Abstract
Background
Abnormal involuntary postures are characteristic of dystonia, but the specific postures observed clinically have not previously been categorized or enumerated. The objective of this study was to determine whether there is a set of specific postures that are common between different children with dystonia.
Methods
Videotapes were examined from all children who were seen in a pediatric movement disorders clinic over a 4‐year period and had a diagnosis of nonpsychogenic dystonia. In total, 179 children were included in the video review.
Results
Visually similar postures were identified in 152 different children. Seven different common postures were identified. All 152 children exhibited at least 1 of these postures, and most had more than 1.
Conclusions
Involuntary postures in childhood dystonia exhibit unexpected similarities despite a wide range of underlying etiology, severity, and developmental experience. This is consistent with the hypothesis that childhood dystonia is a symptom that reflects a shared pathway of expression for multiple anatomic and functional abnormalities.
Keywords: dystonia, pediatric, posture, video review
In children, dystonia is defined as “a movement disorder in which involuntary sustained or intermittent muscle contractions cause twisting and repetitive movements, abnormal postures, or both.”1 Similar definitions exist for adults,2 and all definitions share the observation of abnormal postures as a common element of many forms of dystonia.3, 4, 5 A recent consensus definition notes the “patterned and stereotypical nature of movements within an individual,”6 but it is not known whether there are stereotypical dystonic postures that occur between different individuals. Clinical observation suggests that certain postures occur frequently, and this is reflected in the fact that injection of botulinum toxin for the treatment of dystonia is performed much more commonly in some muscles (e.g., flexor carpi ulnaris, posterior tibialis, sternocleidomastoid) than in others.
There are many causes of dystonia in childhood, including genetic disorders of brain structural development or function, metabolic disorders that affect development or function, and acquired disorders that injure a previously normally developing brain.4, 7 Causes of dystonia with known destructive lesions have been referred to as “secondary dystonia,” although a different classification using the term “acquired” is now preferred according to a recent consensus statement.6 The distinction between “inherited” and “acquired” dystonia remains problematic in children, because many identified inherited disorders that result in dystonia exert their influence through a predisposition to acquired injury with either degenerative or static pathology (examples include mitochondrial disorders, type 1 glutaric aciduria, Fahr's disease, folate transporter deficiency, polymorphism in brain‐derived neurotrophic factor, and many others). The most frequent identified cause of dystonia in childhood in developed countries is perinatal hypoxic‐ischemic injury.1 When an anatomic location of injury is identified, dystonia is most commonly associated with injury to the basal ganglia and thalamus, including the medial globus pallidus. Recently, there has been evidence to suggest that injury to the cerebellum may also be a potential cause of dystonia.8, 9, 10
The mechanism by which injury causes dystonic symptoms is not known. In some forms of adult dystonia, there may be a generalized disinhibition of motor cortical areas,11, 12 leading to the theory that hyperactivation or spread of activation in the cortex might be responsible for excessive muscle activity. Another theory suggests that plasticity in the cortex might cause abnormal expansion or blurring of the representation of nearby muscles, leading to involuntary overflow of activity to muscles that interfere with desired movement.13, 14 Another theory suggests that the normal function of the basal ganglia is to selectively reinforce desired motor programs while inhibiting undesired motor programs, and failure of this focusing mechanism results in the superimposition of desired and undesired muscle activity.15 None of these theories predict that different children share similar dystonic postures. In particular, a theory of generalized disinhibition might predict that overflow of muscle activity would originate in the voluntarily activated muscles and thus would vary, depending on the intended movement. A theory of abnormal experience‐dependent plasticity might predict that postures would reflect the experience of different children and thus would vary, depending on the child's learned movements. A theory of focused action selection and “center‐surround” inhibition might predict that attempted voluntary movement would be corrupted by similar “competing” movements and postures and thus would vary with the intended movement. Other neuromorphic computational models of dystonia have emulated only the activity of a few muscles and thus do not make predictions about specific postures.15, 16, 17, 18
To investigate the set of postures exhibited by children with dystonia, we examined videotapes from all children and young adults with nonpsychogenic dystonia who were seen during a 4‐year period in a pediatric movement disorders clinic. The most common identified cause responsible in approximately one‐half of the children was acute perinatal hypoxic‐ischemic injury. This mechanism is known to have a predilection for varying distribution of injury at specific brain regions, including some or all of the lateral thalamus, medial pallidum, subiculum, substantia nigra pars reticulata, and perirolandic cortex.19
Patients and Methods
Methods were approved by the University of Southern California Institutional Review Board. Video recordings were retrospectively reviewed by a single observer (D.F.) from all children and young adults with dystonia who were seen in the movement disorders clinic between January 2009 and December 2012. Patients without available videos, with a diagnosis of psychogenic dystonia, or who were asymptomatic at the time of their visit were excluded. Consent for video recording and authorization according to the Health Insurance Portability and Accountability Act of 1996 for the use of protected health information were obtained from all adult patients or from adult caregivers of pediatric patients before recording.
