Highlights
-
•
Serum thiols are a marker of oxidative stress.
-
•
High serum thiols are associated with better glycemic control and less complications in T2DM.
-
•
Free thiols have limited capability for the prediction of long-term complications.
Keywords: Type 2 diabetes, Glycemia, Oxidative stress, Thiols, Free sulfhydryl
Abstract
Aims
Oxidative stress is a driver in the development of type 2 diabetes (T2DM) complications. As thiols (R-SH) are oxidized by reactive oxygen and sulfur species, circulating concentrations may directly reflect systemic redox status. We hypothesized that high serum R-SH concentrations are a reflection of a favourable redox status and may therefore positively associate with disease status.
Methods
R-SH were measured in serum of 943 T2DM outpatients (55% males, 65 years and HbA1c of 6.7% (50 mmol/mol)) with a follow-up period of 1.2 years.
Results
In the highest R-SH tertile patients were younger, more often men, had less microvascular complications, lower HbA1c and were more often treated nutritionally or with oral glucose-lowering drugs. Age- and sex adjusted hazard ratios for developing micro-, macro- or any complication plus death were 0.994, 0.992 and 0.993: even after adjustment for potential confounders. The Harrell’s C statistic to predict microvascular complications or any complication plus death was higher in the models with R-SH than in those without R-SH.
Conclusions
Although R-SH concentrations were associated with a favourable disease status, it did not add to the predictive capacity for long-term complications. Based on the current data R-SH seems unsuitable as a prognostic marker in T2DM.
Introduction
Hyperglycemia promotes a state of systemic oxidative stress, in which disproportionate levels of reactive oxygen species (ROS) cause an increase in insulin resistance and β-cell dysfunction, thereby contributing to the progression of type 2 diabetes mellitus (T2DM) [1], [2]. Oxidative stress also plays a key role in the pathogenesis of microvascular and macrovascular complications of diabetes, all of which are associated with significant morbidity and mortality as well as reduced quality of life [2], [3], [4], [5], [6].
At physiological levels, ROS play essential roles in cell signalling and homeostasis [7], [8]. An elaborate network of endogenous antioxidant mechanisms exists to prevent cellular damage by removing excess ROS and containing the action radius close to their sites of production. Oxidative stress results from an imbalance between ROS production and antioxidant defence capacity favouring the former [9]. Under these conditions, ROS can oxidize and damage cellular macromolecules including nucleic acids, lipids and proteins, thereby changing the properties of the cell membrane and intracellular constituents including DNA and enzymes, and affecting cellular function and viability [3].
Thiols, compounds with a free sulfhydryl (R-SH) moiety, occur in the form of proteins containing one or more free cysteine groups or low-molecular-weight compounds (e.g. glutathione) in cells and extracellular fluids. In serum, the concentration of all thiols added together is lower than that intracellular, with albumin being the most abundant thiol [10]. These R-SH groups are readily oxidized by ROS and other reactive species. The circulating concentrations of total R-SH has recently been proposed to directly reflect the whole-body redox status: a decrease in circulating R-SH concentration may reflect an increased oxidative poise and therefore be indicative of oxidative stress [5], [11], [12]. High R-SH serum concentrations have previously been shown to also be associated with a beneficial cardiovascular risk profile and a better patient and graft survival in renal transplant recipients [13]. This may indicate its potential usefulness as a low-cost, high-throughput screening tool for whole-body redox status in translational studies; it may also be a promising target for intervention. Moreover, in an exploratory study we demonstrated a favourable association of serum R-SH with markers of heart failure and disease outcome in non-T2DM individuals [11]. It has been demonstrated in a small cohort that serum R-SH are reduced in T2DM patients as compared to healthy adults [14]. Another small study found that R-SH concentrations are lower in T2DM patients with complications as compared to those without complications [15]. However, this study lacked relevant clinical data including glycemic control and the longitudinal relationship between R-SH and outcomes is still unknown. Nevertheless, these findings suggest that elevated R-SH concentrations may play a favourable role in the pathophysiology and prognosis of T2DM. Given the antioxidant properties of R-SH and the possibility of supplementation affecting circulating thiol concentrations, this may have implications for future therapeutic interventions [16].
