Abstract
Many insurers are using formulary design to influence opioid prescribing, but it is unclear if these changes lead to reduced use or just substitution between opioids. We evaluated the effect of a new prior authorization process implemented in July of 2015 for extended release (ER) oxycodone by Blue Shield of California. Compared to other commercially-insured Californians, among 880,000 enrollees, there was a 36% drop in monthly rates of ER opioid initiation among Blue Shield enrollees relative to control group members, driven entirely by decreases in ER oxycodone without any substitution towards other ER opioids. This reduction was offset by a 1.4% relative increase in the rate of short-acting opioid fills. There was no significant change in the overall use of any opioids prescribed, measured as morphine milligram-equivalents. This suggests that though insurers can play a meaningful role in reducing prescribing of high-risk ER opioids, a formulary change focused on ER opioids alone is insufficient to decrease total opioid prescribing.
Clinician prescribing of opioids has played a central role in fueling a growing national epidemic of opioid dependence and abuse.1–4 From 1991 to 2011, the volume of opioids prescribed in the U.S. more than tripled.5 This dramatic rise in opioid prescribing coincided with a significant increase in the rate of opioid overdose deaths, with over 42,000 deaths in 2016 alone.6 The prescribing of extended release (ER) opioids to treat chronic non-cancer pain is of particular concern given that they may be ineffective in treating pain and are associated with high rates of mortality and overdose.7–10 Also, treatment initiation with ER opioids is associated with a significantly higher risk of opioid overdose than treatment initiation with short-acting opioids.10 The use of the ER form of oxycodone, OxyContin, has been specifically implicated as an important factor in the rise of prescription drug abuse across the U.S.1,11,12
The opioid crisis has prompted state and federal governments, health insurers and health systems to create numerous policies aimed at reducing opioid prescribing. In particular, prior authorizations, which are effective in restricting medication use in other contexts,13–15 are now increasingly being used for opioid prescribing.16,17 Unfortunately, there has been little rigorous research on the impact of such policies.18 Given that overall opioid prescribing is now declining in the U.S.,19 any observational evaluation must use a control group to account for underlying trends and avoid overestimating the impact of any one policy change.
To address the challenge of opioid overuse for non-cancer pain, in 2015 Blue Shield of California, a large commercial insurer with over 4 million members, implemented a “Narcotic Safety Initiative” with a goal of cutting opioid use in half by 2018. The Narcotic Safety Initiative (NSI) includes multiple evidence-based interventions including formulary design, risk-based case management, and partnering with local physician groups to reduce high risk prescribing patterns and prevent patients newly starting opioid therapy from progressing to chronic use unnecessarily. The first wave of NSI interventions began in July of 2015 and targeted the prescribing of ER narcotics by implementing a prior authorization requirement for ER oxycodone (OxyContin). This was the most commonly prescribed ER opioid in the Blue Shield of California population and one of the most frequently abused opioid formulations in the US.20
Using data from other commercially insured Californians as a control group, we evaluated the impact of this prior authorization on opioid prescribing. One concern with limiting access to ER opioids is that physicians could substitute other opioids without reducing total opioid use or that patients could switch to other health plans to avoid the prior authorization restriction.11,12 To address these concerns, we assessed the impact of the intervention on prescribing of ER oxycodone, total ER opioid use, short-acting opioids, and total opioid prescribing. We also assessed health plan disenrollment.
METHODS
Data Sources and Study Sample
We used two separate sources of administrative medical and pharmacy claims in this analysis. For the intervention population we used claims databases from Blue Shield of California from 2014–2016. For the control group, we used the Truven MarketScan database, which includes 2014–16 medical claims for a national sample of private health plans and self-insured employers who elect to contribute data to the database.21
Our study sample included adults aged 21 and older enrolled in a commercial health insurance plan with Blue Shield of California (the intervention group) or an insurer in the MarketScan database (the control group) residing in California. Our main analyses focused on the cohort of individuals who were continuously enrolled in insurance coverage from 2014–2016 in Blue Shield of California and MarketScan databases. In the Blue Shield of California database, we sampled every individual who received any opioid prescription from 2014–2016, and a 20% sample of individuals who did not receive opioids in that period. We weighted the sample of non-opioid users to obtain population-level estimates for the Blue Shield of California cohort. We excluded individuals with any cancer diagnosis or hospice enrollment during the study period because they were excluded from opioid reduction policies. This study was deemed exempt from review by the institutional review board at Harvard T. H. Chan School of Public Health.