Recordings from 179 children and young adults (ages 5 months to 18 years) met criteria and were reviewed to determine whether specific recurring patterns could be identified in more than 2 children. These patterns were categorized and labeled. Subsequently, all recordings were re‐reviewed, and single frames showing categorized patterns were extracted from each recording in which they were observed. Similarity of postures was visually determined. Postures that could not be clearly identified or categorized or that occurred in fewer than 3 children were not included in the categorization.
Results
In total, 7 postures were identified and labeled as follows:
“Swan neck” finger posture (present in 98 children) (Video S1): flexion of the metacarpophalangeal joint with extension of the proximal interphalangeal joint in at least 1 finger, often associated with flexion of the distal interphalangeal joint;
“One‐two‐five” finger posture (present in 57 children) (Video S2): simultaneous extension of the metacarpophalangeal joints of the thumb, index, and little fingers, with flexion of the metacarpophalangeal joints of the middle and ring fingers;
Internal rotation and extension of the shoulder (present in 51 children) (Video S3): internal rotation and extension at the shoulder, extension at the elbow, and flexion at the wrist;
Fisting (present in 58 children) (Video S4): flexion of the metacarpophalangeal joints and (proximal) interphalangeal joints of all digits, including the thumb;
“Striatal toe” (present in 24 children) (Video S5): extension of the metatarsophalangeal joint of the great toe without extension of the other toes;
Foot inversion (present in 53 children) (Video S6): Medial (varus) rotation at the subtalar joint; and
Opisthotonos (present in 7 children) (Video S7): cervical and thoracic spine extension, often associated with lumbar spine extension and anterior rotation of the pelvis.
Examples of each posture are provided in Figure 1. Of the 179 included cases, 152 children exhibited at least 1 of the 7 identified common postures: 50 children exhibited exactly 1, 49 exhibited exactly 2, 23 exhibited exactly 3, 20 exhibited exactly 4, 9 exhibited exactly 5, and 1 child had exactly 6 of the identified common postures. No child exhibited all 7, and 27 children did not exhibit any of the identified common postures.
Figure 1.
