., Zhang X., Yang S., Jin T., He F., Kim Y.J., Mac J., Lu Z., Wang S., et al. Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin. Cancer Res. 2017;23:6982–6992. doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 110.Locke F.L., Westin J.R., Miklos D.B., Herrara A.F., Jacobson C.A., Lee J., Rossi J.M., Bot A., Xue A., Navale L., et al. Phase 1 results from ZUMA-6: Axicabtagene ciloleucel (axicel; KTE-C19) in combination with atezolizumab for the treatment of patients with refractory diffuse large b cell lymphoma (dlbcl) Blood. 2017;130:2826. [Google Scholar]
- 111.Odorizzi P.M., Pauken K.E., Paley M.A., Sharpe A., Wherry E.J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 2015;212:1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lo A., Wang L.S., Scholler J., Monslow J., Avery D., Newick K., O’Brien S., Evans R.A., Bajor D.J., Clendenin C., et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hou W., Chen H., Rojas J., Sampath P., Thorne S.H. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int. J. Cancer. 2014;135:1238–1246. doi: 10.1002/ijc.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sampath P., Li J., Hou W., Chen H., Bartlett D.L., Thorne S.H. Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Mol. Ther. 2013;21:620–628. doi: 10.1038/mt.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in b cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu Y., Tan Y., Ou R., Zhong Q., Zheng L., Du Y., Zhang Q., Huang J. Anti-CD19 chimeric antigen receptor-modified T cells for B-cell malignancies: A systematic review of efficacy and safety in clinical trials. Eur. J. Haematol. 2016;96:389–396. doi: 10.1111/ejh.12602. [DOI] [PubMed] [Google Scholar]
- 117.Gargett T., Yu W., Dotti G., Yvon E.S., Christo S.N., Hayball J.D., Lewis I.D., Brenner M.K., Brown M.P. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol. Ther. 2016;24:1135–1149. doi: 10.1038/mt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao X., Ahmadzadeh M., Lu Y.C., Liewehr D.J., Dudley M.E., Liu F., Schrump D.S., Steinberg S.M., Rosenberg S.A., Robbins P.F. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–5696. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X., et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sommermeyer D., Hudecek M., Kosasih P.L., Gogishvili T., Maloney D.G., Turtle C.J., Riddell S.R. Chimeric antigen receptor-modified t cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng W., O’Hear C.E., Alli R., Basham J.H., Abdelsamed H.A., Palmer L.E., Jones L.L., Youngblood B., Geiger T.L. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32:1157–1167. doi: 10.1038/s41375-017-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fraietta J.A., Beckwith K.A., Patel P.R., Ruella M., Zheng Z., Barrett D.M., Lacey S.F., Melenhorst J.J., McGettigan S.E., Cook D.R., et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chmielewski M., Abken H. Trucks: The fourth generation of cars. Expert Opin. Biol. Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 124.Chmielewski M., Abken H. CAR T cells releasing IL-18 convert to T-Bet(high) FoxO1(low) effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 2017;21:3205–3219. doi: 10.1016/j.celrep.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 125.Koneru M., Purdon T.J., Spriggs D., Koneru S., Brentjens R.J. IL-12 secreting tumor-targeted chimeric antigen receptor t cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koneru M., O’Cearbhaill R., Pendharkar S., Spriggs D.R., Brentjens R.J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shum T., Omer B., Tashiro H., Kruse R.L., Wagner D.L., Parikh K., Yi Z., Sauer T., Liu D., Parihar R., et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hurton L.V., Singh H., Najjar A.M., Switzer K.C., Mi T., Maiti S., Olivares S., Rabinovich B., Huls H., Forget M.A., et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific t cells. Proc. Natl. Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohammed S., Sukumaran S., Bajgain P., Watanabe N., Heslop H.E., Rooney C.M., Brenner M.K., Fisher W.E., Leen A.M., Vera J.F. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol. Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gonen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fraietta J.A., Nobles C.L., Sammons M.A., Lundh S., Carty S.A., Reich T.J., Cogdill A.P., Morrissette J.J.D., DeNizio J.E., Reddy S., et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K., et al. Molecular remission of infant B-all after infusion of universal talen gene-edited car t cells. Sci. Transl. Med. 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]