The Narcotic Safety Initiative
Through its Narcotic Safety Initiative (NSI), Blue Shield of California implemented a series of interventions to promote safe opioid prescribing and reduce the overall burden of opioid use in the insured population starting in 2015 and continuing through the date of publication (Appendix Exhibit 1).22
The prior authorization intervention requires the prescribing physician to submit the following information to Blue Shield: the patients’ diagnosis; previous treatments for pain received by the patient, including non-drug treatments, non-opioid pain medications, and opioid-containing medications past and present; the medical necessity for using an opioid versus non-opioid pain medication, and a treatment plan for managing pain, monitoring for opioid effects, and tapering the dose. Blue Shield reviewers, who are licensed clinicians, considered the patient’s history and proposed treatment’s consistency with published recommendations for chronic opioid use and made a final determination. Of note, ER morphine (the second most-commonly used ER opioid among plan members) remained available without prior authorization at doses below 120 morphine milligram equivalents (MME) daily. Prior to these dates, prior authorization was not required for new ER opioid prescriptions, or for adding or switching to a new ER opioid prescription.
We considered the ER oxycodone prior authorization to be the most salient intervention “start date,” and used January-June of 2014 as a baseline risk adjustment period, and then defined July 2014-June 2015 as the pre-intervention period. We defined July 2015-December 2016 as our post-intervention period with a control group. There was a second wave of prior authorizations for other ER opioids in September 2015, but it targeted opioids comprising a small amount of total prescriptions (6.2% of targeted opioids vs. 48.4% for oxycodone, Appendix Exhibit 2).22
Outcomes
Our primary outcome was monthly rates per 10,000 members of new initiation of an ER opioid, defined as a prescription for an ER opioid to a patient having no ER opioid prescriptions in the preceding 6 months, including short-acting opioids. We defined opioids using national drug codes from previously published work,23–25 excluding methadone and buprenorphine because the majority of their use is for opioid use disorder treatment, not treatment of chronic pain. We also excluded opioid-containing cough syrup formulations not used for pain treatment. We also examined monthly rates of all short acting and ER opioid fills and monthly use of all opioid prescriptions (expressed in morphine milligram equivalent doses – gram-MMEs – using standard conversion tables).26 We divided all measures of opioid use separately into oxycodone and non-oxycodone-containing opioids to assess the differential impacts of the ER prior authorization requirement and potential substitution effects. We also separated opioids into ER and short-acting categories for some analyses.
Additionally, to assess whether the NSI was associated with accelerated disenrollment of opioid users, we compared trends in individuals dropping Blue Shield of California coverage during 2014–2016 between non-opioid users vs. those with any ER opioid fill in the first six months of 2014.
Study Variables
We captured several characteristics of the intervention and control populations, including age, sex, and 31 comorbidities (though 3 cancer-related comorbidities were excluded by definition) as defined by the Elixhauser index using claims from the baseline risk-adjustment period, the first 6 months of 2014 (Exhibit 1).27 We also captured geography of residence defined by metropolitan statistical areas (MSAs)28 (the smallest geographic unit available in both databases).
