Abstract
In commercial practice, broiler chickens may be exposed to Fusarium mycotoxins either during specific growth stages or throughout the entire production cycle. A 34-day feeding trial was conducted to identify sensitive periods for mycotoxin effects during the growth cycle of broiler chickens. A total of 420 newly-hatched Ross 308 male broilers were randomly assigned to 60 cages with 7 birds/cage. Sources of clean wheat (<0.5 mg/kg deoxynivalenol [DON]) and Fusarium-contaminated wheat (11.4 mg/kg DON) were used to formulate the starter diets (0.41 and 6.62 mg/kg DON) provided from 1 to 21 d of age and the grower diets (0.54 and 7.90 mg/kg DON) provided from 22 to 34 d. Control and DON diets were provided to broilers according to treatments (control, DON 1 to 14 d, DON 15 to 21 d, DON 22 to 34 d and DON 1 to 34 d). Birds were monitored daily for morbidity or mortality. Broiler growth performance (body weight, average daily gain, average daily feed intake and feed to gain ratio) was measured weekly. Segments of duodenum, jejunum and ileum were collected at 21 and 34 d and morphometric parameters (villus height, crypt depth, villus width, muscularis thickness and villi:crypt ratio) were measured. Birds fed the DON starter diet during the first 14 d did not exhibit any changes in growth performance; however, growth performance was suppressed in birds fed DON-contaminated diets during the grower period (22 to 34 d). At 34 d, birds that received the DON grower diet (DON 22 to 34 d and DON 1 to 34 d) were lighter (1,433 vs. 1,695 g) than birds fed the control diet. Feed to gain ratio was higher in birds fed the DON grower diet from 22 to 28 d (1.77 vs. 1.56) and 28 to 34 d (2.24 vs. 1.85) compared with corresponding controls. These results suggest that providing older broiler chicks (22 to 34 d) feed contaminated with Fusarium mycotoxins (specifically DON) may result in production losses. Histopathological analysis of the ileum region revealed that birds provided the DON diets throughout the entire trial (1 to 34 d) had shorter villi (506 vs. 680 μm) and shallower crypt (85 vs. 115 μm) than control birds. Taken together, these results indicate that DON-induced growth suppression may be a result of adverse effects on intestinal morphology during later growth phases of broilers.
Keywords: Deoxynivalenol, Chicken, Sensitive period, Feed contamination, Intestine
1. Introduction
Fusarium fungi infect a wide range of cereal grains and corn worldwide (Yli-Mattila, 2010, Tittlemier et al., 2013a, Tittlemier et al., 2013b, Terzi et al., 2014). Fusarium-damaged grains that are down-graded cannot be used for human consumption and are often used in animal feed production. Fusarium fungi can produce several types of mycotoxins, such as trichothecenes, zearalenone and fumonisin (Bryden, 2012, D'Mello et al., 1999, Döll and Dänicke, 2011, Escrivá et al., 2015). A recent global survey of mycotoxins in feed (1,384 samples) indicated that the trichothecene deoxynivalenol (DON) was the most prevalent mycotoxin, with 79% of the samples testing positive at an average concentration of 0.6 mg/kg and a maximum concentration of 8.4 mg/kg (Kosicki et al., 2016). Deoxynivalenol is consistently the most predominant mycotoxin in Fusarium-infected grains in Canada (Tittlemier et al., 2013a, Tittlemier et al., 2013b) and often co-contaminates with other trichothecenes, such as 3-acetyl DON (3ADON) and/or 15-acetyl DON (15ADON) (Girgis and Smith, 2010, Maresca, 2013, Miller and Richardson, 2013). Fusarium mycotoxins induce a wide range of adverse effects in food-production animals with symptoms ranging from vomiting and feed refusal to estrogenic effects and reduced performance, depending on the toxin and sensitivity of the animal species (Osweiler, 2014). In general, feed contamination with mycotoxins such as DON leads to economic losses in animal production (Wu, 2007).
For broiler chickens, dietary trichothecene exposure is known to increase mortality, alter immune function, increase disease susceptibility and reduce growth performance (Andretta et al., 2011, Andretta et al., 2012, Osselaere et al., 2013, Escrivá et al., 2015). However, reported effects of DON on growth performance are often inconsistent with some studies reporting no impacts of feeding high levels (9 to 15 mg/kg) of DON (Eriksen and Pettersson, 2004, Awad et al., 2012, Grenier and Applegate, 2013) while others found significant effects of DON-contaminated feed on feed intake and weight gain, even at levels as low as 2 mg/kg (Awad et al., 2011, Yunus et al., 2012). The adverse effects of DON observed in poultry research trials depend on several factors including length of exposure, timing of exposure as well as whether the poultry feed is spiked with DON or if DON is incorporated using naturally-contaminated grain (Awad et al., 2012, Ghareeb et al., 2015). In commercial feed production, different sources of grains are contaminated with various levels and types of mycotoxins, resulting in uneven mycotoxin distribution within a batch of grain and subsequent variability in the feed produced (Yli-Mattila, 2010, Tittlemier et al., 2013a, Miller et al., 2014). This means that broilers can be subjected to dietary mycotoxins during specific stages of growth or throughout the entire production cycle. In addition, duration and level of exposure can influence the severity of effects observed with dietary mycotoxin exposure. For example, chronic DON exposure is most associated with decreased performance and nutritional efficiency in swine, while intake of 1 to 2 mg/kg vs. 12 mg/kg DON can mean a difference between decreased feed intake and total feed refusal (Pierron et al., 2016). Identifying the most sensitive period for performance effects of mycotoxins is necessary in order to better understand the mycotoxin challenges faced in commercial production.
The gastrointestinal tract is the main site of nutrient digestion and absorption and also serves as a vital immune organ to combat feed-borne pathogens. A healthy, fully functioning intestine is important for developing broilers to reach their genetic potential. It is well known that DON can alter the intestinal morphology of poultry, impairing nutrient uptake, which can adversely affect energy and nutrient availability and, consequently, reduce growth performance (Awad et al., 2012, Awad et al., 2014, Grenier and Applegate, 2013, Maresca, 2013, Pinton and Oswald, 2014, Ghareeb et al., 2015). This influence of DON on gut morphology and function in broilers can be observed at relatively low levels, even before an impairment of growth performance is observed (Awad et al., 2011, Ghareeb et al., 2015). Therefore, morphological changes in intestinal structures can be used as a sensitive biological endpoint to evaluate dietary DON toxicosis.
The aim of the current study was to identify the sensitive period for adverse effects of mycotoxins during the growth cycle of broiler chickens. Diets were formulated with grain naturally-contaminated with Fusarium mycotoxins (primarily DON) and provided to broilers during different stages of the growth cycle. We then evaluated the effects of timing and duration of exposure on growth performance and intestinal morphology throughout the growth cycle.
2. Materials and methods
Permission was granted for all experimental work by the University of Saskatchewan Animal Care Committee (protocol # 20130043), with all procedures following the recommendations of the Canadian Council on Animal Care (1993).
