Abstract
Experimental modelling to identify specific inhalation hazards for nanomaterials has in the main focussed on in vivo approaches. However, these models suffer from uncertainties surrounding species specific differences and cellular targets for biological response. In terms of pulmonary exposure, approaches which combine ‘inhalation-like’ nanoparticulate aerosol deposition with relevant human cell and tissue air-liquid interface cultures are considered an important complement to in vivo work. In this study, we utilized such a model system to build on previous results from in vivo exposures, which highlighted the small airway epithelium as a target for silver nanoparticle (AgNP) deposition. RNA-SEQ was used to characterise alterations in mRNA and miRNA within the lung. Organotypic reconstituted 3D human primary small airway epithelial cell cultures (SmallAir) were exposed to the same spark generated AgNP and at the same dose used in vivo, in an aerosol-exposure air-liquid-interface (AE-ALI) system. Adverse effects were characterised using lactate, LDH release and alterations in mRNA and miRNA. Modest toxicological effects were paralleled by significant regulation in gene expression, reflective mainly of specific inflammatory events. Importantly, there was a level of concordance between gene expression changes observed in vitro and in vivo. We also observed a significant correlation between AgNP and mass equivalent silver ion (Ag+) induced transcriptional changes in SmallAir cultures. In addition to key mechanistic information relevant for our understanding of the potential health risks associated with AgNP inhalation exposure, this work further highlights the small airway epithelium as an important target for adverse effects.
Keywords: Small Airway, Inflammation, Genomics, Mechanistic Toxicology
INTRODUCTION
Respiratory hazards from nanomaterials are an important consideration in circumstances of inadvertent inhalation exposure. As a consequence of their anti-microbial activity, among other properties, silver nanoparticles (AgNPs) are widely used and increasingly incorporated into consumer products (Piccinno et al., 2012, Wang et al., 2014). An understanding of how AgNPs may exert adverse effects on the lung after inhalation has derived mainly from studies of in vivo and in vitro exposure models. Rodent models have been used extensively for toxicity characterisation and distribution assessment. One inhalation study in rats found that sub-chronic exposures to AgNPs across 90 days produced histological changes within the lung, associated with mixed inflammatory cell infiltrate and chronic alveolar inflammation (Sung et al., 2009). Additional sub-chronic studies have observed similar effects, including those on lung function (Ji et al., 2007, Sung et al., 2008, Song et al., 2013). Shorter term inhalation exposures have also revealed that smaller sized AgNPs induce significantly more pulmonary inflammation than larger sized particles (Braakhuis et al., 2014). This difference was attributed in part to increased alveolar dose deposition and dissolution rate of the smaller particles producing increased levels of ionic silver. More recently, we set out to investigate the acute effects of AgNPs inhalation in more detail (Seiffert et al., 2016). Similar to the previous study, we found increased neutrophilia as well as increased inflammatory and tissue injury markers 1 day after exposure, which were diminished at 7 days. We also found dose dependent AgNP accumulation within macrophages. This localisation has been suggested to initiate inflammatory events as a consequence of particle accumulation (Mukherjee et al., 2014).
Growing evidence suggests that the predominant mode of toxicity for AgNP is a consequence of the magnitude of soluble Ag ion release and their interaction with biological systems (Hadrup and Lam, 2014). It is suggested that lysosomal degradation of internalised AgNPs is an important control point for Ag+ release (Gliga et al., 2014, Jiang et al., 2014, Setyawati et al., 2014, Arai et al., 2015). Additional mechanisms have been suggested, including the production of reactive oxygen species (ROS) as a consequence of mitochondrial damage (AshaRani et al., 2009, Foldbjerg et al., 2011). This mode of injury has also been suggested to cause DNA damage resulting in cell cycle arrest at the G2M phase (AshaRani et al., 2009, Lee et al., 2011, Asharani et al., 2012). Other studies however have proposed ROS independent effects on cellular injury and cell cycle arrest (Chairuangkitti et al., 2013, Sweeney et al., 2016). Investigations are still needed to determine the full profile of how AgNPs exert their effects.
In addition to macrophage NP phagocytosis and activation, airway epithelial cells have also been proposed as a primary site for mediating AgNP adverse effects within the lung. Much of this investigation has focussed on the bronchial and alveolar compartments (Foldbjerg et al., 2012, Cronholm et al., 2013, Braakhuis et al., 2014). Interestingly however, little attention has been devoted to study of the bronchiolar compartment composing the small airways. This is despite evidence that inhalation of AgNP results in deposition in this region. Anderson and colleagues demonstrated Ag deposition in the terminal bronchioles localised to club cells and the sub-epithelial layer after AgNP instillation (Anderson et al., 2015b). Further observations revealed that inhalation of AgNP also resulted in Ag localisation mainly to the terminal bronchiole / alveolar duct region (Anderson et al., 2015a). Ag staining on the luminal surface and the sub-epithelial connective tissue of the terminal bronchiole was also observed after AgNP inhalation (Seiffert et al., 2016). The suggestion is that clearance of AgNP through epithelial uptake may point towards a mechanism through which these cells receive doses of Ag capable of eliciting adverse effects and directing adaptive tissue responses, such as the initiation of inflammation.
It was the aim of this current study to investigate the contribution of the small airway epithelium to pulmonary responses to inhaled AgNPs. Initial steps involved characterisation of global transcriptomic responses within the rat lung after inhalation of AgNPs. Cataloguing both mRNA and microRNA (miRNA) allows for a comprehensive analysis of both adverse pathology and mechanisms of action of AgNP pulmonary effects. This analysis was carried out on lung tissue after bronchoalveolar lavage to focus as much as possible on the resident epithelial compartments. Following on from this we compared these tissue responses to small airway epithelial cells exposed to the same spark generated AgNPs across the same dose range using an aerosol exposure – air liquid interface (AE-ALI) deposition device. The cells comprised a newly developed model of differentiated primary human small airway epithelial cells displaying organotypic 3D structure and club cells typical of small airways found in humans. Correlation between in vivo and in vitro model transcriptomic responses was observed.
METHODS
AgNP Generation and Characterisation
AgNP aerosols were produced using a spark generator (DNP 4000, Palas, Karlsruhe, Germany) by the homogeneous nucleation of vapor produced between two electrodes (5mm x 1mm silver wire; Premion™ 99.999 % purity, Alfa Aesar™, Heysham, UK) in an inert argon atmosphere. The rate of primary particle production and final size was dependent on the sparking frequency (90–300 Hz). The particles were passed through a krypon-85 charge neutralizer (Model 3077A, TSI Incorporated, Shoreview, MN, USA) prior to dilution and experimental exposure. A condensation particle counter (CPC model 3775, TSI Inc., Shoreview, MN, USA) continuously monitored the aerosol particle number concentration during exposures and aerosol mass concentrations were determined gravimetrically using Pallflex® emfab™ filters (Pall Life Sciences, Ann Arbor, MI, USA) with the aerosol drawn at 2 L min−1, the results are presented in Table 1. The average number-weighted aerosol particle size distribution was determined using a scanning mobility particle sizer (SMPS; model 3936N76, TSI Inc., Shoreview, MN, USA) and for in vitro exposures results are displayed in supplementary Figure 1A. The shape of the aerosol particles was determined with high resolution transmission electron microscopy (TEM) (JEOL 3000F, JEOL Inc., Tokyo, Japan). A representative TEM image of particles is shown in Supplementary Figure 1B. Detailed AgNP characterisation results for in vivo exposures are previously reported (Seiffert et al., 2016).