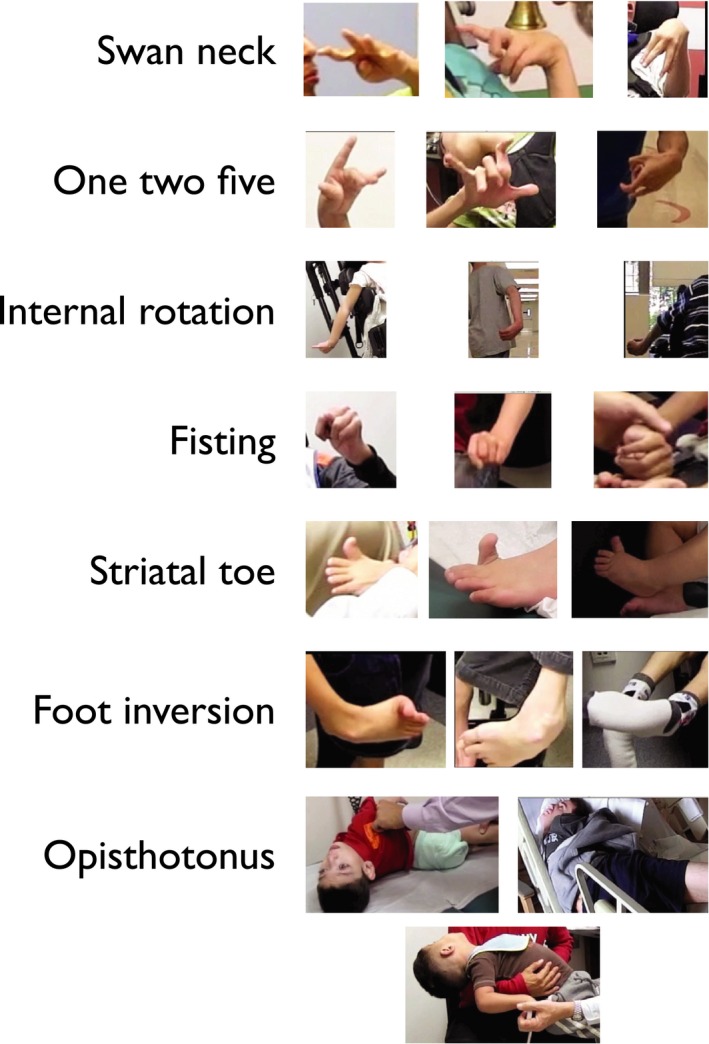
These are selected examples of the identified common postures.
Of the 152 children and young adults who exhibited the common postures, causes of dystonia included perinatal hypoxic‐ischemic injury (N = 72), glutaric aciduria type 1 (N = 10), central nervous system inflammatory disorders (N = 8), kernicterus (N = 8), perinatal intraventricular hemorrhage (N = 5), traumatic brain injury (N = 5), arteriovenous malformation with hemorrhage (N = 3), Torsin 1 gene (DYT‐TOR1A)‐positive dystonia (N = 3), dopamine transporter deficiency (N = 2), stroke (N = 2), acqueductal stenosis (N = 1), arteriovenous malformation without hemorrhage (N = 1) ataxia‐telangiectasia (N = 1), cerebral folate deficiency (N = 1), genetic deletion of unknown significance (N = 1), glucose transporter deficiency type 1 (N = 1), hypoglycemic event (N = 1), methylmalonic aciduria (N = 1), methyl‐tetrahydrofolate reductase deficiency (N = 1), neurofibromatosis type 1 (N = 1), N‐methyl D‐aspartate receptor mutation GRIN2B (glutamate receptor, ionotropic, N‐methyl‐D‐aspartate subunit 2B) (N = 1), Rett syndrome (N = 1), tumor (N = 1), and unknown etiologies (N = 21).
Of the 27 children without identified common postures, diagnoses included perinatal hypoxic‐ischemic injury (N = 7), DYT‐TOR1A‐positive dystonia (N = 2), myoclonus dystonia (N = 2), central nervous system inflammatory disorders (N = 2), arteriovenous malformation (N = 1), ataxia telangiectasia (N = 1), glutaric aciduria type 1 (N = 1), traumatic brain injury (N = 1), vitamin E deficiency (N = 1), and unknown causes (N = 9).
Determination of diagnosis and etiology was made based on clinical records, imaging studies, and neurologic examination of each child (T.D.S.). The video segments show the complete set of images of common postures from all included children.
Conclusion
Dystonic postures in children with dystonia are not random; rather, they exhibit significant commonalities between different children with inherited, acquired, and idiopathic etiologies of dystonia. Dystonic postures do not reflect the full range of movements achievable by each child. This finding has not been predicted by current theories of the mechanism of dystonia.
Examination of dystonic postures reveals that joints are frequently not at joint limits. This suggests a very different mechanism from spasticity seen in corticospinal injury. In moderate‐to‐severe spasticity, joints usually are maintained in full extension or full flexion.1 However, dystonic postures appear to be more nuanced, in the sense that the balance of agonist and antagonist muscle activity required to maintain the posture is maintained against gravity or any attempted movement by the examiner. In a previous study, we demonstrated that hypertonia in dystonia is often maintained by a careful balance of length‐dependent tonic reflexes, such that the posture can be preserved not by nonspecific co‐contraction of antagonist muscles, but by precise adjustment of stretch reflexes, similar to the mechanism used for maintenance of voluntary posture.20 We suggested that dystonia thus co‐opts the normal postural mechanism. In the context of our current results, this suggests the hypothesis that a few specific postures are involuntarily activated and superimposed on attempted voluntary activity. Because dystonic postures usually are triggered by attempts at voluntary movement, this could give the appearance of “overflow” of activity from the voluntary movement to the muscles involved in the superimposed involuntary posture.