2.1. Diets
Diets were formulated to meet or exceed the nutrient requirements for the growing birds (Aviagen, 2014a). Starter diets were provided to birds from 1 to 21 d of age and grower diets were provided from 22 to 34 d. Wheat contaminated with a relatively low level of mycotoxin (<0.5 mg/kg DON) was used for the control diets. Fusarium-damaged wheat was used to prepare mycotoxin-contaminated diets. Samples from each wheat source were analyzed for 16 common mycotoxins (Prairie Diagnostics Services Inc., Saskatoon, SK, Canada) via ultra-high-performance liquid chromatography (Agilent 1100, Agilent Technologies, Santa Clara, CA, USA) and mass spectrometry (Micromass Quattro Ultima Platinum Mass Spectrometer, Waters, MA, USA). The mycotoxin suite included DON and metabolites (3- and 15-acetyl DON), α-zearalenol, diacetoxyscirpenol, T-2, HT-2, nivalenol, ochratoxin A, β-zearalenol, zearalenone, and aflatoxin B1. Deoxynivalenol was the major mycotoxin identified in the Fusarium-damaged wheat (11.4 mg/kg). Diets were formulated to contain DON at 7.5 mg/kg, and the control diets were formulated to contain DON <1.0 mg/kg. After the diets were manufactured, 2 kg samples from each diet were collected. Mycotoxin levels were measured by Prairie Diagnostics Services Inc. as described above (Saskatoon, SK, Canada) and nutrient composition (dry matter, crude fiber, ether extract, calcium, and phosphorous) were analyzed at Central Testing Laboratory Ltd. (Winnipeg, MB, Canada). The apparent metabolic energy (AME) was calculated as AME = 53 + 38× {(Crude protein) + (2.25 × Fat) + [100 – (Crude protein) + (Crude fiber) + (Fat) + (Ash) + (Moisture)] × 0.9} (Central Testing Laboratory Ltd, MB, Canada). The diet ingredients, analyzed and calculated nutrients, and analyzed mycotoxin levels are shown in Table 1. The control starter and grower diets contained 0.41 and 0.54 mg/kg DON, respectively. The starter and the grower diets formulated with Fusarium-damaged grain contained 6.62 and 7.90 mg/kg DON, respectively, and are henceforth referred to as DON-contaminated diets. All diets were prepared in mash form and were visually similar upon inspection.
Table 1.
Feed formulation, nutrient composition and analyzed mycotoxins for the control and deoxynivalenol (DON)-contaminated starter (1 to 21 d) and grower (22 to 34 d) diets, as-fed basis.
| Item | Starter diet |
Grower diet |
||
|---|---|---|---|---|
| Control | DON-contaminated | Control | DON-contaminated | |
| Ingredient, % | ||||
| Control wheat 1 | 70.3 | 0 | 75.7 | 0 |
| Contaminated wheat 2 | 0 | 70.3 | 0 | 75.7 |
| Soybean meal | 20.0 | 20.0 | 14.0 | 14.0 |
| Fish meal | 1.0 | 1.0 | 1.0 | 1.0 |
| Corn-gluten meal | 2.0 | 2.0 | 3.0 | 3.0 |
| Canola oil | 2.0 | 2.0 | 2.0 | 2.0 |
| Dicalcium phosphate | 0.9 | 0.9 | 0.5 | 0.5 |
| Limestone | 1.2 | 1.2 | 1.2 | 1.2 |
| Salt (as NaCl) | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin/mineral premix 3 | 0.5 | 0.5 | 0.5 | 0.5 |
| Choline chloride | 0.1 | 0.1 | 0.1 | 0.1 |
| DL-methionine | 0.5 | 0.5 | 0.5 | 0.5 |
| L-lysine | 0.4 | 0.4 | 0.4 | 0.4 |
| Celite | 0.8 | 0.8 | 0.8 | 0.8 |
| Enzyme 4 | 0.05 | 0.05 | 0.05 | 0.05 |
| Total | 100 | 100 | 100 | 100 |
| Analyzed composition 5, % | ||||
| Crude Protein | 23.8 | 21.5 | 21.1 | 19.9 |
| AME, kcal/kg | 3,096 | 3,251 | 3,091 | 3,085 |
| Calcium | 0.68 | 0.70 | 0.80 | 0.84 |
| Phosphorus | 0.63 | 0.59 | 0.63 | 0.59 |
| Crude fiber | 3.63 | 3.48 | 2.60 | 2.60 |
| Fat | 3.88 | 6.50 | 3.57 | 3.57 |
| Ash | 5.66 | 5.14 | 5.54 | 5.43 |
| Calculated amino acid, % | ||||
| Lysine | 1.38 | 1.31 | 1.22 | 1.18 |
| Methionine | 0.56 | 0.51 | 0.54 | 0.52 |
| Methionine + Cysteine | 1.33 | 1.23 | 1.30 | 1.25 |
| Analyzed mycotoxin6, mg/kg | ||||
| DON | 0.41 | 6.62 | 0.54 | 7.90 |
| 3-acetyl DON | <0.025 | 0.41 | <0.025 | 0.58 |
| 15-acetyl DON | <0.025 | 0.20 | <0.025 | 0.030 |
| HT-2 toxin | 0.055 | 0.40 | 0.059 | 0.12 |
Mycotoxin concentrations in control wheat: aflatoxin B1, not detected (ND); 3-acetyl DON, ND; 15-acetyl DON, ND; α-zearalenol, ND; DON, 352 μg/kg; diacetoxyscirpenil, ND; HT-2 toxin, ND; nivalenol, ND; ochartoxin A, ND; T-2 toxin, ND; β-zearalenol, ND; zearalenone, ND.
Mycotoxin concentrations in contaminated wheat: aflatoxin B1, ND; 3-acetyl DON, 764 μg/kg; 15-acetyl DON, ND; α-zearalenol, ND; DON, 11,470 μg/kg; diacetoxyscirpenil, ND; HT-2 toxin, 107 μg/kg; nivalenol, 59.2 μg/kg; ochartoxin A, ND; T-2 toxin, ND; β-zearalenol, ND; zearalenone, ND.
One kg premix contains 2,200,000 IU vitamin A, 440,000 IU vitamin D, 6,000 IU vitamin E, 400 mg menadione, 300 mg thiamine, 1,200 mg riboflavin, 800 mg pyridoxine, 4 mg vitamin B12, 12,000 mg niacin, 2,000 mg pantothenic acid, 120 mg folic acid, 30 mg biotin. 2,000 mg copper, 16,000 mg manganese, 160 mg iodine, 16,000 mg zinc, 60 mg selenium, 100,000 mg calcium carbonate, 125 mg antioxidant, 807,879 mg wheat midds (DSM Nutritional Products Canada Inc. ON, Canada).
Enzyme: 1,000 units/g beta-glucanase, 1,500 units/g xylanase (GNC Bioferm Inc., Saskatoon, SK, Canada).
Nutrient compositions were analyzed at Central Testing Laboratory Ltd., (Winnipeg, MB, Canada). AME = 53 + 38 × [(Crude protein) + (2.25 × Fat) + (100 – Crude protein + Crude fiber + Fat + Ash + Moisture) × 0.9].
Mycotoxins were analyzed at Prairie Diagnostics Services Inc., (Saskatoon, SK, Canada) via ultra-high performance liquid chromatography (Agilent 1100, Agilent Technologies, Santa Clara, CA, USA) and mass spectrometry (Micromass Quattro Ultima Platinum Mass Spectrometer, Waters, Milford, MA, USA).
2.2. Experimental procedures
A total of 420 newly hatched, non-vaccinated, male broiler chicks (Ross 308) were obtained from a local commercial hatchery. Birds were randomly assigned to 60 battery cages (29 cm high × 48 cm wide × 83 cm long; providing 800 cm2/bird floor space) with 7 birds/cage in a temperature-controlled room at the University of Saskatchewan Poultry Research Center, Saskatoon, SK, Canada.
