Abstract
Purpose
To describe a case of bilateral choroidal osteoma (CO) in a patient with a history of langerhans cell histiocytosis (LCH).
Methods
A 24-year-old man complaining of gradually decreasing visual acuity in both eyes is presented. He had a history of lymphadenopathy, respiratory symptoms, and pathology-proven diagnosis of LCH.
Results
Ophthalmic clinical and imaging studies revealed bilateral CO.
Conclusion
In this patient, we suggest a possible relationship between LCH and CO.
Keywords: Choroidal osteoma, Langerhans cell histiocytosis, Bilateral
Introduction
Langerhans cell histiocytosis (LCH) is a clonal proliferation of dendritic langerhans cells which often involves multiple organs.1 Although ocular involvement in LCH usually manifests as a slowly growing orbital mass, histiocytes could infiltrate the choroid, too.2 Patton et al. reported a case of presumed solitary choroidal LCH manifested as a posterior pole choroidal mass, which showed low internal reflectivity in B-scan and subretinal exudation in optical coherence tomography (OCT).1 Gass was the first who described choroidal osteoma (CO) in 1978.3 It usually involves young healthy women. It is unilateral in 75% of cases. Although the exact pathogenesis of CO remains unknown, choriostomatous origin, inflammatory, traumatic, metabolic, environmental or hereditary causes have been proposed. In this case report, we will describe a case of bilateral CO associated with LCH.
Case report
The patient was a 24-year-old man complaining of decreased vision, mainly in the left eye, since about 4 months earlier. He had a history of axillary and inguinal lymph adenopathy and severe chronic cough since the age of 17 months old. The laboratory tests had revealed anemia. LCH was diagnosed definitely by pathologic examination and confirmed by demonstrating CD1a positive cells in lymph node specimens. He was treated with systemic prednisolone and vinblastine, which was tapered after clinical improvement. At the time of presentation to the retina clinic of Farabi Eye Hospital, the BCVA was 20/20 in the right eye and 20/30 in the left eye. The external eye examination was unremarkable, and eye motility and eye alignment were normal. Detailed ocular examination showed bilateral minimal posterior subcapsular cataract and peripapillary pseudopode-like subretinal yellow white mass with geographic margins (Fig. 1). Multiple peripheral atrophic retinochoroidal scars, similar to the scars of panretinal photocoagulation, were visible at the presentation. Fluorescein angiography (FA) showed patchy diffuse early and late hyperfluorescence (Fig. 2). In OCT, the retinal architecture seemed preserved but nasal to the fovea a hyperreflective choroidal mass with dome shaped pushing effect on the overlying retina, and posterior shadowing was present (Fig. 3). Nasal to the fovea there was ellipsoid zone disruption in the right eye. In B-scan, a highly echogenic, slightly elevated mass with back shadowing was seen. Lowering the gain of the instrument was in favor of the calcified structure of the lesion (Fig. 4). In computed tomography scan (CT-scan), the choroidal calcification was seen, but there was no orbital or elsewhere involvement. The patient was diagnosed as bilateral calcified CO.
Fig. 1.
Fundus color photography of the right eye (1a) and left eye (1b) showing a pseudopode-like peripapillary subretinal yellow white mass.
Fig. 2.
Late phase of fluorescein angiography (FA) of the right eye (2a) and left eye (2b) showing patchy diffuse hyper-fluorescence.
Fig. 3.
Optical coherence tomography (OCT) of the right eye (3a) and left eye (3b) with intact inner retinal layers but patchy outer retina involvement mostly in left eye. Meanwhile mass induced diffuse choroidal and some posterior low reflectivity is visible.