What mechanism could account for similarity of postures between different children? A relevant observation in nonhuman primates is that stimulation of the motor or premotor cortex with long pulse trains (range, 500–1000 msec) leads to fixed postures.21 Similar to what is observed in dystonia, these stimulated postures in nonhuman primates modulate muscle stretch response to maintain joints at mid‐ranges of angle,22 suggesting that exogenously stimulated activity in the cortex has the ability to create and maintain postures against gravity or perturbations. Despite initial claims that this could be a general mechanism for posture or movement, only a few different postures have been experimentally achieved, perhaps because stimulation activates a contiguous local region of the cortex without the ability to modulate detailed patterns of neural firing. This suggests the hypothesis that similarity of postures in dystonia might be due to involuntary activation of a contiguous region of premotor or motor cortex, superimposed on the normal pattern of activity. It further suggests the conjecture that there could be a much wider anatomic basis for the origin of dystonia than is currently believed. Any brain region that has the ability to stimulate premotor or motor cortex (including cerebellum, thalamus, prefrontal cortex, supplementary motor area, or parietal cortex) could potentially cause involuntary postures, yet the postures might remain similar despite the varying origins of the abnormal activity.
This study is necessarily limited by the lack of an objective method for identifying and distinguishing postures. Unfortunately, current automated video and motion analysis remains insensitive relative to the eye of a trained clinician, and no objective substitute yet exists. Many children exhibited dystonic postures that were not identified as 1 of our common postures, so we cannot exclude an influence of experience or other factors. Also, it is important to realize that we examined only postures that could be identified on single, still frames extracted from video recordings. Therefore, we do not know whether the hyperkinetic movements that are also seen in dystonia might share common patterns. It is also possible that referral of children to this clinic does not reflect the statistical distribution of children seen in other movement disorders clinics.
Thus there is much additional research to be done, and categorization of additional postures and movements in different forms of dystonia will be essential. Our results hopefully will be helpful in the diagnosis of dystonia, and they may contribute to an understanding of the mechanisms of dystonia. When combined with data from nonhuman primate physiology, our results suggest the conjecture that different children could have very different anatomic origins of dystonia despite similar phenotypes. This may have important consequences for the selection of surgical targets for deep‐brain stimulation surgery and for the identification of medical therapy that can more selectively target causative brain regions in different children.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
T.D.S.: 1A, 3A, 3B
D.F.: 1B, 1C, 2A, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: This project was supported by the Children's Hospital of Los Angeles Department of Neurology. The authors report no conflicts of interest.
Financial Disclosures for the previous 12 months: Terence D. Sanger reports grants from the National Institutes of Health, the National Science Foundation, the Carter Foundations, the Lee/Ramo Chair in Health Technology and Engineering; medicolegal compensation from Tetreault & Crist LLP, Kitch Drutchas LLP, and Higgs, Fletcher & Mack LLP; consulting fees from Cala Health Inc.; and holds a patent (US8311623B2: Systems and methods for estimating surface electromyography). Diana Ferman reports no sources of funding and no conflicts of interest.
Supporting information
A video accompanying this article is available in the supporting information here.
Video S1. This is a concatenated, complete set of still images from all children with “swan neck” posture (present in 96 children).
Video S2. The “one‐two‐five” finger posture is shown (present in 56 children).
Video S3. Internal rotation and extension of the shoulder are shown (present in 50 children).
Video S4. Fisting is shown (present in 60 children).
Video S5. “Striatal toe” is shown (present in 22 children).
Video S6. Foot inversion is shown (present in 55 children).
Video S7. Opisthotonos is shown (present in 5 children).