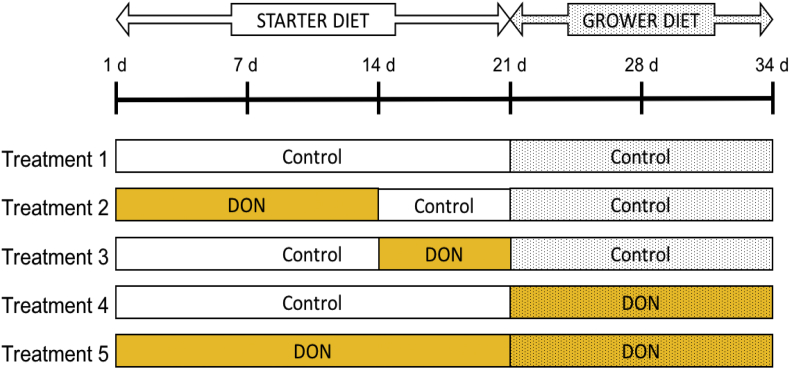
There were 5 treatments in this experiment and each treatment was replicated 12 times. For each treatment, the control and the DON-contaminated diets were provided during different periods of growth cycle of broilers from 1 to 34 d (Fig. 1). Treatment 1 was given the control diet throughout the feeding trial (1 to 34 d). Treatments 2, 3, and 4 were given the DON-contaminated diet during 1 to 14 d, 15 to 21 d and 22 to 34 d, respectively. Treatment 5 was given the DON-contaminated diets throughout the entire trial. Birds had free access to drinking water and feed during the whole trial. All mortality and culled birds were necropsied for cause of death or morbidity by Prairie Diagnostics Services Inc. (Saskatoon, SK, Canada).
Fig. 1.
Schematic representation of the timing of different diets (control and deoxynivalenol [DON]-contaminated) fed to broiler chicken during a 34-day feeding trial. The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON) and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). The DON diets were prepared with wheat that was naturally-contaminated with Fusarium mycotoxins.
2.3. Growth performance
Broiler body weight (BW, g) of each bird was recorded at 14, 21 and 34 d. Feed consumption was measured at 14, 21 and 34 d or as mortality occurred. Average daily gain (ADG, g/d), average daily feed intake (DFI, g/d) and feed to gain ratio (F:G, g feed/g weight gain) of each cage were then calculated. Average daily gain, DFI, F:G were calculated as follows: ADG (period a–b) = (BW day b – BW day a)/Days period a–b, DFI = (Feed weight day a – Feed weight day b)/Days period a–b and F:G = DFI/ADG.
2.4. Organ weight and length
One bird from each cage was randomly selected for tissue sampling at 21 and 34 d. Birds were euthanized by cervical dislocation. The liver and spleen were weighed. Weights of the individual segments of the gastrointestinal tract were recorded after removing the surrounding fat. The intestine was divided into the duodenum, jejunum and ileum for measuring the length of each segment. The duodenum was defined as the segment encompassing the duodenal loop (Denbow, 2015). The ileum was defined as the distal segment of the small intestine tract, which starts at Meckel's diverticulum and ends at ileum cecal junction, and the jejunum was defined as the segment in between of duodenum and ileum (Denbow, 2015). Organ weights were expressed in g/kg BW (relative weight). The weight to length ratio of each intestinal segment was also calculated as an indicator of intestine density (g/cm).
2.5. Intestinal histology
Intestinal morphology was assessed by histology at 21 and 34 d. On 21 d, 2-cm segments of duodenum, jejunum and ileum were collected from 6 birds randomly selected from both the control group and from the group fed the DON diet from 1 to 21 d. On 34 d, 6 birds from each of the 5 treatments were randomly selected and intestinal segments collected. Tissues were fixed in 10% neutral buffered formalin and then transferred to ethanol until processing. Tissues were paraffin embedded, sectioned at 5-μm thickness and stained with hematoxylin and eosin by Prairie Diagnostic Services Inc. (Saskatoon, SK, Canada). Two cross sections of each tissue were prepared for evaluation. Slides were examined under an Axiostar plus light microscope (Carl Zeiss Microscopy, LLC, NY, USA) and pictures of intestinal epithelial structures were acquired with × 5 objective. Measurements were done on 10 to 16 individual villi and associated crypt per bird. All 3 intestinal regions were evaluated for changes in villus height, crypt depth, villus width, muscularis thickness and villi:crypt ratio.
2.6. Statistical analysis
Statistical analyses were performed using the PROC MIXED procedure (Littell, 2006) of SAS version 9.4 (SAS Institute Inc., Cary, NC). The experimental unit for performance was the cage, and for other analyses, the bird was used as the experimental unit. Growth performance data were analyzed as repeated measures with time as a factor, and the appropriate covariance structure was selected by comparing the Akaike's Information Criteria. Where interactions with time were significant (P = 0.05), data were sliced by time and analyzed separately. Intestinal histological data were analyzed by using ANOVA with 6 replications (Littell, 2006). Significant treatment effects (P = 0.05) were analyzed using the Tukey–Kramer test to differentiate the means.
3. Results
3.1. Growth performance
The overall mortality in the current study was 7%. The mortality in each treatment at the end of the feeding trial (35 d) was 8%, 10%, 7%, 2% and 6% for control, DON 1 to 14 d, DON 15 to 21 d, DON 22 to 34 d, and DON 1 to 34 d, respectively. Mortality occurred early (within the first week) and was equal across treatments (the control diet fed birds vs. the DON diet fed birds). Mortality was most often attributed to poor chick quality (i.e. yolk sec infection and polyserositis) and was likely not attributable to the presence of mycotoxins in the diet.
The initial BW of chicks did not differ among treatments at the start of the trial (P > 0.05). Body weight was not affected by treatment during first 2 weeks (P > 0.05, Table 2). At 21 d, birds in treatment 5 (DON 1 to 34 d) were lighter than control birds (P < 0.01, Table 2). At 34 d, the birds from treatment 4 (DON 22 to 34 d) and treatment 5 (DON 1 to 34 d) were lighter when compared with birds fed control diets during the same time period (P < 0.01, Table 2). During the first 2 weeks of the trial, ADG was not affected by presence of dietary mycotoxins (P > 0.05, Table 3); however, when assessed at 21 d, ADG was decreased (P < 0.01) for birds consuming DON-contaminated diets (treatment 5) compared with same-age birds fed control diets (Table 3). In addition, ADG (from 22 to 34 d) was decreased (P < 0.01) in birds consuming DON-contaminated diets both throughout the entire growth period and during only the grower phase when compared with birds fed control diets (Table 3). From 22 to 34 d, the birds fed DON diets since hatch (DON 1 to 34 d) or during the grower stage (DON 22 to 34 d) consumed less feed than the birds fed DON diet only during the starter (1 to 21 d) stages (P < 0.01, Table 4). Feed to gain ratios (F:G) were not affected by the presence of dietary DON during the starter (1 to 21 d) stages (P > 0.05, Table 5). During 22 to 34 d, DON-fed birds had higher F:G than the birds fed control diets (P < 0.01, Table 5).
Table 2.
Effects of feeding deoxynivalenol (DON)-contaminated diets1 during different growth stages on body weight of male broilers during a 34-day growth trial.
| Treatment | Body weight, g |
||
|---|---|---|---|
| 14 d | 21 d | 34 d | |
| Control | 359.5 ± 10.0 | 724.6 ± 14.2a | 1,695.0 ± 22.5a |
| DON 1 to 14 d | 330.9 ± 14.1 | 673.4 ± 17.8ab | 1,720.0 ± 26.4a |
| DON 15 to 21 d | 368.9 ± 9.8 | 707.0 ± 17.1ab | 1,717.0 ± 28.5a |
| DON 22 to 34 d | 356.7 ± 8.6 | 706.6 ± 10.4ab | 1,517.5 ± 21.0b |
| DON 1 to 34 d | 332.4 ± 7.3 | 650.8 ± 13.9b | 1,433.7 ± 24.9b |
| ANOVA | P = 0.43 | P < 0.01 | P < 0.01 |
a, b Within a column, means ± SE with different superscripts are significantly different (P ≤ 0.05, one-way ANOVA followed by Tukey–Kramer test; n = 12/treatment).