Given the potential of R-SH as a modifiable biomarker of ROS-mediated damage in the progression of T2DM and associated complications, we aimed to investigate the association between circulating free thiols and T2DM in a large cohort of stable patients.
Subjects, materials and methods
This is a prospective, observational cohort study. Baseline data and blood samples were obtained from the e-VitaDM study, which was designed to assess the feasibility of using an online platform in routine primary healthcare for subjects with T2DM. As a pre-specified part of the e-VitaDM study, patients were assessed in a long-term follow-up. This prospective arm was nested within the Zwolle Outpatient Diabetes project Integrating Available Care (ZODIAC) study. Both the e-VitaDM and the ZODIAC study are described in detail elsewhere [17]. The primary aim of the present study was to investigate the association between baseline serum concentrations of R-SH with indices of T2DM management, in particular HbA1c. A secondary, hypothesis-generating aim was to analyse the longitudinal relationship between baseline R-SH concentrations and the risk of death and/or complications (both micro- and macrovascular).
This study was conducted in general practices that are connected to the Care Group Drenthe in the Drenthe-region (located in the North-East) of the Netherlands. Fifty-two out of the 110 general practices in this care group agreed to participate. In these practices, approximately 8300 patients with T2DM were treated between 2012 and 2014. Patients were recruited during a regular check-up by their practice nurse and included from May 2012 until September 2014 [17], [18]. Patients with T2DM, aged ≥18 years and the general practitioner as main care provider for T2DM were eligible for participation [17]. For the e-VitaDM study there were no exclusion criteria. A total of 1710 out of 3988 patients, who were asked to participate in the eVita-DM study, gave written informed consent. Of these patients, 764 were not included in the present study due to the inability to match blood samples taken in the e-VitaDM with the clinical data from the ZODIAC study. A further 3 individuals were excluded from the final analysis because apparent R-SH concentrations were below the detection limit. Consequently, the final study cohort consisted of 943 patients.
Baseline demographic data included gender, age, duration of diabetes, BMI, alcohol intake and smoking habits. Information concerning alcohol intake and smoking habits was derived from questionnaires at baseline. Medical data were extracted from the diabetes-specific database at the Diabetes Centre of the Isala hospital (Zwolle, The Netherlands). This centre gathers data of primary care-treated patients with T2DM in a large part of the Netherlands on an annual basis, to provide benchmark information to general practitioners. This database includes information on physical examination, use of medication, and laboratory blood and urine tests. The following data were extracted: date of diabetes diagnosis, height, weight, diastolic and systolic blood pressure, cholesterol, HbA1c, estimated glomerular filtration rate (eGFR) based on the modification of diet in renal disease (MDRD) equation, urine creatinine, urine albumin, urine albumin:creatinine ratio, and the presence of macrovascular- and microvascular complications.
Macrovascular complications were defined as (a history of) angina pectoris (AP), myocardial infarction (MI), percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass grafting (CABG), cerebrovascular accident (CVA) or transient ischemic attack (TIA). Microvascular complications were defined as diabetic retinopathy, albuminuria (both micro- and macroalbuminuria) and diabetic peripheral neuropathy. Microalbuminuria was defined as 20–200 mg/L albumin or an albumin:creatinine ratio between 2.5 and 25 mg/mmol in men and 3.5–35 mg/mmol in women. Macro-albuminuria was defined as >200 mg/L albumin or a albumin:creatinine ratio greater than 25 mg/mmol and 35 mg/mmol for men and women, respectively [19]. An ophthalmologist determined the presence of diabetic retinopathy biannually. Foot sensibility was tested with 5.07 Semmes-Weinstein monofilaments. Neuropathy was defined as two or more errors in a test of three, affecting at least one foot. Blood glucose-lowering therapy was categorized into: dietary measures only, oral blood glucose lowering drugs (OBGLDs) including metformin, sulfonylurea derivatives, thiazolidinediones and DDP4 inhibitors, and insulin therapy. HbA1c is expressed in both derived NGSP units (as %) and IFCC-recommended (as mmol/mol) units.