Table 1.
Parameters for in vivo and in vitro aerosol exposures.
| Group | Exposure Length |
Mass conc. (μg/m3) |
Number conc. (#/cm3) |
CMD (nm) |
GS D |
Estimated Dose (ng/cm2) |
Estimated Dose (#/μm2) |
|
|---|---|---|---|---|---|---|---|---|
| In vivo | 3hours/day for 4 days | 670 ± 49 | 4.6 ± 0.7 X 107 | 14.1 ± 2.3 | 1.58 | 4.9 | 3.3 | |
| In vitro | Low | 7 min | 37.4* | 1.2 ± 0.3 X 106 | 16.5 ± 1.1 | 1.33 | 1.5 | 0.4 |
| Medium | 20 min | 37.4* | 1.0 ± 0.3 X 106 | 16.0 ± 0.9 | 1.36 | 4.4 | 1.3 | |
| High | 60 min | 37.4* | 1.1 ± 0.1 X 106 | 15.8 ± 0.2 | 1.36 | 13.2 | 3.8 |
The mass concentration is an average for all the in vitro exposures. (CMD; Count Median Diameter, GSD; Geometric Standard Deviation)
In vivo AgNP exposure
The lung tissues processed for transcriptomic analysis were collected from a previous exposure study (Seiffert et al., 2016). Briefly, male Sprague–Dawley rats (10–12 weeks, 250–320 g) (Harlan, UK) were exposed to nose only aerosols of AgNPs under UK home office approval (Project Licence number: PPL 70/7581) with exposure set up and schematic as previously described (Seiffert et al., 2016). Animals were exposed for 3 hrs on four consecutive days to AgNPs with an estimated lung deposition of 4.9 ng/cm2 (Table 1). Animals were sacrificed at 1 day or 7 days. Control animals were exposed to air using the same system.
In vitro AgNP exposure
Organotypic reconstituted primary human small airway epithelial cell cultures (SmallAir™), differentiated and maintained at an air liquid interface on 6.5mm diameter tissue culture inserts were obtained from Epithelix (Geneva, Switzerland). Cultures were maintained at 37°C in a 5% CO2 humidified atmosphere with basolateral media (SmallAir™ culture media # EP65SA) exchange and apical washing every 2–3 days. 5 different donors without any reported pathology were used for experimental exposures. Cultures were exposed to AgNPs using a Cultex® RFS exposure module (Cultex® Laboratories GmbH, Hannover, Germany) with an EDD (Electrical Deposition Device) to enhance the particle deposition efficiency. With temperature at 37°C, Oxygen, CO2, Nitrogen, and relative humidity were maintained at 20%, 5%, 75% and 80% respectively. A schematic of the aerosol exposure – air liquid interface (AE-ALI) set up is shown in Supplementary Figure 2. Exposures were designed to provide deposition doses in a range including that estimated for our in vivo rat exposures (Seiffert et al., 2016) to the same spark generated material. This resulted in exposure times of 7, 20 and 60 minutes. Details of how these exposure doses and therefore durations were estimated, including the calculation method can be found in the supplementary methods with the results summarised in Table 1. ALI cultures of SmallAir were also exposed to solutions of Ag+ in the form of AgNO3 (Sigma Aldrich, Dorset, UK). Ag mass dose equivalents to AgNP exposures were applied to the apical surface of the cells in 30μl volume of cell culture media. This minimal volume, recommended by SmallAir manufacturer to have little impact on ALI differentiation status, did not have any significant effects on toxicological endpoints when compared to air alone exposure. After exposure, ALI cultures were returned to fresh culture media under normal culture conditions for a further 6 or 24 hours, after which, samples were processed for gene expression and toxicity assessment as detailed in Figure 4.
Figure 4: Schematic representation of small airway in vitro cell exposure and toxicological assessment.
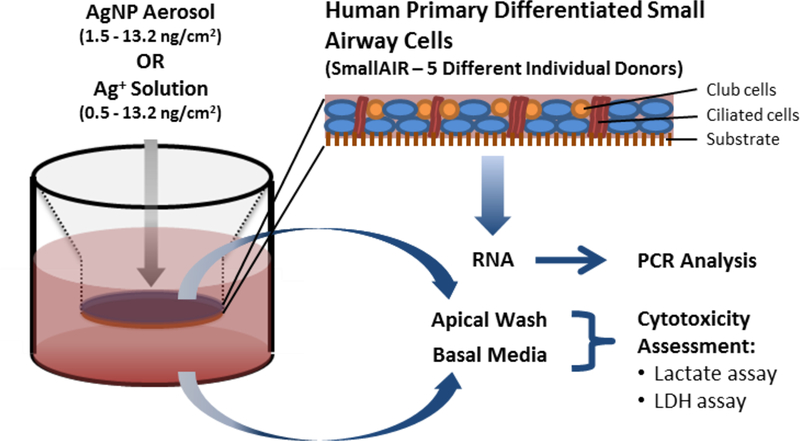
Endpoint Analysis was carried out 6hrs and 24hrs post exposure.
In vivo rat inhalation RNA-SEQ Analysis
After removal of bronchoalveolar lavage (BAL) cells through consecutive washes in PBS, total RNA was extracted from whole lung tissue using the miRNeasy Mini Kit (QIAGEN). RNA quality was determined using an Agilent 2100 Bioanalyser and those samples with RIN above 8.0 were used for library preparation. For mRNA sequencing analysis samples were processed using the TruSeq™ RNA sample preparation method (Illumina, San Diego, USA) as previously described (Meldrum et al., 2016). Processing and sequencing was carried out in conjunction with BGI Tech Solutions (Hong Kong) using 101PE sequencing on the Illumina HiSeq™ 2000 platform. Raw sequence data was initially processed to remove adaptor sequences, contamination and low-quality reads. 20 million clean reads for each sample was obtained and brought forward for further processing using CLC Genomics Workbench software (CLCBIO, Aarhus, Denmark). Paired end reads were trimmed to remove remaining adapter and other variable sequences followed by annotation using the Rnor_5.0 (Ensembl release 79) rat reference genome build. For normalization across gene and sample reads, RPKM (Reads Per Kilobase per Million mapped reads) values > 0.1 in at least one treatment condition were selected for differential expression analysis. Further comparative analysis and visualization of differentially regulated transcripts was carried out using Qlucore Omics Explorer software (Qlucore, Lund, Sweden). Pathway analysis of differently regulated transcripts was carried out using ingenuity pathway analysis (IPA) (Ingenuity Systems, http://www.ingenuity.com) software (Redwood, CA, USA). For microRNA sequencing analysis, small RNA between 18 and 30 nucleotides was fractionated and processed using TruSeq™ small RNA library preparation followed by 101 PE sequencing. Raw data was processed including adaptor removal and annotation using CLC Genomics Workbench software with 10 million clean reads brought forward. Annotation was carried out against the rat miRBase – Release 20 reference dataset.
ICP-MS measurements
Lung tissue was fixed in formalin and 5μm sections were analysed for 13C and 107Ag spatial distribution by laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) using a New Wave Research NWR213 laser ablation system (Electro Scientific Industries, Portland, Oregon, USA) linked to an iCAP Q ICPMS (Thermo Fisher Scientific, Hemel Hempstead, UK). AgNP deposition quantification on transwell inserts after AE-ALI exposure in the absence of cells was carried out by acid digestion of the culture membrane prior to iCAP Q ICP-MS analysis. In addition to 107Ag, 193Ir content was used as an internal standard. Further details can be found in the supplementary methods.