The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON), and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). The DON-diets were prepared with wheat naturally contaminated with Fusarium mycotoxins.
Table 3.
Effects of feeding deoxynivalenol (DON)-contaminated diets during different growth stages on average daily gain (ADG) of male broilers during a 34-day growth trial.1
| Treatment | ADG, g/d |
|||
|---|---|---|---|---|
| 1 to 14 d | 15 to 21 d | 22 to 34 d | 1 to 34 d | |
| Control | 22.5 ± 0.7 | 52.2 ± 1.2a | 82.8 ± 2.2a | 49.9 ± 2.1a |
| DON 1 to 14 d | 20.4 ± 1.0 | 48.9 ± 1.4ab | 86.6 ± 1.9a | 48.8 ± 1.8a |
| DON 15 to 21 d | 23.1 ± 1.1 | 48.3 ± 2.0ab | 82.6 ± 2.0a | 48.6 ± 1.9a |
| DON 22 to 34 d | 22.2 ± 0.8 | 50.0 ± 1.3ab | 66.3 ± 2.0b | 42.9 ± 1.5b |
| DON 1 to 34 d | 20.6 ± 0.6 | 45.5 ± 1.1b | 66.4 ± 1.5b | 41.3 ± 1.6b |
| ANOVA | P = 0.50 | P = 0.04 | P < 0.01 | P < 0.01 |
a, b Within a column, means ± SE with different superscripts are significantly different (P ≤ 0.05, one-way ANOVA followed by Tukey–Kramer test; n = 12/treatment).
The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON) and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). The DON-diets were prepared with wheat naturally contaminated with Fusarium mycotoxins.
Table 4.
Effects of feeding deoxynivalenol (DON)-contaminated diets during different growth stages on daily feed intake (DFI) of male broilers during a 34-day growth trial.1
| Treatment | DFI, g/d |
|||
|---|---|---|---|---|
| 1 to 14 d | 15 to 21 d | 22 to 34 d | 1 to 34 d | |
| Control | 30.7 ± 0.7 | 74.5 ± 1.8a | 147.2 ± 1.7a | 84.2 ± 2.8a |
| DON 1 to 14 d | 30.6 ± 1.0 | 66.5 ± 1.8b | 144.1 ± 2.1a | 81.2 ± 2.2ab |
| DON 15 to 21 d | 30.5 ± 1.1 | 73.5 ± 2.3a | 144.5 ± 2.1a | 82.8 ± 2.2a |
| DON 22 to 34 d | 31.1 ± 0.8 | 74.2 ± 1.4a | 137.6 ± 2.7b | 80.7 ± 2.1ab |
| DON 1 to 34 d | 28.9 ± 0.6 | 67.3 ± 2.2b | 135.5 ± 2.3b | 77.6 ± 2.4b |
| ANOVA | P = 0.91 | P < 0.01 | P < 0.01 | P < 0.01 |
a, b Within a column, means ± SE with different superscripts are significantly different (P ≤ 0.05, one-way ANOVA followed by Tukey–Kramer test P ≤ 0.05; n = 12/treatment).
The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON) and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). The DON-diets were prepared with wheat naturally contaminated with Fusarium mycotoxins.
Table 5.
Effects of feeding deoxynivalenol (DON)-contaminated diets during different growth stages on feed to gain ratio (F:G) of male broilers during a 34-day growth trial.1
| Treatment | F:G, g feed/g gain |
|||
|---|---|---|---|---|
| 1 to 14 d | 15 to 21 d | 22 to 34 d | 1 to 34 d | |
| Control | 1.37 ± 0.02 | 1.46 ± 0.01 | 1.70 ± 0.03a | 1.69 ± 0.07a |
| DON 1 to 14 d | 1.41 ± 0.02 | 1.39 ± 0.03 | 1.64 ± 0.03a | 1.67 ± 0.07a |
| DON 15 to 21 d | 1.33 ± 0.02 | 1.52 ± 0.02 | 1.70 ± 0.03a | 1.71 ± 0.05a |
| DON 22 to 34 d | 1.41 ± 0.03 | 1.43 ± 0.01 | 1.98 ± 0.02b | 1.89 ± 0.05b |
| DON 1 to 34 d | 1.41 ± 0.03 | 1.48 ± 0.03 | 1.98 ± 0.05b | 1.89 ± 0.05b |
| ANOVA | P = 0.22 | P = 0.08 | P < 0.01 | P < 0.01 |
a, b Within a column, means ± SE with different superscripts are significantly different (P ≤ 0.05, one-way ANOVA followed by Tukey–Kramer test P < 0.05; n = 12/treatment).
The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON) and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). The DON-diets were prepared with wheat naturally contaminated with Fusarium mycotoxins.
3.2. Organ weights and intestinal density
The means of the relative organ weights (g/kg BW) and density (g/cm) of intestinal sections for birds in each dietary treatment are presented in Table 6. Among birds sampled at 21 d, relative organ weights (liver, spleen, duodenum, jejunum and ileum) were not affected by treatment (P > 0.05). Birds fed DON-contaminated diets from 1 to 21 d had lower jejunum density (0.20 ± 0.01 g/cm) compared with control birds (P < 0.05). At 34 d, relative weight of duodenum, jejunum, ileum and spleen as well as density of intestinal segments were not affected by treatment (P > 0.05). Birds fed DON-contaminated diets during the grower period (DON 1 to 34 d or DON 22 to 34 d) had greater relative liver when compared with the control group or groups fed DON-contaminated diets during the early starter period (1 to 14 d).
Table 6.