Exhibit 1:
Patient Characteristics
| Blue Shield of California Enrollees (n = 471,337) |
MarketScan Commercially Insured Enrollees (n=409,268) |
|
|---|---|---|
| Mean age in years | 45.6 | 43.2 |
| Percent female | 55.9 | 57.6 |
| Number of Elixhauser conditions | 0.95 | 0.84 |
| Presence of comorbidity, % | ||
| Cardiac Arrhythmias | 3.9 | 3.0 |
| Chronic Pulmonary Disease | 8.6 | 8.1 |
| Depression | 7.4 | 10.1 |
| Diabetes, Complicated | 2.3 | 2.2 |
| Diabetes, Uncomplicated | 8.7 | 7.5 |
| Hypertension, Uncomplicated | 22.1 | 17.9 |
| Hypothyroidism | 9.1 | 7.7 |
| Liver Disease | 3.1 | 2.6 |
| Obesity | 9.4 | 8.0 |
| Rheumatoid Arthritis | 2.6 | 2.4 |
| MSA, % | ||
| Los Angeles-Long Beach-Santa Ana | 28.9 | 29.5 |
| San Francisco-Oakland-Fremont | 14.6 | 22.7 |
| Sacramento-Arden-Arcade-Roseville | 7.2 | 10.5 |
| Riverside-San Bernardino-Ontario | 8.6 | 6.3 |
| San Diego-Carlsbad-San Marcos | 4.4 | 7.3 |
| Other | 33.7 | 21.9 |
| Non-MSA (i.e. Rural) | 2.6 | 1.6 |
Source: Authors’ analysis of Blue Shield of California and Truven MarketScan databases, 2014–2016.
Notes: MSA is metropolitan statistical area. The 10 most common of 31 Elixhauser comorbidities are shown. Comorbidities were assessed using diagnoses from any claims January-June 2014, prior to the pre-intervention period. For all comparisons, p<0.001.
Abbreviations: metropolitan statistical area (MSA), standard deviation (SD)
All comparisons p<0.001
The most common 10 of 31 Elixhauser comorbidities shown. Comorbidities assessed using diagnoses from any claims January-June 2014, prior to the pre-intervention period.
Statistical Analysis
For our multivariable regression models, we used a difference-in-differences study design at the individual-month level. In the intervention and control groups, we compared changes in individual-level outcomes from the pre-intervention period (July 2014-June 2015) to the post-intervention period (July 2015-December 2016).
To estimate the key outcome of the differential change in the intervention group compared to the control group in the post-intervention period, we used an interaction term of an indicator for intervention group membership and an indicator for the post-intervention period. These regression models adjusted for patient characteristics, MSA fixed effects, and year and month fixed effects to control for trends over time and seasonal variation. These regression models estimate the change in each outcome attributable to the prior authorization in the intervention population compared to what could be expected in the absence of this intervention, holding region constant and adjusting for patient characteristics. We tested for the key assumption that trends in the intervention and control groups did not diverge meaningfully in the pre-intervention group. We clustered standard errors at the individual level to account for correlation in individual-level outcomes over time.
To compare disenrollment trends, we used Cox proportional hazard regression (survival analysis) to compare time to disenrollment for any cause between the full intervention population and those with ER opioid prescriptions in the baseline period.
We performed additional sensitivity analyses to assess the robustness of our findings. First, to confirm that there was no change in the Blue Shield population impacting all prescriptions, we conducted a falsification test in which we examined trends in two drug classes unrelated to the prior authorization policy – statins and selective serotonin reuptake inhibitors. Second, we examined trends in the use of gabapentinoids, a potential alternative drug class for chronic pain management, to assess whether clinicians used these drugs as a substitute for ER opioids. Last, we examined whether our decision in the main analysis to exclude buprenorphine and methadone, which can be used for chronic pain management or opioid use disorder treatment, affected our findings. To do so, we replicated our ER opioid analyses including these medications.
Analyses were performed in Stata (v. 15). The 95% confidence interval around reported estimates reflects 0.025 in each tail or P≤0.05.