In vitro cytotoxicity assessment
The metabolite lactate was measured in the cell culture media on the basolateral side as well as from a media wash (100μl/Transwell) sample of the apical side of the cultures after treatment. This was used as an indicator of cell stress and altered metabolic activity (Limonciel et al., 2011). Further details of how this assay was performed can be found in the supplementary methods. Levels of lactate dehydrogenase in these media samples were also assessed as an indicator of compromised membrane activity and direct cytotoxicity. This was carried out using a kit from Roche Diagnostics as per manufacturer’s instructions.
In vitro PCR analysis
Total RNA was isolated using the miRNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. For mRNA, cDNA was synthesised from 1μg RNA using the QuantiTect Reverse Transcription Kit (QIAGEN). RT-PCR reaction mixtures contained 1X Fast SYBR® Green Master Mix (Life Technologies), primer pairs at a concentration of 200 nM and 2 ng of cDNA in 20 μl reaction volume. Primers were designed using Primer3 software and obtained from Integrated DNA Technologies. Primer Sequences can be found in supplementary table 1. Human HPRT1 levels were used to normalise expression data using the ΔΔCT method. No signals were detected in no-template controls. For miRNA, reverse transcription was carried out using a TaqMan MicroRNA reverse transcription kit (Applied Biosystems) according to the manufacturer’s protocol. hsa-miR-146b, hsa-miR-224, hsa-miR-1298–3p, and hsa-miR-21 were detected using TaqMan MicroRNA assays (Applied Biosystems). Expression levels were normalized to RNU6B. Standard 40 cycle PCR reactions were used and fluorescent detection was performed using the QuantStudio® 6 Flex Real-Time PCR System (Applied Biosystems). Genes were selected for analysis in vitro based on those which were differentially regulated in vivo by AgNP. In total 50 genes and 4 miRNAs were chosen to cover the most highly regulated transcripts and miRNAs as well as the most prominent pathways identified from RNA-SEQ analysis. miRNAs were selected also on the basis of comparison with IPA regulator analysis and overlap with miRNA-SEQ results (Supplementary Figure 4).
Statistical analysis
For in vitro exposure on HPSAE cultures, all data for cytotoxicity assays and PCR analysis is presented as mean +/− standard error of the mean (S.E.M.) for 5 different donors. Statistical significance was evaluated based on ratio paired t-test unless otherwise stated.
RESULTS
Lung transcriptomic profiles after AgNP inhalation.
To establish the degree to which lung tissue was impacted by AgNP exposure over the short term we examined global mRNA and miRNA levels using RNA-SEQ. This type of analysis allows for the capture of global transcriptional changes including miRNAs, whose function is to modulate transcriptional events, in order to provide insight into the most relevant cellular and tissue responses underlying the response to toxicological insult. Initial bioinformatic analysis was carried to identify those statistically relevant transcripts, which can be used to provide confident assessment of tissue injury and resolution responses. Importantly, this type of global analysis is used to provide detailed mechanistic information into how toxicological insult occurs and the cellular and molecular level. Hierarchical clustering of 17,804 mRNA transcripts across groups (N=6 animals per group) revealed 1 outlier at Day 7 control (Air) exposure, which was removed. Differential expression criteria of 1.5 fold change and a statistical p value of 0.005 (ANOVA) was applied and resulted in 493 transcripts at day 1 and 97 transcripts at day 7. PCA analysis of these transcripts (Figure 1A) demonstrates separation of AgNP treatment groups with control air exposure groups clustering together. Hierarchical clustering of this data visualised as a heatmap (Figure 1B) demonstrates substantially more up regulated than down regulated transcripts with AgNP treatment. The top 20 up regulated transcripts at day 1 are listed (Figure 1C) and can be broadly characterised as part of inflammatory processes. In addition to the immune cell recruitment signals within these top 20, there was a large representation of genes involved in regulating type II inflammation. The most significant pathways associated with all 493 differentially regulated transcripts were involved in regulating inflammatory processes (Figure 1D). Also prominent were pathways involved in DNA damage and cell cycle regulation. A full list of differentially expressed transcripts can be found in supplementary Figure 3. Examination of transcripts for consistent regulation across day 1 and day 7 post exposure, revealed 16 transcripts including Mt1a, Chia, Duox1 and CD68. This would indicate a persistent response to AgNPs within the lung involving regulation of metal response pathways, oxidative stress and macrophage activity. For miRNA expression analysis small RNA sequences were annotated to 390 known miRNAs. Hierarchical clustering of miRNA revealed an outlier within the Day 7 control (Air) group, which was removed from further processing. PCA analysis of these miRNA (Figure 2A) was used to select for those miRNA that separate treatment groups based on ANOVA, using a false discovery rate of 0.05 (p value (0.001)) (Figure 2A). This resulted in 12 miRNA sequences and similar to mRNA transcript analysis, hierarchical clustering visualised as a heatmap revealed more upregulated miRNA with AgNP treatment than downregulated (Figure 2B). The magnitude of change induced by AgNP was below 3 fold (Figure 2C).
Figure 1: AgNP aerosol exposure alters rat lung mRNA expression.
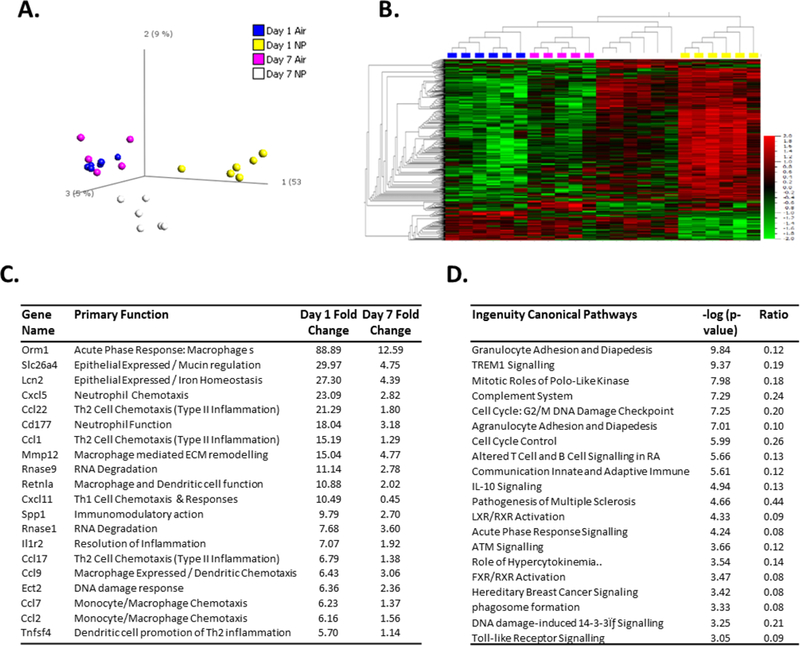
Sprague Dawley rats (n=5–6 per group) were exposed to control gas (Air) or AgNP Aerosol (4.9 ng/cm2) (NP). Bronchoalveolar lavage was performed and lung tissue was removed for mRNA expression analysis by RNA-SEQ, 1 and 7 days post exposure. PCA analysis (A) and hierarchical clustering (B) were used to visualise patterns of differentially expressed transcripts. The most highly upregulated transcripts by AgNP are displayed as fold over control (C) and ordered according to Day 1 regulation. Pathway analysis of differentially regulated transcripts by AgNP (Day 1 and 7 combined) is displayed as a function of significant association (D).
Figure 2: AgNP aerosol exposure alters rat lung miRNA expression.