Effects of feeding deoxynivalenol (DON)-contaminated diets during different growth stages on relative organ weights and density of small intestine sections in male broilers at 21 and 34 d.1
| Treatment | Relative organ weight, g/kg BW |
Intestine density, g/cm |
||||||
|---|---|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Liver | Spleen | Duodenum | Jejunum | Ileum | |
| 21 d | ||||||||
| Control | 7.41 ± 0.54 | 15.55 ± 0.88 | 11.10 ± 0.44 | 33.18 ± 1.27 | 0.94 ± 0.08 | 0.28 ± 0.02 | 0.23 ± 0.01a | 0.17 ± 0.01 |
| DON 1 to 14 d | 7.32 ± 0.63 | 14.88 ± 0.43 | 10.59 ± 0.43 | 31.37 ± 1.78 | 0.92 ± 0.06 | 0.26 ± 0.01 | 0.21 ± 0.01ab | 0.15 ± 0.01 |
| DON 15 to 21 d | 7.74 ± 0.49 | 15.42 ± 0.66 | 10.89 ± 0.67 | 33.43 ± 1.13 | 0.84 ± 0.06 | 0.28 ± 0.02 | 0.23 ± 0.01a | 0.17 ± 0.01 |
| DON 22 to 34 d | 7.44 ± 0.42 | 16.0 ± 0.36 | 10.90 ± 0.25 | 31.60 ± 1.27 | 1.02 ± 0.09 | 0.27 ± 0.02 | 0.23 ± 0.01ab | 0.16 ± 0.01 |
| DON 1 to 34 d | 6.84 ± 0.66 | 14.70 ± 0.67 | 11.0 ± 0.50 | 34.38 ± 1.37 | 0.89 ± 0.07 | 0.23 ± 0.02 | 0.20 ± 0.01b | 0.16 ± 0.01 |
| ANOVA | P = 0.85 | P = 0.58 | P = 0.16 | P = 0.50 | P = 0.54 | P = 0.27 | P = 0.04 | P = 0.13 |
| 34 d | ||||||||
| Control | 5.56 ± 0.27 | 10.83 ± 0.59 | 7.71 ± 0.26 | 21.91 ± 1.01b | 1.04 ± 0.05 | 0.35 ± 0.01 | 0.28 ± 0.01 | 0.21 ± 0.01 |
| DON 1 to 14 d | 5.77 ± 0.30 | 11.56 ± 0.35 | 7.89 ± 0.35 | 21.67 ± 0.47b | 1.07 ± 0.06 | 0.36 ± 0.01 | 0.29 ± 0.01 | 0.21 ± 0.01 |
| DON 15 to 21 d | 5.58 ± 0.24 | 11.50 ± 0.36 | 8.56 ± 0.29 | 22.95 ± 0.57ab | 1.06 ± 0.10 | 0.36 ± 0.01 | 0.30 ± 0.01 | 0.23 ± 0.01 |
| DON 22 to 34 d | 5.78 ± 0.15 | 11.81 ± 0.43 | 8.84 ± 0.39 | 25.24 ± 0.59a | 1.03 ± 0.05 | 0.34 ± 0.01 | 0.28 ± 0.01 | 0.23 ± 0.01 |
| DON 1 to 34 d | 5.87 ± 0.26 | 11.98 ± 0.056 | 8.26 ± 0.28 | 25.28 ± 0.77a | 0.97 ± 0.07 | 0.35 ± 0.01 | 0.28 ± 0.01 | 0.21 ± 0.01 |
| ANOVA | P = 0.88 | P = 0.41 | P = 0.14 | P < 0.01 | P = 0.32 | P = 0.46 | P = 0.54 | P = 0.14 |
a, b Within a column, mean ± SE with different superscripts are significantly different (P ≤ 0.05, one-way ANOVA followed by Tukey–Kramer test P ≤ 0.05; n = 12/treatment).
The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON) and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). The DON-diets were prepared with wheat naturally contaminated with Fusarium mycotoxins.
3.3. Intestine morphological structures
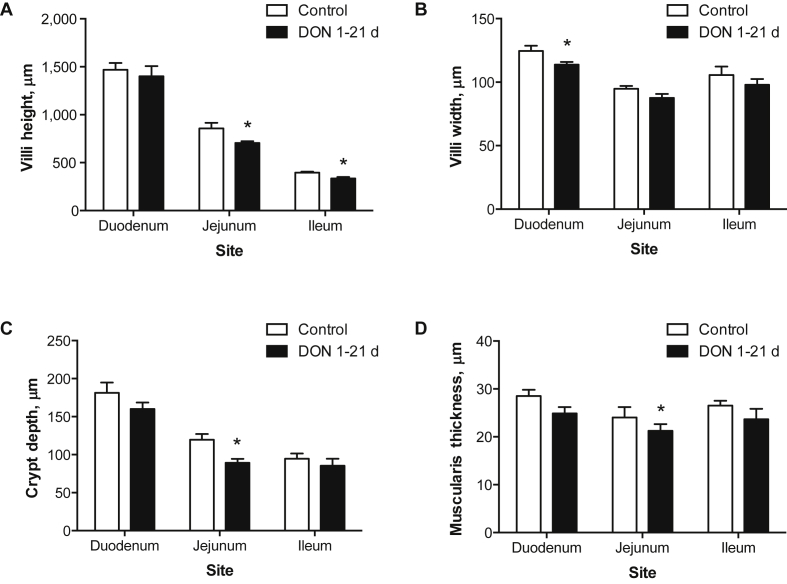
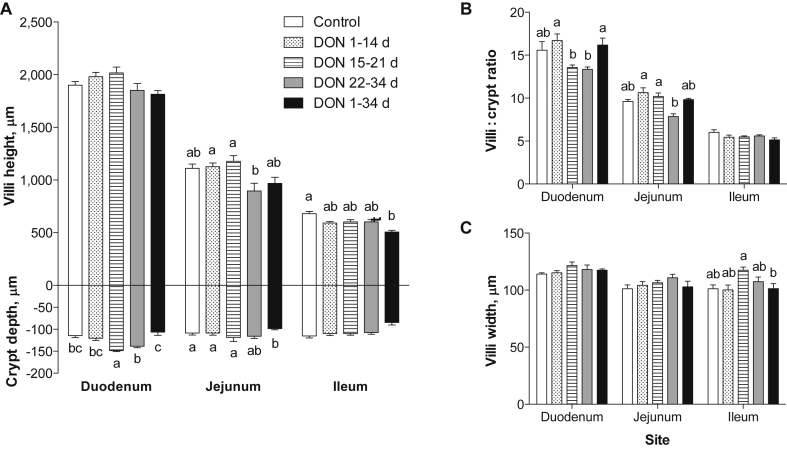
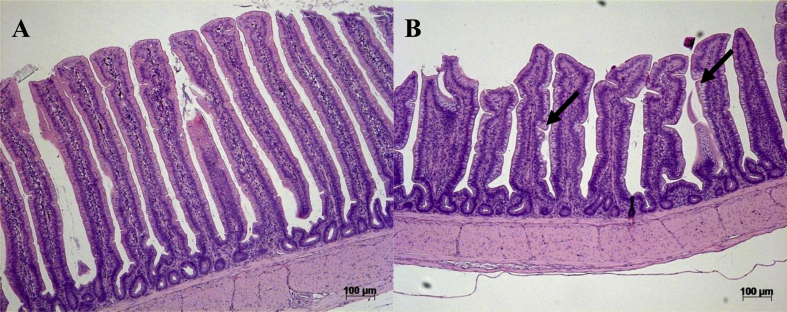
On d 21, birds fed the DON-contaminated diet had shorter villi in the jejunum (P = 0.03, Fig. 2A) and ileum (P = 0.01, Fig. 2A) and more narrow villi in the duodenum region (P = 0.04, Fig. 2B) compared with control birds. On d 34, birds fed the DON-contaminated diets throughout the entire trial had shorter villi (P < 0.01, Fig. 3A) and shallower crypts (P = 0.01, Fig. 3A) in the ileum region compared with birds fed control diets. Ileum villi:crypt ratios were not affected by treatment (P > 0.05, Fig. 3B). On d 34, birds that received the DON diet during the first 14 d had higher villi:crypt ratio in the duodenum compared with those fed the DON-diets later in the growing period (15 to 21 d and 22 to 34 d; P = 0.03, Fig. 3B). Birds receiving the DON grower diet from 21 to 34 d had shorter jejunum villi (P < 0.01, Fig. 3A) and higher jejunum villi to crypt ratio (P = 0.01, Fig. 3B) than birds receiving the DON diet from 1 to 14 d and 15 to 21 d. Jejunum crypt depth was not affected by treatment (P > 0.05, Fig. 3B). The effects of feeding DON-contaminated diets for 1 to 34 d on ileum mucosal structures are depicted in the histological pictures in Fig. 4.
Fig. 2.
Effects of feeding a deoxynivalenol [DON]-contaminated diet from 1 to 21 d on (A) villus height, (B) villus width, (C) crypt depth and (D) muscularis thickness in different intestinal regions of broiler chickens. Bars represent means ± standard error of the mean (SEM) with n = 6 animals. White bars represent birds fed the control diet and black bars represent birds fed the DON-contaminated diet. Asterisk (*) represents a significant difference within intestinal region (ANOVA, P ≤ 0.05). Villi to crypt ratio was not affected by DON treatment (ANOVA, P > 0.05).