Limitations
Our study has several limitations. First, our follow-up period was limited to 12 months, so it is possible that the changes we assess may not persist or could change over time. For example, patients may resort to extended release opioid use after an initial drop. Second, our analysis focused on a commercially insured population in California. Our results may not generalize to other settings. Third, our analysis centered on a single formulary change in the context of multiple interventions around opioid prescribing both in Blue Shield and across California. For example, 3 months after implementation of the ER oxycodone prior authorization requirement, Blue Shield added prior authorization requirements for other less commonly used extended release opioids (details in Appendix).22 Fifth, we lack data on formulary changes affecting our control population of enrollees in other California insurance plans. To the degree they were also implementing initiatives to address extended release opioid prescribing, we may be underestimating the impact of the Blue Shield intervention. Sixth, because the commercial insurers available in the MarketScan database are masked, we cannot estimate the overlap, if any, between the Blue Shield and the MarketScan samples. To the extent that the samples, this may result in underestimation of the impact of the intervention. Last, we were unable to assess patient health outcomes such as opioid overdose events. Future analyses examining the impact of opioid formulary changes would benefit from the ability to link to statewide databases of opioid overdose events to more robustly assess the potential of unintended consequences from policy changes.
RESULTS
There were no statistically significant pre-intervention differences in trends of monthly rates of ER opioid fills between the intervention and control populations (Appendix Exhibit 3).22 For total MME-gram and short-acting opioid MME-gram outcomes, in the pre-intervention period there were diverging trends with slight increases in the intervention group compared to the control group that were statistically significant but small in magnitude.
Our final study sample contained 471,337 and 409,268 individuals in the intervention (Blue Shield of California) and control (MarketScan enrollees in California) populations, respectively. There were multiple statistically significant baseline differences between the intervention and control groups (Exhibit 1).Compared to the control population, the intervention population was on average slightly older (45.6 vs 43.2 years) and had more comorbidities (mean count 0.95 vs. 0.84).
Changes in opioid prescribing after intervention
New ER opioid starts dropped significantly in the intervention group after the intervention onset. (center panel, Exhibit 2 and Appendix Exhibit 4).22 Overall, new ER opioid starts per 10,000 members decreased by 1.28 in the intervention group compared to 0.30 in the control group (Exhibit 3). After adjustment, this represented a differential change of -0.97 new starts per 10,000 members (p<0.001), a 36% decrease in overall new ER opioid starts over the study period relative to the control group. This change was almost exclusively attributable to a decrease in new ER oxycodone starts (illustrated in Exhibit 2). New ER oxycodone starts dropped 63% from 1.51 to 0.13 new starts per 10,000 members (p<0.001 for differential change). There was no statistically significant change in new starts for non-oxycodone ER opioids (p=0.80).
Exhibit 2: Unadjusted Trends in New Extended Release Opioid Starts Relative to Implementation of Prior Authorization.
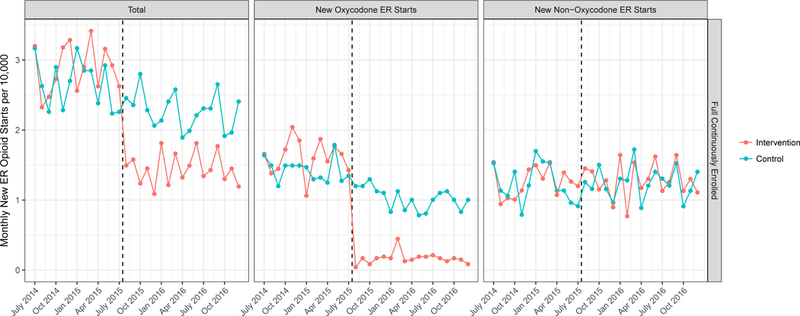
Source: Authors’ analysis of Blue Shield of California and Truven MarketScan databases, 2014–2016.
Notes: Unadjusted trends in monthly new extended release opioid fill rates per 10,000 persons divided into all ER opioids (right panel), oxycodone ER opioids (center panel) and non-oxycodone ER opioids (right panel). “New” prescribing is defined as a new fill among those without any opioid use in the prior 6 months. Trends are shown for the intervention population (Blue Shield of California, orange line) and the control population (MarketScan, blue line). The dashed black line indicates the time of oxycodone ER prior authorization implementation, July 2015. ER is extended release.