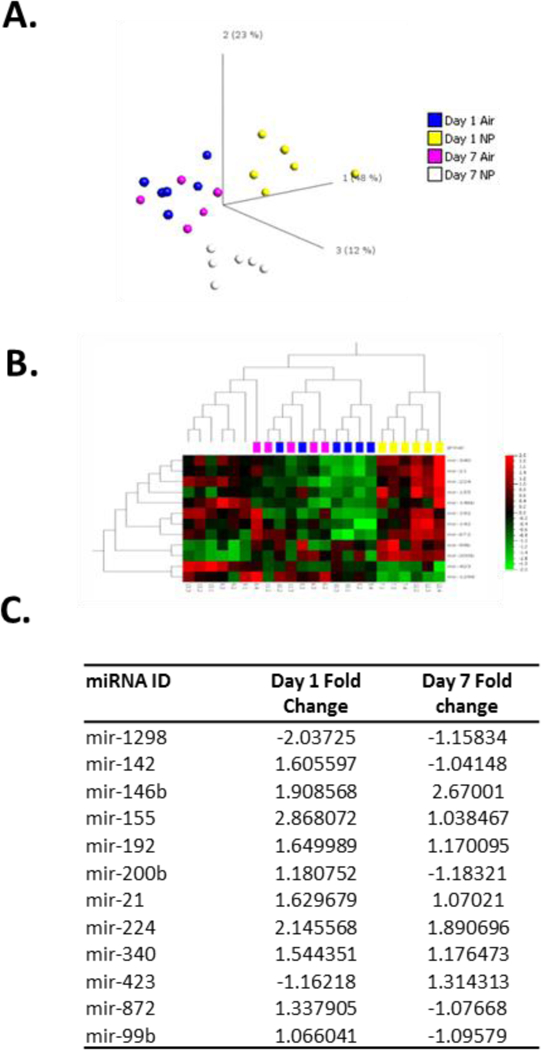
Sprague Dawley rats (n=5–6 per group) were exposed to control gas (Air) or AgNP Aerosol (4.9 ng/cm2) (NP). Bronchoalveolar lavage was performed and lung tissue was removed for miRNA expression analysis by RNA-SEQ, 1 and 7 days post exposure. PCA analysis (A) and hierarchical clustering (B) were used to visualise patterns of differentially expressed miRNAs. Details of AgNP induced fold expression changes for miRNAs are also displayed (C).
Analysis of Ag levels within lung
To evaluate levels of Ag distribution within the distal rat lung one day after inhalation of AgNPs, laser ablation ICP-MS was used. Initial observations revealed that the small airway bronchiolar sized airways appeared to display higher levels of 107Ag than surrounding alveolar structures (Figure 3A). Quantification of 107Ag content normalised to 13C within the airway walls confirmed higher levels within the small airway bronchiolar structures when compared to alveolar walls (Figure 3B).
Figure 3: Analysis of 107Ag levels with rat lung after AgNP exposure.
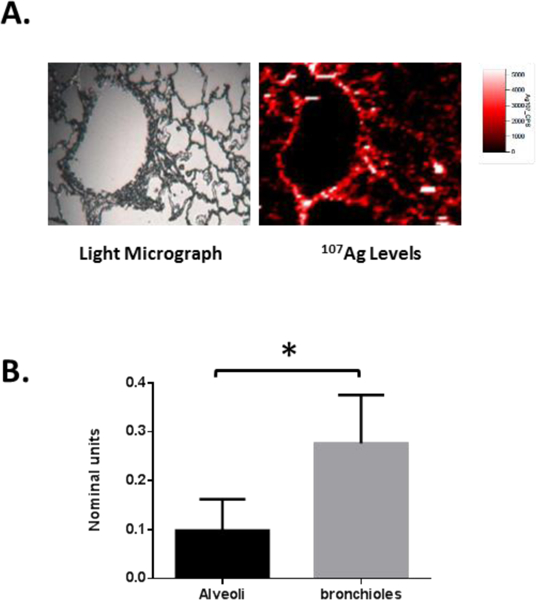
After AgNP exposure, lung tissue was fixed and processed for laser ablation ICP-MS assessment of 107Ag levels. Representative light microscopy image (A; left panel) and associated map of spatial distribution of 107Ag (A; right panel) for the distal lung. Quantification of airway wall 107Ag as a proportion of 13C content was carried out for both bronchiolar and alveolar structures for 4 separate airway structure types in each animal (n=3 animals) (B). Results are expressed as mean ± SEM normalised units and statistical analysis performed by t-test (* p<0.05).
Small Airway Epithelium exposure to AgNP aerosol in vitro
SmallAir epithelial cultures are organotypic differentiated cultures of human primary small airway epithelial cells (Huang et al., 2017). These cultures are maintained at the air liquid interface and comprise ciliated, secretory as well as basal cells within a pseudostratified structure and express CC10, a marker of small airway club cells. Considering this in vitro culture as a close representative of the human small airway epithelium in vivo, we investigated the effects of AgNP aerosol exposure using an air liquid interface particle deposition device. In order to directly compare effects with our in vivo inhalation observations we used the same spark generated material for our in vitro ALI exposures. The size of the AgNPs used for in vitro exposure was slightly larger than used for in vivo exposure (~16nm v 14nm) (Table 1). We used three different exposure times (7–60mins) to the AgNP aerosol to provide a range of dose estimates for both mass and particle number that would cover the dose estimate for in vivo lung exposure (Table 1). In addition to estimates of in vitro exposure dose for these times (Table 1), we also quantified the amount of Ag deposited onto cell culture inserts in the absence of biological material using ICP-MS. The quantities for deposited 107Ag were 1.56 ± 0.24 ng/cm2 for 7 min exposure, 2.49 ± 0.15 ng/cm2 for 20 min exposure, and 4.59 ± 0.24 ng/cm2 for 60 min exposure (n=3 for all measurements).
Adverse effects of AgNP exposure
Exposure of SmallAIR cultures to AgNP resulted in no significant alterations in gross cytotoxicity indicators with cell stress marker lactate unaltered across the range of exposures (Figure 5A). LDH assessment however did reveal significant differences compared to control after 6hrs at the highest 13.2 ng/cm2 dose (Figure 5B). Basolateral levels at both 6 and 24hrs indicated an increase with exposure, which did not reach statistical significance. Gene expression analysis was also carried across these exposure ranges. Air only exposures at 7mins, 20mins and 60mins were performed to control for system exposure effects and analyzed for a range of stress responses genes at 24hrs post exposure. For 20mins and 60mins, exposure in the AE-ALI system resulted in significant alterations in stress response genes (Supplementary Figure 5). As the 7mins exposure did not result in such stress responses and since we cannot be confident of how system exposure may alter or confound gene expression results, further interrogation was only carried at the lower dose of AgNP (7mins; 1.5 ng/cm2). The majority of the 50 transcripts chosen from the in vivo RNA-SEQ analysis were upregulated by AgNP exposure (Figure 5C; right hand panel). Of these 50 genes, 24 displayed significant alterations in expression in response to in vitro AgNP at 24hrs (Figure 5C; left hand panel). Of these 24, 20 were regulated in the same direction as in vivo. While these in vitro changes may capture the airway epithelial cell response to AgNP treatment, the level of correlation between in vitro and in vivo was not statistically different (Pearson Correlation). Two of the 4 miRNAs selected for analysis were significantly altered by AgNP in vitro (Figure 5C). These were however not regulated in the same direction as in vivo.