Fig. 3.
Effect of feeding deoxynivalenol [DON]-contaminated diets during different growth stages on (A) villi height and crypt depth, (B) villi to crypt ratio and (C) villi width in different intestinal regions of broiler chickens at 34 d. Bars represent means ± standard error of the mean (SEM) with n = 6 animals. The starter diets were fed from 1 to 21 d (0.41 and 6.62 mg/kg DON) and the grower diets were fed from 22 to 34 d (0.54 and 7.90 mg/kg DON). Figure legend in Fig. 3A applies to all subfigures. The bars with different letters a and b are significantly different (P ≤ 0.05, one-way ANOVA followed by Tukey–Kramer test; n = 6/treatment). The muscular thickness did not differ among treatments (P > 0.05, one-way ANOVA).
Fig. 4.
Effects of feeding deoxynivalenol [DON]-contaminated diets to broilers for 34 d on morphology of ileum mucosal structures ( × 10, hematoxylin and eosin staining). (A) Ileum of a control bird at 34 d with normal villi. (B) Ileum from a bird fed DON-contaminated diet (∼7 mg/kg DON) from 1 to 34 d showing villi flattening and increased goblet cells (arrows).
4. Discussion
We investigated the impacts of timing of exposure to Fusarium mycotoxin-contaminated feed in broiler chickens in order to determine specific periods of sensitivity to adverse effects. Broilers may be frequently exposed to Fusarium mycotoxin-contaminated diets during specific parts of their growth cycle under production settings. In previous studies examining the growth performance effects of mycotoxins in broilers, contaminated diets were usually provided throughout the entire trial period (e.g. 1 to 35 d; Yunus et al., 2012 and 1 to 56 d, Swamy et al., 2002, Swamy et al., 2004a) and therefore, would not have addressed the influence of different exposure scenarios potentially encountered in industry. Thus, it was of interest to use targeted, stage-specific dietary exposure to determine whether broiler chickens were more sensitive to Fusarium mycotoxin-contaminated diets (specifically DON) during specific periods of growth.
In the current study, broilers that were provided contaminated diets in the later growth stage (22 to 34 d) had lower body weight, average daily gain, feed intake and increased F:G than birds fed contaminated diets at early growth stages or those fed clean diets. In fact, this short-term feeding of DON-contaminated diets to older birds with no previous exposure to DON resulted in the same degree of growth suppression when compared to birds chronically fed DON-contaminated diets. These findings suggest that broilers are more sensitive to DON-contaminated diets during later stage of growth, and adverse effects on growth performance are not necessarily cumulative with continuous exposure to DON-contaminated diets. A recent meta-analysis by Andretta et al. (2011) also concluded that the effect of mycotoxins on broiler weight gain was not constant across all growth phases; however, contrary to our results, they found that the effect of mycotoxins on growth was greater in young broilers. Our data does support findings by Swamy et al., 2002, Swamy et al., 2004a, where DON-contaminated diets were provided during a longer feeding trial (1 to 56 d); reduction in body weight and feed intake were observed during mid- (21 to 42 d, Swamy et al., 2004a) and later (43 to 56 d, Swamy et al., 2002) growth stages. Given that older broilers (22 to 34 d) consume significantly more feed than younger birds (1 to 21 d), and previous studies show that feed conversion ratio increases with age in broilers (Aviagen, 2014b, Zuidhof et al., 2014), performance effects of DON in older birds may be due to the fact that older birds consume more total mycotoxins, which is estimated by feed intake (kg) × dietary mycotoxin levels (mg/kg). Other maturational factors can influence an animal's response to mycotoxins, including age-related differences in uptake, distribution, metabolism and excretion of the compounds and metabolites. Older broilers (22 to 34 d) have higher basal metabolic rates than younger birds (Kuenzel and Kuenzel, 1977) and so one could hypothesize that mature broilers would metabolize DON faster than younger birds. Previous research in rodents found that older mice (8 to 10 weeks old) could metabolize orally administrated DON more quickly than younger mice (3 to 4 weeks old, Pestka and Amuzie, 2008); however, no studies to date have specifically compared toxicokinetics following DON exposure across growth stages in poultry.
Partial feed refusal was also observed in birds provided DON-contaminated diets during the grower phase and this response matched that of birds fed contaminated diets through the entire feeding trial. Trichothecenes are known to suppress feed intake through multiple pathways, such as inducing taste aversion, release of anorexia peptides, inducing inflammatory responses and altering liver function (Maresca, 2013, Osweiler, 2014, Lebrun et al., 2015). Long-term (1 to 56 d) feeding of Fusarium mycotoxin-contaminated diets to broilers increase levels of serotonin, a strong satiety neurochemical (Swamy et al., 2004b), which corresponded with DON-induced partial feed refusal and growth suppression observed in related studies (Swamy et al., 2002, Swamy et al., 2004a). Broilers exhibit increased responsiveness to peripheral administration of serotonin throughout early chick development, as indicated by greater suppression of feed intake in older birds (Baranyiova, 1990). Although we did not measure serotonin, it is possible that the stage-specific response of partial feed refusal is due to elevated serotonin levels or increased sensitivity to serotonin in later growth stages as there is no effect of DON on feed intake during the early growth phase.
Deoxynivalenol exposure appeared to have accumulative adverse effects on broiler intestinal mucosa structures, as birds fed the DON-contaminated diets throughout the trial had the most severe ileum mucosa structure alterations, while other stage-specific treatments were intermediate. Specifically, we observed decreased villi height and crypt depth in the jejunum of broilers fed the DON-contaminated diets. Villi flattening is probably due to the impairment of cell proliferation and our data agrees with previous studies in piglets and broiler chickens that showed a significant decrease in villi height in duodenum and jejunum after ingestion of DON-contaminated diets (Awad et al., 2006, Awad et al., 2011, Ghareeb et al., 2015). However, our results also indicate that DON intake during the starter phase is sufficient to affect intestinal mucosa structures. Broilers can metabolize DON rapidly (Osselaere et al., 2013, Broekaert et al., 2015) and individuals can rapidly (1 to 2 d) recover from DON-induced feed refusal and growth suppression if clean diets are provided (Osweiler, 2014). This was also evident in our trial where birds provided the DON-contaminated diets during the starter phase did not exhibit any long-term effects on feed intake or growth performance.
The gastrointestinal tract is the site of nutrient digestion and absorption and a fully functioning, healthy intestine is essential for fast-growing broilers to achieve maximum growth rates with superior feed efficiency (Jin et al., 1998, Denbow, 2015). In the current study, a decrease in villi height in the ileum region was observed. Feeding broilers DON-contaminated diets is known to reduce villi height, crypt depth and villi surface area (Awad et al., 2006, Awad et al., 2011, Ghareeb et al., 2015). Decreased villi height is associated with a reduction of nutrient digestion and absorption (Ruttanavut and Yamauchi, 2010). Although dietary nutrient digestibility was not directly measured in the present study, the negative effects of DON on ileum mucosa structures suggest a reduction in nutrient absorption. This hypothesis is supported by our observed reduction in feed efficiency.