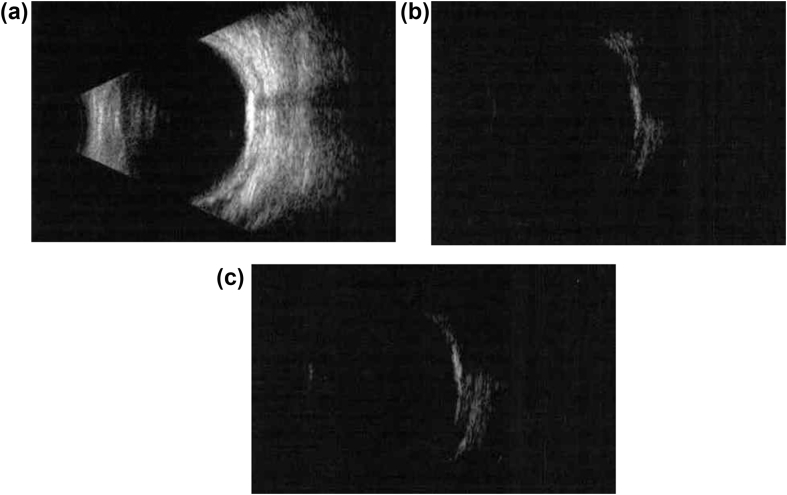
Fig. 4.
B-scan of the right eye (4a) demonstrates a highly echogenic, slightly elevated mass with posterior acoustic shadowing. Lowering the gain of the instrument (4b: right eye; 4c: left eye) proved the calcified structure of the lesion.
Discussion
CO is a benign tumor which usually happens in young healthy women in their second or third decades of life. While CO is sometimes asymptomatic, possible symptoms include blurred vision, metamorphopsia, or visual field defects corresponding to the tumor location. It is unilateral in 75% of cases. In bilateral cases, the tumors could be symmetric or asymmetric, with different stages of growth or calcification. The initially unilaterally presented tumor could rarely involve the other eye, too.1, 3 Although the exact mechanism of CO remains to be defined, some theories were given. Gass et al.3 described three possible theories: 1. Osseous metaplasia of pigment epithelium or primitive mesoderm, 2. Osseous metaplasia in a cavernous hemangioma, and 3. Trauma or inflammation-induced secondary ossification.3
Noticing the possible theories about the CO pathogenesis, chronic inflammation may have a role,3 and LCH is the most common type of histiocytic disorder, characterized by abnormal accumulation of CD1A+/CD207+ mononuclear phagocytes within granulomatous lesions, affecting nearly all organ systems.4 The presence of the choroidal infiltrated histiocytes has previously been shown in LCH.2
In LCH, the infiltrated monocytes and macrophages would secrete tumor necrosis factor α (TNF α) and interleukine 1 (IL1) which would stimulate the retinal pigment epithelial (RPE) cells to produce TGF β. The TGF β, including the subfamily of bone morphogenetic proteins (BMPs), have different roles in mesenchymal epithelial transformation and bone formation,5 e.g. some of the BMPs could trigger the mesenchymal cell transformation into the osteoprogenitors.5 Toyran et al. showed that in ocular ossification, adjacent to bone metaplastic areas, there is moderate intensification of BMP-7 and Growth Differentiation Factor 5 (GDF-5) reactivity in the RPE cells and fibrous metaplasia.6 They concluded that the mesenchymal cell/dormant osteoprogenitor cells have been stimulated by BMPs and GDF-5 to differentiate into osteoblasts. They claimed that the IL1 and TNF α secreted by monocytes and microphages would trigger the RPE cells to secrete TGF β1 and BMP-7. The TGF β1 would initiate fibrous metaplasia of RPE cells and BMP-7 would transform the metaplastic RPE cell into osteoblasts.6
The potential of re-programmed mesenchymal cells to differentiate into osteogenic lines has been proven, and heterotopic calcification in LCH has been reported in other organ involvements, too.7, 8 Ducassou et al.7 found thymic and mediastinal node calcification in patients with systemic LCH, and Caruso et al.8 reported biliary wall calcification in LCH liver involvement. On the other hand, CO has been associated with other inflammatory eye diseases, like Behçet's disease, too.9
To our knowledge, there are few case reports of bilateral CO associated with LCH.10, 11
Kline et al. reported an 11-year-old boy with choroidal plexus mass, multiple infiltrating lesions in both hemispheres, and bilateral CO.10 Although according to the pathologic examination the probable diagnosis of histiocytosis X was made, they proposed infectious or toxic etiologies, too.