Figure 5: Exposure of small airway epithelium to AgNP aerosol.
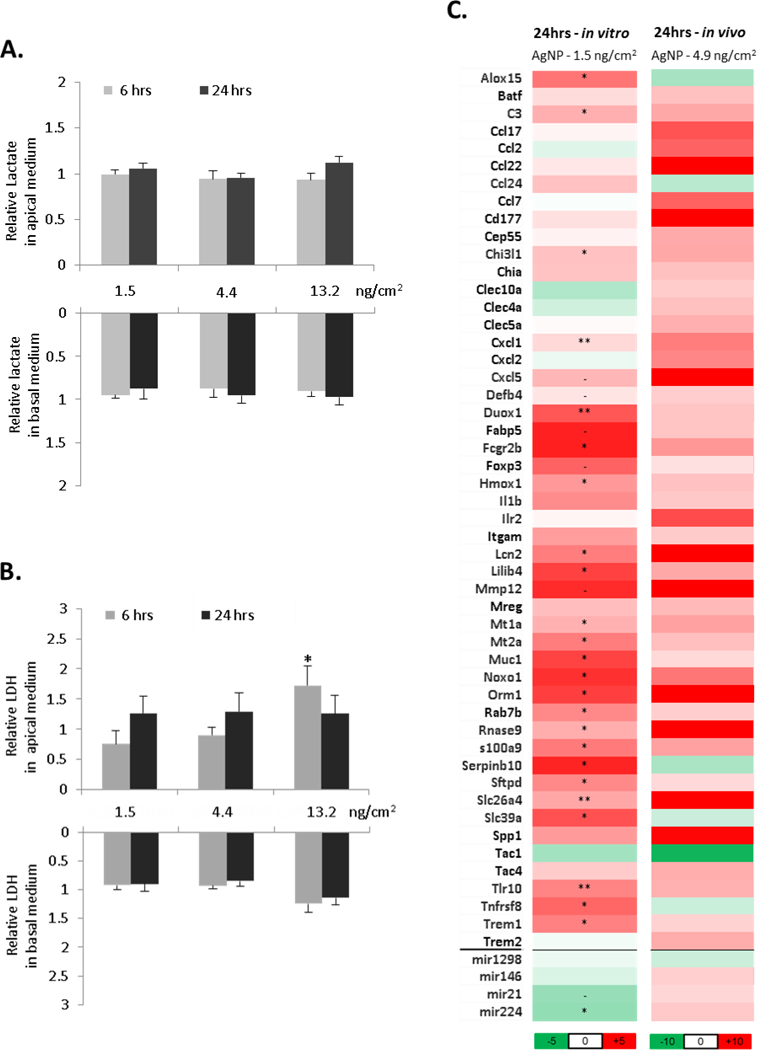
Human primary small airway epithelial cell cultures (SmallAIR) were exposed to AgNP aerosols using an AE-ALI deposition device. After 7, 20 or 60 mins of exposure (respective doses 1.5, 4.4 and 13.2 ng/cm2), cultures were incubated for 6 or 24hrs and apical wash and basolateral media was assessed for levels of lactate (A) or LDH (B). RNA was also assessed for relative expression of 50 mRNA and 4 miRNA transcripts displayed as fold over control values for 7 min exposure (C; left panel). For comparison, in vivo lung AgNP transcript fold change alterations from RNA-SEQ data were also included (C; right panel). All AgNP measurements were normalised to air exposed control cultures. Statistical significance was calculated between unexposed and exposed conditions using paired t-test (p-value; - < 0.1, * < 0.05, ** < 0.01).
The mechanisms through which AgNPs have been suggested to mediate their effects include NP dissolution and cellular alterations in response to silver ion (Ag+) levels. To investigate whether Ag+ impact cellular responses to the same extent as AgNP, we exposed SmallAIR cultures to a range of AgNO3 doses covering both in vivo and in vitro AgNP exposure ranges. Levels of lactate from these exposures were observed as significantly decreased at both 6 and 24hr exposure, indicating an impact on normal cellular metabolism (Figure 6A). Apical LDH levels were increased at 24hrs at the highest Ag+ dose indicating some level of toxicity (Figure 6B). Genes were selected for Ag+ analysis from those which were significantly regulated by AgNP in vitro and in vivo. For the most part Ag+ treatment displayed minimal effect on gene expression at 6hrs, while increased significantly for 25 out of 28 genes analyzed at 24hrs exposure (Figure 6C). Direct comparison of Ag+ gene expression to AgNP in vitro revealed a significant correlation (Pearson correlation coefficient r=0.4124; p value = 0.019).
Figure 6: Exposure of small airway epithelium to Ag+ solution.

Human primary small airway epithelial cell cultures (SmallAIR) were exposed to AgNO3 at a dose range of 0.5 – 13.2 ng/cm2 for either 6 or 24hrs. Apical media wash and basolateral media was assessed for levels of lactate (A) or LDH (B). RNA was also assessed for relative expression of 28 mRNA and 4 miRNA transcripts displayed as fold over control values (C). All Ag+ measurements were normalised to air exposed control cultures. Statistical significance was calculated between unexposed and exposed conditions using paired t-test (p-value; * < 0.05, ** < 0.01, *** < 0.001).
DISCUSSION
Acute pulmonary exposure to AgNP in vivo causes a substantial increase in neutrophil accumulation within the lung at day 1, diminished at day 7, with little effect on other inflammatory cell recruitment (Seiffert et al., 2016). This response was paralleled by changes in neutrophil chemoattractant transcripts within lung tissue, including cxcl5 as the 4th highest regulated after 1 day, again diminished after day 7. Other transcripts for genes associated with neutrophils were also regulated in a similar way, including cxcl1, cxcl2 and cd177. Macrophage levels were modestly increased on AgNP exposure (Seiffert et al., 2016). Ccl22 and ccl1, which regulate monocyte and macrophage recruitment, were among the top 10 most highly regulated genes at day 1. This together with changes in expression of genes associated with macrophage function, mmp12, retnla and trem1 indicate the involvement of these cells in addition to neutrophils as primary responders to AgNP exposure. Complement genes including C3 were also increased to a significant level. In addition there was a significant proportion of transcripts involved in regulating type II inflammation within the top 20 genes. This may be important for pulmonary hazard assessment given the prominence this immune profile has in asthma and allergic airway disease (Meldrum et al., 2017). In addition to pro-inflammatory events, there was also evidence of inflammation resolution processes with the induction of IL1R2 expression.
SLC26A4 is an epithelial cell expressed anion transporter that has been suggested as a regulator of mucin production and inflammatory events in airway conditions such as asthma and COPD (Nakagami et al., 2008, Nakao et al., 2008, Gorrieri et al., 2016, Suzuki et al., 2016). Expression of this gene was observed as the second most highly regulated transcript (Fig 1C). Duox1 expression was also increased in our study and has been previously observed to mediate small airway epithelial repair and bronchiolar reepithelialisation in a model of naphthalene induced small airway injury (Gorissen et al., 2013). The Club cell marker CLDN10 (Zemke et al., 2009, Fukumoto et al., 2016) was also significantly altered in the lungs of these animals by AgNP. Regulation of other epithelial specific genes including ect2, sftpa1, sftpd, muc1 and cftr was also observed. This data suggests the airway epithelium including the small airway epithelium as responding to NP inhalation exposure. We addressed whether Ag was deposited in the distal airways, in particular the bronchiolar and alveolar regions, using laser ablation ICP-MS detection. We found higher levels of Ag in the bronchiolar wall when compared to alveolar (Fig 3). All of this evidence strongly suggests the small airway epithelium as a target for AgNP inhalation exposure.