The reported effects of dietary Fusarium mycotoxins on relative organ weights in poultry are very inconsistent across studies (Swamy et al., 2004a, Girgis and Smith, 2010, Awad et al., 2011). In the present study, feeding the DON-contaminated diets during different growth stages had no effect on relative organ weights, whether evaluated at the end of the starter period (21 d) or at the end of the trial (34 d). The only exception was an increase in relative liver weight at 34 d in individuals given the DON-contaminated feed throughout the entire trial. Increased liver weight has been reported in mice given DON by oral gavage (Pestka, 2007, Sobrova et al., 2010). Liver is the major site of DON detoxification (Sobrova et al., 2010, Maresca, 2013) and so increased relative liver weight may be due to enhanced hepatic detoxification activity. Liver is also the major organ for nutrient metabolism (Humphrey and Klasing, 2004) and DON exposure negatively affects nutrient metabolism in the liver (Pestka, 2007, Maresca, 2013, Lebrun et al., 2015). Reduced feed efficiency reported herein in birds fed DON-contaminated diets may be the result of such toxic effects of DON on liver.
It is important to note that broilers used in the current study were lighter than the suggested body weight at the same age (Aviagen, 2014b). The unexpected slower growth rate may be attributed to the starter diets (1 to 21 d) having lower than recommended (Aviagen, 2014a) calcium levels (0.70% vs. 0.96%). Calcium is an important nutrient for modern broilers to reach maximum growth performance (Rama Rao et al., 2003, Driver et al., 2005). Feeding broilers low calcium diets (approximately 80% of requirement) results in suppression of feed intake, reduced weight gain and feed efficiency (Driver et al., 2005). The calcium levels in our starter diets were comparable in both clean and DON-contaminated diets; thus, any growth suppression effects observed in birds received DON-diets are most likely the result of DON toxicity.
Another concern is that the DON-contaminated diets contained approx. 10% lower crude protein compared to control diets, which may be attributed to different wheat (control vs. naturally Fusarium-damaged wheat) used in the diet preparation (Kim et al., 2005, Amerah, 2015, Kautzman et al., 2015). In the current study, naturally Fusarium-damaged grains were used to better reflect the mycotoxin challenges faced in broiler industry. Since lower crude protein level is characteristic of Fusarium-damaged grains (Kautzman et al., 2015), naturally-contaminated feed encountered in broiler production systems would conceivably contain less crude protein as compared to uncontaminated feed. It is important to note that across all experimental diets, protein levels and amino acids were sufficient to meet broiler growth requirement (Aviagen, 2014a). Previous studies reported that broiler growth performance was not affected by diets containing crude protein at 10% less than recommended levels when the essential amino acids met nutrient requirements (Bregendahl et al., 2002, Aftab et al., 2006). We are confident that the growth suppression along with altered intestinal morphology observed in the current study is due to intake of DON-contaminated feed. However, in future studies, the protein levels of wheat should be measured prior to feed formulation, and dietary nutrient levels within manufactured complete feed should be confirmed prior to feeding experiments.
The current study demonstrated that broiler chickens are more sensitive to dietary Fusarium mycotoxins (primarily DON) during the later stages of growth as exhibited by reduced feed intake, weight gain and feed efficiency when compared to effects at early stages (starter period). The experimental design used in this study, with DON exposure at specific periods of growth, showed that broilers can recover from performance effects due to early DON exposure while late exposure can have performance effects equivalent to those in chronically-fed birds. The current study also indicates that growth suppression induced by intake of DON-contaminated diets is not cumulative. Mycotoxin-induced adverse effects on growth performance may be the result of partial feed refusal and alteration of intestinal mucosa structures. Further studies are required to determine the predominant mechanisms (possibly taste aversion, anorexia effects or inflammatory responses) underlying the Fusarium mycotoxin-induced feed refusal in broiler chickens. Regardless, in order to achieve maximum growth performance, Fusarium mycotoxin-contaminated diets should be avoided, especially during in the later stage of broiler growth.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by Natural Sciences and Engineering Council of Canada (NSERC) (CRDPJ 452505-13) Collaborative Research and Development Grant (N. Hogan) and the Canadian Poultry Research Council (AMN059). Stipend support to A. Wang was provided by a University of Saskatchewan Devolved Scholarship. The authors are grateful to Denise Beaulieu for comments on the manuscript. The authors thank the staff at Canadian Feeds Research Centre for assistance in preparing diets, the staff and students at the University of Saskatchewan Poultry Research Center for help with animal trials, and students in the Hogan Lab for help with sampling.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Anhao Wang, Email: anhao.wang@usask.ca.
Natacha S. Hogan, Email: natacha.hogan@usask.ca.
References
- Aftab U., Ashraf M., Jiang Z. Low protein diets for broilers. Worlds Poult Sci J. 2006;62:688–701. [Google Scholar]
- Amerah A.M. Interactions between wheat characteristics and feed enzyme supplementation in broiler diets. Anim Feed Sci Technol. 2015;199:1–9. [Google Scholar]
- Andretta I., Kipper M., Lehnen C.R., Hauschild L., Vale M.M., Lovatto P.A. Meta-analytical study of productive and nutritional interactions of mycotoxins in broilers. Poult Sci. 2011;90:1934–1940. doi: 10.3382/ps.2011-01470. [DOI] [PubMed] [Google Scholar]
- Andretta I., Kipper M., Lehnen C.R., Lovatto P.A. Meta-analysis of the relationship of mycotoxins with biochemical and hematological parameters in broilers. Poult Sci. 2012;91:376–382. doi: 10.3382/ps.2011-01813. [DOI] [PubMed] [Google Scholar]
- Aviagen . 2014. Ross 308 nutrition specifications.http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross308BroilerNutritionSpecs2014-EN.pdf [Google Scholar]
- Aviagen . 2014. Ross 308 broiler performance objectives.http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-308-Broiler-PO-2014-EN.pdf [Google Scholar]
- Awad W.A., Böhm J., Razzazi-Fazeli E., Ghareeb K., Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. 2006;85:974–979. doi: 10.1093/ps/85.6.974. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess M., Twaruzek M., Grajewski J., Kosicki R., Böhm J., Zentek J. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int J Mol Sci. 2011;12:7996–8012. doi: 10.3390/ijms12117996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Böhm J. The toxicity of Fusarium mycotoxin deoxynivalenol in poultry feeding. Worlds Poult Sci J. 2012;68:651–668. [Google Scholar]
- Awad W.A., Ghareeb K., Zentek J. Mechanisms underlying the inhibitory effect of the feed contaminant deoxynivalenol on glucose absorption in broiler chickens. Vet J. 2014;202:188–190. doi: 10.1016/j.tvjl.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Baranyiova E. Effects of serotonin on the food intake in chickens in the post-hatching period. Acta Vet Brno. 1990;59:23–33. [Google Scholar]
- Bregendahl K., Sell J.L., Zimmerman D.R. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult Sci. 2002;81:1156–1167. doi: 10.1093/ps/81.8.1156. [DOI] [PubMed] [Google Scholar]
- Broekaert N., Devreese M., De Mil T., Fraeyman S., Antonissen G., De Baere S. Oral bioavailability, hydrolysis, and comparative toxicokinetics of 3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol in broiler chickens and pigs. J Agric Food Chem. 2015;63:8734–8742. doi: 10.1021/acs.jafc.5b03270. [DOI] [PubMed] [Google Scholar]