In our report, the diagnosis of LCH was proven by immunohistochemistry. One of the Gass theories was inflammation-induced secondary heterotopic calcification.3 By assuming all the previous works we propose that in this case, the inflammatory mediators secreted by choroidal infiltrated monocytes/macrophages might have caused osseous metaplasia and finally CO formation, but further investigation with larger samples is necessary for better clarifying the pathogenesis of CO.
Footnotes
Authors have not received any grant support or research funding, and there is no proprietary interest in the materials described in the article.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Patton N., Lai T., Robbins P., Holthouse D., Barry C., Constable I. Presumed choroidal langerhans cell histiocytosis following a previously resected solitary central nervous system lesion in an adult. Arch Ophthalmol. 2006;124(8):1193–1195. doi: 10.1001/archopht.124.8.1193. [DOI] [PubMed] [Google Scholar]
- 2.Kim I.T., Lee S.M. Choroidal Langerhans' cell histiocytosis. Acta Ophthalmol Scand. 2000;78(1):97–100. doi: 10.1034/j.1600-0420.2000.078001097.x. [DOI] [PubMed] [Google Scholar]
- 3.Gass J.D., Guerry R.K., Jack R.L., Harris G. Choroidal osteoma. Arch Ophthalmol. 1978;96:428–435. doi: 10.1001/archopht.1978.03910050204002. [DOI] [PubMed] [Google Scholar]
- 4.Berres M.L., Merad M., Allen C.E. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to Histiocytosis X? Br J Haematol. 2015;169(1):3–13. doi: 10.1111/bjh.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munteanu M., Munteanu G., Giuri S., Zolog I., Motoc A.G. Ossification of the choroid: three clinical cases and literature review of the pathogenesis of intraocular ossification. Rom J Morphol Embryol. 2013;54(3 suppl):871–877. [PubMed] [Google Scholar]
- 6.Ducassou S., Seyrig F., Thomas C. Thymus and mediastinal node involvement in childhood langerhans cell histiocytosis: long-term follow-up from the French National Cohort. Pediatr Blood Cancer. 2013;60:1759–1765. doi: 10.1002/pbc.24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso S., Miraglia R., Maruzzelli L., Luca A., Gridelli B. Biliary wall calcification in Langerhans cell histiocytosis: report of two cases. Pediatr Radiol. 2008 Jul;38(7):791–794. doi: 10.1007/s00247-008-0809-x. [DOI] [PubMed] [Google Scholar]
- 8.Toyran S., Lin A.Y., Edward D.P. Expression of growth differentiation factor-5 and bone morphogenic protein-7 in intraocular osseous metaplasia. Br J Ophthalmol. 2005;89(7):885–890. doi: 10.1136/bjo.2004.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casaroli-Marano R.P., Molina J.J., Adán A., Corretger X. Bilateral choroidal osteoma associated with optic neuritis in Behçet's disease. Ophthalmic Surg Lasers Imaging. 2010 Mar 9:1–4. doi: 10.3928/15428877-20100215-78. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Kline L.B., Skalka H.W., Davidson J.D., Wilmes F.J. Bilateral choroidal osteoma associated with fatal systemic illness. Am J Ophthalmol. 1982;93(2):192–197. doi: 10.1016/0002-9394(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 11.Okada K., Minamoto A., Sakata H., Mizote H. Bilateral choroidal osteoma associated with histiocytosis X. Jpn J Ophthalmol. 1996;40(1):111–115. [PubMed] [Google Scholar]