Exhibit 3:
Estimates from difference-in-difference analysis showing the impact of prior authorization on opioid prescribing
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Monthly Rates per 10,000 Members |
Pre- period |
Post- period |
Pre to Post Change |
DiD | Relative Change vs. Blue Shield Pre- Period |
p-value |
| New ER Opioid Starts | ||||||
| All ER Opioids | ||||||
| Blue Shield | 2.70 | 1.42 | −1.28 | −0.97 | −35.9%**** | <0.001 |
| MarketScan | 2.51 | 2.21 | −0.3 | − | ||
| Oxycodone-containing | ||||||
| Blue Shield | 1.51 | 0.13 | −1.38 | −0.95 | −62.9%**** | <0.001 |
| MarketScan | 1.31 | 0.88 | −0.43 | − | ||
| Non-oxycodone containing | ||||||
| Blue Shield | 1.19 | 1.28 | 0.09 | −0.02 | −1.7% | 0.804 |
| MarketScan | 1.21 | 1.32 | 0.11 | − | ||
| Monthly ER Opioid Fills | ||||||
| All ER Opioids | ||||||
| Blue Shield | 32.05 | 29.67 | −2.38 | −3.48 | −10.9%**** | <0.001 |
| MarketScan | 28.08 | 29.18 | 1.1 | − | ||
| Oxycodone-containing | ||||||
| Blue Shield | 15.41 | 11.55 | −3.86 | −3.90 | −25.3%**** | <0.001 |
| MarketScan | 11.40 | 11.45 | 0.05 | − | ||
| Non-oxycodone containing | ||||||
| Blue Shield | 16.64 | 18.12 | 1.48 | 0.42 | 2.5% | 0.385 |
| MarketScan | 16.67 | 17.73 | 1.06 | − | ||
| Monthly Short Acting Opioid Fills | ||||||
| All Short Acting Opioids | ||||||
| Blue Shield | 487.3 | 476.0 | −11.3 | 7.0 | 1.4%**** | 0.001 |
| MarketScan | 425.1 | 406.8 | −18.3 | − | ||
| Oxycodone-containing | ||||||
| Blue Shield | 77.5 | 85.0 | 7.5 | 1.9 | 2.5%* | 0.057 |
| MarketScan | 63.8 | 69.4 | 5.6 | − | ||
| Non-oxycodone containing | ||||||
| Blue Shield | 426.3 | 407.3 | −19 | 5.1 | 1.2%** | 0.014 |
| MarketScan | 374.4 | 350.3 | −24.1 | − | ||
| Total gram-MME Dispensed (all prescriptions) | ||||||
| All Opioids | ||||||
| Blue Shield | 558.3 | 544.2 | −14.1 | 0.2 | 0.04% | 0.97 |
| MarketScan | 448.8 | 434.6 | − | − | ||
| ER Opioids | ||||||
| Blue Shield | 144.55 | 127.25 | −17.3 | −6.91 | −4.8%* | 0.063 |
| MarketScan | 120.42 | 110.03 | −10.39 | |||
| Short-Acting Opioids | ||||||
| Blue Shield | 414.3 | 416.9 | 2.6 | 5.6 | 1.4% | 0.013 |
| MarketScan | 327.5 | 324.5 | −3.0 | - | ||
Source: Authors’ analysis of Blue Shield of California and Truven MarketScan databases, 2014–2016.
Abbreviations: extended release (ER), adjusted difference-in-differences estimate (DiD), morphine milligram equivalents (MME)
SIGNIFICANCE:
p < 0.10
p < 0.05
p < 0.01
p < 0.001
* Adjusted estimates show the differential change in prescribing rate between the intervention (Blue Shield of California) and control (MarketScan) groups after vs. before prior authorization implementation, adjusting for patient covariates, MSA fixed effects, and year and month fixed effects. ER is extended release.