To date, the investigation of the small airway epithelium as a primary responder to NP exposure has been exclusively limited to in vitro studies of model cell lines and primary cells in submerged culture, including (Ng et al., 2013, Snyder-Talkington et al., 2013, Sisler et al., 2016, Stueckle et al., 2017). Submerged culture is not optimal for differentiation of airway cells towards in vivo functionality and does not allow for representative NP deposition to occur using aerosolised material. These limitations can be overcome with the use of air-liquid interface (ALI) culture. In our study, we use such an approach to investigate deposited NP aerosol effects on ALI cultures of small airway epithelial cells for the first time utilising SmallAIR (Huang et al., 2017). Using an electrostatic charge coupled particle deposition device we exposed these cultures to the same AgNP aerosol used for an in vivo rat inhalation study to investigate how small airway epithelial cells may account for primary responses within the lung.
Estimation of deposition by ICP-MS analysis compared well with the manufacturer’s estimated deposition efficiency of 40% (Aufderheide et al., 2011). Distribution of Ag across the 6.5mm insert membrane was relatively homogeneous (data not shown) but some caution should be taken using larger inserts due to potential uneven concentric deposition, which may require optimisation. Caution should also be taken with exposure duration within the system, where greater than 7 mins exposure to air alone resulted in adverse cell gene expression. Despite this, when we compared the results from an estimated dose (1.5 ng/cm2) in vitro, which was lower than that estimated in vivo, we still achieved a level of concordance in transcript regulation indicative of similar biological responses. The application of ionic silver to ALI cultures at doses covering the estimates of in vivo exposure to Ag also resulted in concordant transcript expression changes. While comparison of in vitro to in vivo effects must take into consideration multiple factors including species effects, other cell types and particle handling, that may account for incomplete concordance, we believe we have established an in vitro system and exposure set-up that can attribute, in part, the pulmonary stress response to AgNP inhalation to the small airway compartment.
Dosimetry comparisons to real world exposure scenarios are an important component for how our study may be considered for risk assessment. There is limited information on AgNP exposures in the workplace or public exposure from the use of products containing AgNPs. Measurements within a manufacturing facility have indicated typical workplace exposure levels approximately 1μg/m3 with maximum concentrations up to 300μg/m3 (Lee et al., 2012). If one is to consider cumulative dose these concentrations are comparable to those used in our study. Our findings also point towards the mechanisms through which AgNP have their effects at the level of material characteristic, biological stress response and specific cellular injury. Both the nanoparticulate and dissolved component of AgNPs have been suggested to contribute to adverse pulmonary effects (Kawata et al., 2009), with the percentage of Ag+ ion contributing to cellular responses estimated to be between 10–60% (Beer et al., 2012, Maurer-Jones et al., 2013, Comfort et al., 2014). From our SmallAir ALI cultures we found a significant correlation between AgNP and Ag+ regulated RNA transcripts. This is similar to other studies (Bouwmeester et al., 2011, van der Zande et al., 2016) and suggests a high proportion of the effects of AgNP may be attributable to the dissolved fraction. Silver ions are biologically active and are known to interact with cellular components including proteins such as cell membrane receptors and those containing metal ions (Lansdown, 2006). Cellular defence systems are in place to mitigate the effects of metal ion exposure such as the metallothioneins and anti-oxidant response pathways such as Nrf2. Examination of mRNA-SEQ data from rat lung exposed to AgNP aerosol revealed multiple pathways and genes outlining specific stress responses. Metallothioneins aim to sequester metal ions preventing intracellular biochemical disruption and both MT1A and MT2A were upregulated by AgNP. Also prominent among these responses was an oxidative stress response, which included upregulation of SLC7A11, NOXO1, DUOXA1, SOD2 and HMOX1, all Nrf2 dependent genes involved in defence against oxidant and electrophilic stress (Jennings et al., 2013). A mechanism previously proposed to outline AgNP toxicity involves disruption of the mitochondrial respiratory chain by Ag+, leading to production of ROS and interruption of ATP synthesis, in turn causing oxidative DNA damage, which may also involve direct Ag+ interaction with DNA leading to cell cycle arrest in the G(2)/M phase (AshaRani et al., 2009, Lee et al., 2011). Our data would generally support aspects of this mechanism, where we observe markers of oxidative stress and regulation of genes involved in G2/M phase cell cycle arrest. With the alteration in levels of the mitochondrial genes, NOXO1 and SOD2, there is also evidence that mitochondrial disruption occurs with AgNP exposure. We did not observe alterations in lactate levels in our in vitro exposures, indicating mitochondrial injury was insufficient to push metabolism towards glycolysis, as has been previously observed with nano-silver exposure (Chen et al., 2014). Other mechanisms suggested to mediate Ag nanomaterial effects include inhibition of thioredoxin reductase activity and selenium metabolism (Srivastava et al., 2012). We did not observe any alterations in transcripts involved in this pathway.
miRNAs are small endogenous noncoding RNAs of 19–24 nucleotides in length with the potential to control the expression of hundreds of mRNA at the post-transcriptional level. In our study we identified differentially regulated miRNA transcripts using RNA-SEQ and found a small number of miRNAs altered by AgNP exposure. These included mir146, mir155, mir21 and mir224 all of which have been observed regulated as part of inflammatory processes in response to nanoparticulate exposure within the lung (Moschos et al., 2007, Izzotti et al., 2009, Bourdon et al., 2012, Dorhoi et al., 2013, Fossati et al., 2014, Malmhall et al., 2014). Analysis of select miRNAs in our ALI cultures after AgNP exposure revealed differences when compared to in vivo exposure. Only mir224 demonstrated significant regulation in response to AgNP but in the opposite direction to in vivo changes. Regulation of miRNAs by Ag ion treatment displayed alterations in levels with increases at 6hrs and downregulation at 24hrs and may indicate similar effects could occur with AgNP treatment. Given these temporal differences, the limited numbers and the low magnitude of change it is difficult to see how changes in miRNAs may be used as sensitive biomarkers for specific injury or provide substantial insight into mechanisms of toxicological insult from this study.
CONCLUSION
In conclusion, our findings support the suggestion that a component of the in vivo pulmonary response to inhaled AgNP arises from the small airway epithelium. They also support the principle that using differentiated in vitro models in an environment representative of in vivo exposure as a relevant and appropriate model to capture detailed cellular responses when pursuing in vitro culture alternatives. Transcriptomic analysis of lung tissue provided important information on specific cell type responses to AgNP, including small airway effects and immune cell recruitment and regulation. It also allowed us to more clearly define inflammatory phenotypes such as complement activation and type II inflammation responses, as well as inflammation resolution indicators. From a cellular signalling perspective, we observed responses indicative of DNA damage, cell cycle effects, oxidative stress as well as metal overload. This information together with our ability, in part, to capture many of these tissue responses using cultures of small airway epithelial cells exposed to AgNP and Ag+ ions adds significant detail and strengthens our mechanistic understanding of how acute AgNP inhalation exposure impacts the lung.
Supplementary Material
Acknowledgments
Funding
The original in vivo study was part funded by grants from US National Institute for Environmental Health Sciences grant number U19ES019536 (http://www.niehs.nih.gov/research/supported/index.cfm), and from the UK National Environmental Research Council grant NE/H012893 (http://www.nerc.ac.uk/research/). Transcriptomic analysis of lung tissue samples was funded by Public Health England. The in vitro aspect of this research was part funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Health Impact of Environmental Hazards at King’s College London in partnership with Public Health England. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Footnotes
Disclosure Statement
The authors report no conflicts of interests with the work in this article. The authors alone are responsible for the content and writing of the manuscript.