Acknowledgements
We are grateful to Aprille Tongol for assisting with video recordings in the clinic. This work was supported by the Children's Hospital of Los Angeles Division of Neurology.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Sanger TD, Delgado MR, Gaebler‐Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics 2003;111:e89–e97. [DOI] [PubMed] [Google Scholar]
- 2. Burke RE, Fahn S, Jankovic J, Marsden CD, Lang AE, Gollomp S, Ilson J. Tardive dystonia and inappropriate use of neuroleptic drugs [letter]. Lancet 1982;1:1299. [DOI] [PubMed] [Google Scholar]
- 3. Sanger TD, Mink JW. Movement disorders In: Swaiman KF, Ashwal S, Ferriero DM, eds. Pediatric Neurology: Principles and Practice, 4th ed Philadelphia, PA: Mosby; 2006:1271–1311. [Google Scholar]
- 4. Sanger TD. Pediatric movement disorders. Curr Opin Neurol 2003;16:529–535. [DOI] [PubMed] [Google Scholar]
- 5. Sanger TD. Toward a definition of childhood dystonia. Curr Opin Pediatr 2004;16:623–627. [DOI] [PubMed] [Google Scholar]
- 6. Albanese A, Bhatia K, Bressman S, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanger TD. Pathophysiology of pediatric movement disorders. J Child Neurol 2003;18(Suppl 1):S9–S24. [DOI] [PubMed] [Google Scholar]
- 8. Jinnah HA. Hess EJ A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology 2006;67:1740–1741. [DOI] [PubMed] [Google Scholar]
- 9. Neychev VK, Fan X, Mitev V, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 2008;131:2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci 2002;22:7825–7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hallett M. The neurophysiology of dystonia. Arch Neurol 1998;55:601–603. [DOI] [PubMed] [Google Scholar]
- 12. Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain 1998;121(Pt 7):1195–1212. [DOI] [PubMed] [Google Scholar]
- 13. Byl N, Merzenich MM, Cheung S, Bedenbaugh P, Nagarajan SS, Jenkins WM. A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys Ther 1997;77:169–184. [DOI] [PubMed] [Google Scholar]
- 14. Sanger TD, Merzenich MM. Computational model of the role of sensory disorganization in focal task‐specific dystonia. J Neurophysiol 2000;84:2458–2464. [DOI] [PubMed] [Google Scholar]
- 15. Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 1996;50:381–425. [DOI] [PubMed] [Google Scholar]
- 16. Sanger TD. Childhood onset generalised dystonia can be modelled by increased gain in the indirect basal ganglia pathway. J Neurol Neurosurg Psychiatry 2003;74:1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohn WJ, Niu C, Sanger TD. A neuromorphic model of motor overflow in focal hand dystonia due to correlated sensory input. J Neural Eng 2016;13:1741–1751. [DOI] [PubMed] [Google Scholar]
- 18. Sohn WJ, Niu C, Sanger TD. Increased long‐latency reflex activity as a sufficient explanation for childhood hypertonic dystonia: a neuromorphic emulation study [serial online]. J Neural Eng 2015;12:03610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Volpe JJ. Neurology of the Newborn, 4th ed Philadelphia, PA: WB Saunders; 2000. [Google Scholar]
- 20. van Doornik J, Kukke S, Sanger TD. Hypertonia in childhood secondary dystonia due to cerebral palsy is associated with reflex muscle activation. Mov Disord 2009;24:965–971. [DOI] [PubMed] [Google Scholar]
- 21. Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 2002;34:841–851. [DOI] [PubMed] [Google Scholar]
- 22. Graziano MSA, Patel KT, Taylor CSR. Mapping from motor cortex to biceps and triceps altered by elbow angle. J Neurophysiol 2004;25:395–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video S1. This is a concatenated, complete set of still images from all children with “swan neck” posture (present in 96 children).
Video S2. The “one‐two‐five” finger posture is shown (present in 56 children).
Video S3. Internal rotation and extension of the shoulder are shown (present in 50 children).
Video S4. Fisting is shown (present in 60 children).
Video S5. “Striatal toe” is shown (present in 22 children).
Video S6. Foot inversion is shown (present in 55 children).
Video S7. Opisthotonos is shown (present in 5 children).