SIGNIFICANCE:
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Similar to the trends seen in new ER opioid starts, monthly rates of any ER opioid prescription (new start or refills among patients previously on ER opioids) dropped by 2.38 per 10,000 members in the intervention group while increasing by 1.1 in the control group (Exhibit 4 and Appendix Exhibit 5).22 This was an adjusted differential change of -3.48 monthly ER opioid fills per 10,000 members (p<0.001, Exhibit 3), an 11% decrease over the study period relative to the control group.. The differential change in all ER opioid prescriptions (-3.48 per 10,000 members) was larger than the differential change in new ER opioid prescriptions (-0.97 per 10,000 members). Similar to trends in new ER starts, this trend was driven entirely by reductions in ER oxycodone-containing prescriptions. There was no statistically significant differential change in non-oxycodone ER opioid prescribing rates.
Exhibit 4: Unadjusted Trends in All Extended Release Opioid Starts Relative to Implementation of Prior Authorization.
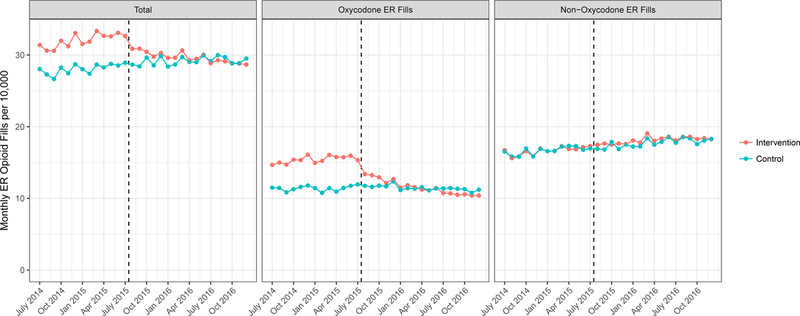
Source: Authors’ analysis of Blue Shield of California and Truven MarketScan databases, 2014–2016.
Unadjusted trends in overall monthly ER opioid fill rates per 10,000 persons divided into all ER opioids (right panel), oxycodone ER opioids (center panel) and non-oxycodone ER opioids (right panel). Trends are shown for the intervention population (Blue Shield of California, orange line) and the control population (MarketScan, blue line). The dashed black line indicates the time of oxycodone ER prior authorization implementation, July 2015. ER is extended release.
There was an offsetting 1.4% relative increase in the monthly rate of short-acting opioid fills associated with the intervention vs. control group (7.0 per 10,000 members, p=0.001, Exhibit 3 and Appendix Exhibit 6).22 There was no statistically significant differential change in the overall use of all opioids (gram-MMEs) in the intervention vs. control groups (Exhibit 3 and Appendix Exhibit 7).22
In sensitivity analyses, there was no significant relationship between the intervention and prescription rates of two medication classes unrelated to the prior authorization for statins (p=0.27) and selective serotonin reuptake inhibitors (p=0.21, Appendix Exhibits 8–9).22 We also observed no relationship between the intervention and prescribing rates of gabapentinoid medications, a potential non-opioid substitute for chronic pain management (p=0.35, Appendix Exhibits 8–9).22 There was minimal change in our results on ER opioid use when we included methadone and buprenorphine as possible ER opioids (Appendix Exhibit 9).22
There was no statistically significant difference in the rate of disenrollment from July 2014 to December 2016 for intervention group members on ER opioids in the baseline period (January-June 2014) compared to all members (Appendix Exhibit 10).22
DISCUSSION
We found that a prior authorization for ER oxycodone as one component of a larger opioid safety initiative resulted in a significant 36% relative reduction in new ER opioid starts and an 11% relative reduction in monthly rates of ER opioid prescribing. These effects were offset by a small increase in the rate of short acting opioid prescribing. To our knowledge, this evaluation is among the first studies of the effects of a statewide opioid formulary change on overall prescribing using a large, contemporaneous control group.17,18 In addition, this evaluation fills an evidence gap identified in a recent CDC systematic review, which called for controlled observational studies of system-level interventions for opioid prescribing.18 The study also extends the literature by examining outcomes that could reflect unintended consequences of reduced prescribing, such as insurance disenrollment or substitution towards alternative opioids.