REFERENCES
- Anderson DS, Patchin ES, Silva RM, Uyeminami DL, Sharmah A, Guo T, Das GK, Brown JM, Shannahan J, Gordon T, Chen LC, Pinkerton KE & Van Winkle LS, 2015a. Influence of particle size on persistence and clearance of aerosolized silver nanoparticles in the rat lung. Toxicol Sci, 144, 366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DS, Silva RM, Lee D, Edwards PC, Sharmah A, Guo T, Pinkerton KE & Van Winkle LS, 2015b. Persistence of silver nanoparticles in the rat lung: Influence of dose, size, and chemical composition. Nanotoxicology, 9, 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Miyayama T & Hirano S, 2015. Difference in the toxicity mechanism between ion and nanoparticle forms of silver in the mouse lung and in macrophages. Toxicology, 328, 84–92. [DOI] [PubMed] [Google Scholar]
- Asharani P, Sethu S, Lim HK, Balaji G, Valiyaveettil S & Hande MP, 2012. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr, 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asharani PV, Low Kah Mun G., Hande MP & Valiyaveettil S, 2009. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano, 3, 279–90. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Scheffler S, Mohle N, Halter B & Hochrainer D, 2011. Analytical in vitro approach for studying cyto- and genotoxic effects of particulate airborne material. Anal Bioanal Chem, 401, 3213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer C, Foldbjerg R, Hayashi Y, Sutherland DS & Autrup H, 2012. Toxicity of silver nanoparticles - nanoparticle or silver ion? Toxicol Lett, 208, 286–92. [DOI] [PubMed] [Google Scholar]
- Bourdon JA, Saber AT, Halappanavar S, Jackson PA, Wu D, Hougaard KS, Jacobsen NR, Williams A, Vogel U, Wallin H & Yauk CL, 2012. Carbon black nanoparticle intratracheal installation results in large and sustained changes in the expression of miR-135b in mouse lung. Environ Mol Mutagen, 53, 462–8. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Poortman J, Peters RJ, Wijma E, Kramer E, Makama S, Puspitaninganindita K, Marvin HJ, Peijnenburg AA & Hendriksen PJ, 2011. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano, 5, 4091–103. [DOI] [PubMed] [Google Scholar]
- Braakhuis HM, Gosens I, Krystek P, Boere JA, Cassee FR, Fokkens PH, Post JA, Van Loveren H & Park MV, 2014. Particle size dependent deposition and pulmonary inflammation after short-term inhalation of silver nanoparticles. Part Fibre Toxicol, 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chairuangkitti P, Lawanprasert S, Roytrakul S, Aueviriyavit S, Phummiratch D, Kulthong K, Chanvorachote P & Maniratanachote R, 2013. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol In Vitro, 27, 330–8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Z, Xu M, Wang X, Liu R, Liu Q, Zhang Z, Xia T, Zhao J, Jiang G, Xu Y & Liu S, 2014. Nanosilver incurs an adaptive shunt of energy metabolism mode to glycolysis in tumor and nontumor cells. ACS Nano, 8, 5813–25. [DOI] [PubMed] [Google Scholar]
- Comfort KK, Maurer EI & Hussain SM, 2014. Slow release of ions from internalized silver nanoparticles modifies the epidermal growth factor signaling response. Colloids Surf B Biointerfaces, 123, 136–42. [DOI] [PubMed] [Google Scholar]
- Cronholm P, Karlsson HL, Hedberg J, Lowe TA, Winnberg L, Elihn K, Wallinder IO & Moller L, 2013. Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. Small, 9, 970–82. [DOI] [PubMed] [Google Scholar]
- Dorhoi A, Iannaccone M, Farinacci M, Fae KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf HJ, Oberbeck-Muller D, Jorg S, Heinemann E, Hahnke K, Lowe D, Del Nonno F., Goletti D, Capparelli R & Kaufmann SH, 2013. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest, 123, 4836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldbjerg R, Dang DA & Autrup H, 2011. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol, 85, 743–50. [DOI] [PubMed] [Google Scholar]
- Foldbjerg R, Irving ES, Hayashi Y, Sutherland DS, Thorsen K, Autrup H & Beer C, 2012. Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol Sci, 130, 145–57. [DOI] [PubMed] [Google Scholar]
- Fossati S, Baccarelli A, Zanobetti A, Hoxha M, Vokonas PS, Wright RO & Schwartz J, 2014. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology, 25, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto J, Soundararajan R, Leung J, Cox R, Mahendrasah S, Muthavarapu N, Herrin T, Czachor A, Tan LC, Hosseinian N, Patel P, Gone J, Breitzig MT, Cho Y, Cooke AJ, Galam L, Narala VR, Pathak Y, Lockey RF & Kolliputi N, 2016. The role of club cell phenoconversion and migration in idiopathic pulmonary fibrosis. Aging (Albany NY), 8, 3091–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga AR, Skoglund S, Wallinder IO, Fadeel B & Karlsson HL, 2014. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol, 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorissen SH, Hristova M, Habibovic A, Sipsey LM, Spiess PC, Janssen-Heininger YM & Van Der Vliet A, 2013. Dual oxidase-1 is required for airway epithelial cell migration and bronchiolar reepithelialization after injury. Am J Respir Cell Mol Biol, 48, 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrieri G, Scudieri P, Caci E, Schiavon M, Tomati V, Sirci F, Napolitano F, Carrella D, Gianotti A, Musante I, Favia M, Casavola V, Guerra L, Rea F, Ravazzolo R, Di Bernardo D & Galietta LJ, 2016. Goblet Cell Hyperplasia Requires High Bicarbonate Transport To Support Mucin Release. Sci Rep, 6, 36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup N & Lam HR, 2014. Oral toxicity of silver ions, silver nanoparticles and colloidal silver--a review. Regul Toxicol Pharmacol, 68, 1–7. [DOI] [PubMed] [Google Scholar]
- Huang S, Boda B, Vernaz J, Ferreira E, Wiszniewski L & Constant S, 2017. Establishment and characterization of an in vitro human small airway model (SmallAir). Eur J Pharm Biopharm, 118, 68–72. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM & De Flora S., 2009. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J, 23, 806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P, Limonciel A, Felice L & Leonard MO, 2013. An overview of transcriptional regulation in response to toxicological insult. Arch Toxicol, 87, 49–72. [DOI] [PubMed] [Google Scholar]
- Ji JH, Jung JH, Kim SS, Yoon JU, Park JD, Choi BS, Chung YH, Kwon IH, Jeong J, Han BS, Shin JH, Sung JH, Song KS & Yu IJ, 2007. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol, 19, 857–71. [DOI] [PubMed] [Google Scholar]
- Jiang X, Miclaus T, Wang L, Foldbjerg R, Sutherland DS, Autrup H, Chen C & Beer C, 2014. Fast intracellular dissolution and persistent cellular uptake of silver nanoparticles in CHO-K1 cells: implication for cytotoxicity. Nanotoxicology. [DOI] [PubMed] [Google Scholar]
- Kawata K, Osawa M & Okabe S, 2009. In Vitro Toxicity of Silver Nanoparticles at Noncytotoxic Doses to HepG2 Human Hepatoma Cells. Environmental Science & Technology, 43, 6046–6051. [DOI] [PubMed] [Google Scholar]