- Bryden W.L. Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Anim Feed Sci Technol. 2012;173:134–158. [Google Scholar]
- Canadian Council on Animal Care (CCAC) 1993. Guidelines on: the care and use of farm animals in research, teaching and testing. Canadian Council on Animal Care, Constitution Square – Tower 2, 1510-130 Albert Street, Ottawa, ON Canada K1R 1B1. [Google Scholar]
- Denbow D.M. Chapter 14 - Gastrointestinal anatomy and physiology. In: Scanes C.G., editor. Sturkie's avian physiology. 6th ed. Academic Press; San Diego: 2015. pp. 337–366. [Google Scholar]
- Döll S., Dänicke S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev Vet Med. 2011;102:132–145. doi: 10.1016/j.prevetmed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Driver J.P., Pesti G.M., Bakalli R.I., Edwards H.M. Calcium requirements of the modern broiler chicken as influenced by dietary protein and age. Poult Sci. 2005;84:1629–1639. doi: 10.1093/ps/84.10.1629. [DOI] [PubMed] [Google Scholar]
- D'Mello J.P.F., Placinta C.M., Macdonald A.M.C. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim Feed Sci Technol. 1999;80:183–205. [Google Scholar]
- Eriksen G.S., Pettersson H. Toxicological evaluation of trichothecenes in animal feed. Anim Feed Sci Technol. 2004;114:205–239. [Google Scholar]
- Escrivá L., Font G., Manyes L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: a review. Food Chem Toxicol. 2015;78:185–206. doi: 10.1016/j.fct.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Böhm J., Zebeli Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J Appl Toxicol. 2015;35:327–337. doi: 10.1002/jat.3083. [DOI] [PubMed] [Google Scholar]
- Girgis G.N., Smith T.K. Comparative aspects of Fusarium mycotoxicoses in poultry fed diets containing naturally contaminated grains. Worlds Poult Sci J. 2010;66:65–86. [Google Scholar]
- Grenier B., Applegate T.J. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B.D., Klasing K.C. Modulation of nutrient metabolism and homeostasis by the immune system. Worlds Poult Sci J. 2004;60:90–100. [Google Scholar]
- Jin S.H., Corless A., Sell J.L. Digestive system development in post-hatch poultry. Worlds Poult Sci J. 1998;54:342–345. [Google Scholar]
- Kautzman M.E., Wickstrom M.L., Scott T.A. The use of near infrared transmittance kernel sorting technology to salvage high quality grain from grain downgraded due to Fusarium damage. Anima Nutr. 2015;1:41–46. doi: 10.1016/j.aninu.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.C., Simmins P.H., Mullan B.P., Pluske J.R. The digestible energy value of wheat for pigs, with special reference to the post-weaned animal [Review] Anim Feed Sci Technol. 2005;122:257–287. [Google Scholar]
- Kosicki R., Błajet-Kosicka A., Grajewski J., Twarużek M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim Feed Sci Technol. 2016;215:165–180. [Google Scholar]
- Kuenzel W.J., Kuenzel N. Basal metabolic rate in growing chicks Gallus domesticus. Pout Sci. 1977;56:619–627. doi: 10.3382/ps.0560619. [DOI] [PubMed] [Google Scholar]
- Lebrun B., Tardivel C., Félix B., Abysique A., Troadec J.D., Gaigé S. Dysregulation of energy balance by trichothecene mycotoxins: mechanisms and prospects. Neurotoxicology. 2015;49:15–27. doi: 10.1016/j.neuro.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Littell R.C. 2nd ed. SAS Institute; 2006. SAS for mixed models. [Google Scholar]
- Maresca M. From the gut to the brain - journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins. 2013;5:784–820. doi: 10.3390/toxins5040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D., Richardson S. Department of Chemistry, Carleton University; 2013. Mycotoxins in Canada: a perspective for 2013. [Google Scholar]
- Miller J.D., Schaafsma A.W., Bhatnagar D., Bondy G., Carbone I., Harris L.J. Mycotoxins that affect the North American agri-food sector: state of the art and directions for the future. World Mycotoxin J. 2014;7:63–82. [Google Scholar]
- Osselaere A., Devreese M., Goossens J., Vandenbroucke V., De Baere S., De Backer P. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem Toxicol. 2013;51:350–355. doi: 10.1016/j.fct.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Osweiler G.D. 2014. Mycotoxicoses; the merck veterinary manual.https://www.merckvetmanual.com/toxicology/mycotoxicoses/overview-of-mycotoxicoses [Google Scholar]
- Pestka J.J. Deoxynivalenol: toxicity, mechanisms and animal health risks. Anim Feed Sci Technol. 2007;137:283–298. [Google Scholar]
- Pestka J.J., Amuzie C.J. Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: comparison of weanling and adult mice. Food Chem Toxicol. 2008;46:2826–2831. doi: 10.1016/j.fct.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron A., Alassane-Kpembi I., Oswald I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016;2:21–29. doi: 10.1186/s40813-016-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Oswald I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: a review. Toxins. 2014;6:1615–1643. doi: 10.3390/toxins6051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Rao S.V., Panda A.K., Raju M.V.L.N., Shyam Sunder G., Praharaj N.K. 2003. Requirement of calcium for commercial broilers and white leghorn layers at low dietary phosphorus levels. Anim Feed Sci Technol. 2003;106:199–208. [Google Scholar]
- Ruttanavut J., Yamauchi K. Growth performance and histological alterations of intestinal villi in broilers fed dietary mixed minerals. Asian-Australas J Anim Sci. 2010;4:96–106. [Google Scholar]
- Sobrova P., Adam V., Vasatkova A., Beklova M., Zeman L., Kizek R. Deoxynivalenol and its toxicity. Interdiscip Toxicol. 2010;3:94–99. doi: 10.2478/v10102-010-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy H.V.L.N., Smith T.K., Cotter P.F., Boermans H.J., Sefton A.E. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on production and metabolism in broilers. Poult Sci. 2002;81:966–975. doi: 10.1093/ps/81.7.966. [DOI] [PubMed] [Google Scholar]
- Swamy H.V.L.N., Smith T.K., Karrow N.A., Boermans H.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on growth and immunological parameters of broiler chickens. Poult Sci. 2004;83:533–543. doi: 10.1093/ps/83.4.533. [DOI] [PubMed] [Google Scholar]
- Swamy H.V.L.N., Smith T.K., MacDonald E.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on brain regional neurochemistry of starter pigs and broiler chickens. J Anim Sci. 2004;82:2131–2139. doi: 10.2527/2004.8272131x. [DOI] [PubMed] [Google Scholar]
- Terzi V., Tumino G., Stanca A.M., Morcia C. 2014. Reducing the incidence of cereal head infection and mycotoxins in small grain cereal species. J Cereal Sci. 2014;59:284–293. [Google Scholar]
- Tittlemier S.A., Gaba D., Chan J.M. Monitoring of Fusarium trichothecenes in Canadian cereal grain shipments from 2010 to 2012. J Agric Food Chem. 2013;61:7412–7418. doi: 10.1021/jf4019257. [DOI] [PubMed] [Google Scholar]
- Tittlemier S.A., Roscoe M., Trelka R., Gaba D., Chan J.M., Patrick S.K. Fusarium damage in small cereal grains from western Canada. 2. Occurrence of Fusarium toxins and their source organisms in durum wheat harvested in 2010. J Agric Food Chem. 2013;61:5438–5448. doi: 10.1021/jf400652e. [DOI] [PubMed] [Google Scholar]
- Wu F. 2007. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim Feed Sci Technol. 2007;137:363–374. [Google Scholar]
- Yli-Mattila T. Ecology and evolution of toxigenic Fusarium species in cereals in northern Europe and Asia. J Plant Pathol. 2010;92:7–18. [Google Scholar]
- Yunus A.W., Ghareeb K., Twaruzek M., Grajewski J., Böhm J. Deoxynivalenol as a contaminant of broiler feed: effects on bird performance and response to common vaccines. Poult Sci. 2012;91:844–851. doi: 10.3382/ps.2011-01873. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.F., Schneider B.L., Schneider B.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]