There have been concerns that prior authorization may simply drive clinicians to another form of ER opioid prescriptions, but we did not observe any such effect. Instead, we observed an offsetting increase in the rate of short-acting opioid prescriptions. We also observed a lack of change in overall MME for opioids.
Given that the magnitude of the increase in prescribing of short-acting opioids was small and there was no clear inflection associated with the time of prior authorization, it is possible that the increase in short-acting opioids may be explained by other differences in the intervention and control populations. The static level of overall MME for opioids may reflect the fact that the pre-authorization policy only targeted new ER opioid starts, and the drop in new ER opioid use will take some time to decrease total use. Our results imply that the administrative hurdle of a prior authorization may be enough to prevent physicians from prescribing a new ER opioid, though they may instead use shorter-acting opioids.
This study raises the larger question of whether prior authorization is an effective strategy for promoting safer opioid prescribing. The impact of the intervention for decreasing new ER starts for the targeted drug is fairly clear. However, this came at the cost of potentially increasing short-acting opioid prescriptions as well as creating an administrative burden for prescribers. Clinically, ER opioid use for non-cancer pain is associated with a high risk of overdose and largely discouraged by guidelines,7 so from that perspective the prior authorization was effective, even if offset by other prescribing. However, if the most important goal of a prior authorization is to reduce total opioid use, then targeting individual drugs as a sole strategy may not be successful given the ease of opioid substitution. The design of the Narcotic Safety Initiative acknowledges this limitation of prior authorization by combining multiple approaches to promote safer prescribing, but longer follow-up will be necessary to assess the effect of the collective set of interventions over time.
There remains the possibility of other unintended consequences from a policy that restricts access to ER opioids, such as seeking illicit opioids. Prior work has identified an increase in heroin overdoses when OxyContin was reformulated into an abuse-deterrent formulation in 2011.11,12 Though we were unable to assess patient outcomes such as opioid overdoses or untreated pain, future research into similar policies should focus on these outcomes for a more complete picture of potentially unintended consequences.
In conclusion, the introduction of a new prior authorization requirement as part of a multi-faceted insurer opioid safety initiative was associated with a substantial decrease in ER opioid prescribing without significant substitution towards alternative ER opioids. This is encouraging evidence that health insurers can play a meaningful role in promoting appropriate opioid prescribing around high-risk ER opioids. However, overall opioid use did not drop substantially due to an offsetting increase in short acting opioid use. Though the new prior authorization led to some changes in behavior, our results show that formulary changes focused on ER opioids alone are likely insufficient to decrease total opioid prescribing. Creating systemic change with lower rates of safer prescribing likely requires a series of complementary policies implemented over a sustained time period.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute on Aging, Blue Shield of California and the California Health Care Foundation.
Contributor Information
Michael L. Barnett, the Department of Health Care Policy and Management, Harvard T. H. Chan School of Public Health, Boston, Massachusetts;; Division of General Internal Medicine and Primary Care, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts;
Andrew R. Olenski, Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts;.
N. Marcus Thygeson, Blue Shield of California, San Francisco, CA, Boston, Massachusetts;.
Denis Ishisaka, Blue Shield of California, San Francisco, CA, Boston, Massachusetts, Boston, Massachusetts;.
Salina Wong, Blue Shield of California, San Francisco, CA, Boston, Massachusetts, Boston, Massachusetts;.
Anupam B. Jena, Department of Health Care Policy, Harvard Medical School; Department of Medicine, Massachusetts General Hospital; National Bureau of Economic Research, Cambridge, Massachusetts; Department of Medicine, Massachusetts General Hospital.
Ateev Mehrotra, Department of Health Care Policy, Harvard Medical School; Department of Medicine, Beth Israel Deaconess Medical Center.