- Lansdown AB, 2006. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol, 33, 17–34. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ahn K, Kim SM, Jeon KS, Lee JS & Yu IJ, 2012. Continuous 3-day exposure assessment of workplace manufacturing silver nanoparticles. Journal of Nanoparticle Research, 14. [Google Scholar]
- Lee YS, Kim DW, Lee YH, Oh JH, Yoon S, Choi MS, Lee SK, Kim JW, Lee K & Song CW, 2011. Silver nanoparticles induce apoptosis and G2/M arrest via PKCzeta-dependent signaling in A549 lung cells. Arch Toxicol, 85, 1529–40. [DOI] [PubMed] [Google Scholar]
- Limonciel A, Aschauer L, Wilmes A, Prajczer S, Leonard MO, Pfaller W & Jennings P, 2011. Lactate is an ideal non-invasive marker for evaluating temporal alterations in cell stress and toxicity in repeat dose testing regimes. Toxicol In Vitro, 25, 1855–62. [DOI] [PubMed] [Google Scholar]
- Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M & Radinger M, 2014. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol, 133, 1429–38, 1438 e1–7. [DOI] [PubMed] [Google Scholar]
- Maurer-Jones MA, Mousavi MPS, Chen LD, Buhlmann P & Haynes CL, 2013. Characterization of silver ion dissolution from silver nanoparticles using fluorous-phase ion-selective electrodes and assessment of resultant toxicity to Shewanella oneidensis. Chemical Science, 4, 2564–2572. [Google Scholar]
- Meldrum K, Gant TW, Macchiarulo S & Leonard MO, 2016. Bronchial epithelial innate and adaptive immunity signals are induced by polycyclic aromatic hydrocarbons. Toxicology Research, 5, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum K, Guo C, Marczylo EL, Gant TW, Smith R & Leonard MO, 2017. Mechanistic insight into the impact of nanomaterials on asthma and allergic airway disease. Part Fibre Toxicol, 14, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG & Lindsay MA, 2007. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics, 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Royce SG, Sarkar S, Thorley A, Schwander S, Ryan MP, Porter AE, Chung KF, Tetley TD, Zhang J & Georgopoulos PG, 2014. Modeling in vitro cellular responses to silver nanoparticles. J Toxicol, 2014, 852890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Favoreto S Jr., Zhen G, Park SW, Nguyenvu LT, Kuperman DA, Dolganov GM, Huang X, Boushey HA, Avila PC & Erle DJ, 2008. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol, 181, 2203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao I, Kanaji S, Ohta S, Matsushita H, Arima K, Yuyama N, Yamaya M, Nakayama K, Kubo H, Watanabe M, Sagara H, Sugiyama K, Tanaka H, Toda S, Hayashi H, Inoue H, Hoshino T, Shiraki A, Inoue M, Suzuki K, Aizawa H, Okinami S, Nagai H, Hasegawa M, Fukuda T, Green ED & Izuhara K, 2008. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J Immunol, 180, 6262–9. [DOI] [PubMed] [Google Scholar]
- Ng CT, Li JJ, Gurung RL, Hande MP, Ong CN, Bay BH & Yung LY, 2013. Toxicological profile of small airway epithelial cells exposed to gold nanoparticles. Exp Biol Med (Maywood), 238, 1355–61. [DOI] [PubMed] [Google Scholar]
- Piccinno F, Gottschalk F, Seeger S & Nowack B, 2012. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. Journal of Nanoparticle Research, 14. [Google Scholar]
- Seiffert J, Buckley A, Leo B, Martin NG, Zhu J, Dai R, Hussain F, Guo C, Warren J, Hodgson A, Gong J, Ryan MP, Zhang JJ, Porter A, Tetley TD, Gow A, Smith R & Chung KF, 2016. Pulmonary effects of inhalation of spark-generated silver nanoparticles in Brown-Norway and Sprague-Dawley rats. Respir Res, 17, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyawati MI, Yuan X, Xie J & Leong DT, 2014. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells. Biomaterials, 35, 6707–15. [DOI] [PubMed] [Google Scholar]
- Sisler JD, Pirela SV, Shaffer J, Mihalchik AL, Chisholm WP, Andrew ME, Schwegler-Berry D, Castranova V, Demokritou P & Qian Y, 2016. Toxicological Assessment of CoO and La2O3 Metal Oxide Nanoparticles in Human Small Airway Epithelial Cells. Toxicol Sci, 150, 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Pacurari M, Dong C, Leonard SS, Schwegler-Berry D, Castranova V, Qian Y & Guo NL, 2013. Systematic analysis of multiwalled carbon nanotube-induced cellular signaling and gene expression in human small airway epithelial cells. Toxicol Sci, 133, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Sung JH, Ji JH, Lee JH, Lee JS, Ryu HR, Lee JK, Chung YH, Park HM, Shin BS, Chang HK, Kelman B & Yu IJ, 2013. Recovery from silver-nanoparticle-exposure-induced lung inflammation and lung function changes in Sprague Dawley rats. Nanotoxicology, 7, 169–80. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Singh S & Self WT, 2012. Exposure to silver nanoparticles inhibits selenoprotein synthesis and the activity of thioredoxin reductase. Environ Health Perspect, 120, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueckle TA, Davidson DC, Derk R, Wang P, Friend S, Schwegler-Berry D, Zheng P, Wu N, Castranova V, Rojanasakul Y & Wang L, 2017. Effect of surface functionalizations of multi-walled carbon nanotubes on neoplastic transformation potential in primary human lung epithelial cells. Nanotoxicology, 11, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Ji JH, Park JD, Yoon JU, Kim DS, Jeon KS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Chang HK, Lee JH, Cho MH, Kelman BJ & Yu IJ, 2009. Subchronic inhalation toxicity of silver nanoparticles. Toxicol Sci, 108, 452–61. [DOI] [PubMed] [Google Scholar]
- Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Kim J, Kim TS, Chang HK, Lee EJ, Lee JH & Yu IJ, 2008. Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal Toxicol, 20, 567–74. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Ogawa M, Ohta S, Nunomura S, Nanri Y, Shiraishi H, Mitamura Y, Yoshihara T, Lee JJ & Izuhara K, 2016. Induction of Airway Allergic Inflammation by Hypothiocyanite via Epithelial Cells. J Biol Chem, 291, 27219–27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney S, Leo BF, Chen S, Abraham-Thomas N, Thorley AJ, Gow A, Schwander S, Zhang JJ, Shaffer MS, Chung KF, Ryan MP, Porter AE & Tetley TD, 2016. Pulmonary surfactant mitigates silver nanoparticle toxicity in human alveolar type-I-like epithelial cells. Colloids Surf B Biointerfaces, 145, 167–75. [DOI] [PubMed] [Google Scholar]
- Van Der Zande M, Undas AK, Kramer E, Monopoli MP, Peters RJ, Garry D, Antunes Fernandes EC, Hendriksen PJ, Marvin HJ, Peijnenburg AA & Bouwmeester H, 2016. Different responses of Caco-2 and MCF-7 cells to silver nanoparticles are based on highly similar mechanisms of action. Nanotoxicology, 10, 1431–1441. [DOI] [PubMed] [Google Scholar]
- Wang X, Ji Z, Chang CH, Zhang H, Wang M, Liao YP, Lin S, Meng H, Li R, Sun B, Winkle LV, Pinkerton KE, Zink JI, Xia T & Nel AE, 2014. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small, 10, 385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N & Stripp BR, 2009. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol, 40, 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