ENDNOTES
- 1.Quinones S Dreamland: The True Tale of America’s Opiate Epidemic. Bloomsbury Publishing; USA; 2015. [Google Scholar]
- 2.Prescription Opioid Overdose Data | Drug Overdose | CDC Injury Center; [Internet]. [cited 2016. August 1];Available from: http://www.cdc.gov/drugoverdose/data/overdose.html [Google Scholar]
- 3.CDC Grand Rounds: Prescription Drug Overdoses — a U.S. Epidemic [Internet]. [cited 2016. August 1];Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.htm [PubMed]
- 4.Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 2015;(36):559–74. [DOI] [PubMed] [Google Scholar]
- 5.National Institute on Drug Abuse. America’s Addiction to Opioids: Heroin and Prescription Drug Abuse [Internet]. 2014 [cited 2018. June 7];Available from: https://web.archive.org/web/20180526233121/https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse
- 6.Centers for Disease Control and Prevention. Mortality in the United States, 2016 [Internet]. 2017 [cited 2018. February 8];Available from: https://www.cdc.gov/nchs/products/databriefs/db293.htm
- 7.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain. JAMA 2016;15(315):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;2(152):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;7(171):686–91. [DOI] [PubMed] [Google Scholar]
- 10.Miller M, Barber CW, Leatherman S, Fonda J, Hermos JA, Cho K, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med 2015;4(175):608–15. [DOI] [PubMed] [Google Scholar]
- 11.Cicero TJ, Ellis MS, Surratt HL. Effect of Abuse-Deterrent Formulation of OxyContin. N Engl J Med 2012;2(367):187–9. [DOI] [PubMed] [Google Scholar]
- 12.Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Rates of Opioid Dispensing and Overdose After Introduction of Abuse-Deterrent Extended-Release Oxycodone and Withdrawal of Propoxyphene. JAMA Intern Med 2015;6(175):978–87. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med 2004;21(351):2187–94. [DOI] [PubMed] [Google Scholar]
- 14.Ross JS, Jackevicius C, Krumholz HM, Ridgeway J, Montori VM, Alexander GC, et al. State Medicaid programs did not make use of prior authorization to promote safer prescribing after rosiglitazone warning. Health Aff Proj Hope 2012;1(31):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starner CI, Fenrick B, Coleman J, Wickersham P, Gleason PP. Rosiglitazone prior authorization safety policy: a cohort study. J Manag Care Pharm JMCP 2012;3(18):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuels EA, Ross JS, Dhruva SS. Medicare Formulary Coverage Restrictions for Prescription Opioids, 2006 to 2015. Ann Intern Med 2017;12(167):895–6. [DOI] [PubMed] [Google Scholar]
- 17.García MC. Declines in Opioid Prescribing After a Private Insurer Policy Change — Massachusetts, 2011–2015. MMWR Morb Mortal Wkly Rep [Internet] 2016 [cited 2018. June 4];(65). Available from: https://www.cdc.gov/mmwr/volumes/65/wr/mm6541a1.htm [DOI] [PubMed] [Google Scholar]
- 18.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend 2014;(145):34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy GP. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep [Internet] 2017 [cited 2017. July 7];(66). Available from: https://www.facebook.com/cdcmmwr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J 2011;(8):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truven Health Analytics. The Truven Health MarketScan Databases for Health Services Researchers [Internet]. 2017 [cited 2018. June 7];Available from: https://truvenhealth.com/portals/0/assets/2017_MarketScan_Databases_Health_Services_Researchers.pdf
- 22.Note: To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 23.Barnett ML, Olenski AR, Jena AB. Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use. N Engl J Med 2017;7(376):663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ 2014;(348):g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jena AB, Goldman D, Karaca-Mandic P. Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med 2016;7(176):990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Pharmacopeial Convention [Internet]. [cited 2017. Jun 22];Available from: http://www.usp.org/
- 27.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A Modification of the Elixhauser Comorbidity Measures into a Point System for Hospital Death Using Administrative Data. Med Care 2009;6(47):626–33. [DOI] [PubMed] [Google Scholar]
- 28.2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas [Internet]. Fed. Regist.2010 [cited 2017. November 29];Available from: https://www.federalregister.gov/documents/2010/06/28/2010-15605/2010-standards-for-delineating-metropolitan-and-micropolitan-statistical-areas [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