Patients were instructed to be in a fasted state when the blood samples were collected. Venous blood samples were collected into BD Vacutainer™ serum tubes, centrifuged and the serum was stored directly in aliquots at −80 °C until measurement. R-SH were detected in serum aliquots not subjected to any prior freeze/thaw cycle as previously described, with minor modifications [20], [21]. Briefly, 75 µl serum was diluted 1:4 in 0.1 M Tris buffer (pH 8.2) and then transferred to a 96-well plate. Using a Sunrise microplate reader (Tecan Trading AG, Männedorf, Switzerland), background absorption was measured at 412 nm with a reference filter at 630 nm. Subsequently, 10 µl 3.8 mM 5,5′ -dithio-bis (2-nitrobenzoic acid) (DTNB; Sigma Aldrich, Zwijndrecht, Netherlands) in 0.1 M phosphate buffer (pH 7) was added to the samples. Following 20 min of incubation at room temperature, absorption was read again. The concentration of R-SH in the samples was determined by comparing their absorbance readings to a standard curve of L-cysteine (15–1000 µM; Fluka Biochemika, Buchs, Switzerland) in 0.1 M Tris and 10 mM EDTA (pH 8.2). Measurements were performed in January 2017 at the department of pathology and medical biology of the university medical centre Groningen (The Netherlands).
Statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and STATA version 15 (StataCorp. 2015. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP.). Multiple imputations were used for missing data on the independent variables, assuming data was missing (completely) at random. Five imputed datasets were created and the pooled results are described. The distributions of all variables were examined using histograms and Q-Q plots. Results were expressed as mean (with standard deviation (±SD)) or median (with interquartile range [IQR]) for normally distributed and non-normally distributed data, respectively. Nominal data are presented as n (with percentage (%)). A significance level of 5% (two-sided) was used. The study population was subdivided into tertiles based on the concentrations of serum R-SH to visualize associations between R-SH and clinical parameters. Differences in baseline characteristics between the groups were analysed using one-way ANOVA for normally distributed continuous data, the Kruskal-Wallis test for non-normally distributed continuous data and the Chi-Square test for nominal data. Linear regression was used to test the association between R-SH concentrations and individual parameters. For the hypothesis generating part we performed Cox regression analyses to assess the risk of death and complications (both micro- and macrovascular), microvascular complications or macrovascular complications during follow-up. Four models were used: (1) a crude model, (2) a model that included age, gender and R-SH, (3) a fully adjusted (duration of diabetes, smoking (yes/no), BMI, systolic blood pressure, HbA1c, log serum creatinine, cholesterol-HDL ratio, use of OBGLD and use of insulin) model in which R-SH was included, and (4) the fully adjusted model without R-SH. There are no data regarding variables influencing R-SH concentrations in T2DM; we therefore chose the variables in the current models based on their known influence on complications in T2DM. Proportional-hazards assumption were confirmed by Stata’s PH-test. The Harrel’s C and R2 statistics were used to investigate the capability of each model to predict mortality [22]. The Harrel’s C statistic ranges from 0 to 1, with 1 indicating a perfect prediction capacity of the model. Calibration, a measure to evaluate how well predicted probabilities agree with observed risks, was assessed using the Grønnesby and Borgan ‘goodness-of-fit’ likelihood-ratio test, a non-significant result means an acceptable calibration [23].
The study protocol was registered prior to the start of the study (study ID METC 11.10117) and approved by the Medical Ethical Committee of the Isala hospital. The protocol was also registered on clinicaltrials.gov (study ID NCT01570140). All patients gave written informed consent.
Results
Baseline characteristics of the 943 patients with T2DM are presented in Table 1. The patient cohort had a mean age of 65 (±10) years, 55% were male, diabetes duration was 6.5 [2.9–10.1] years and HbA1c concentration was 6.7 ± 0.7% (50 (±9) mmol/mol). At baseline, 29.2% of patients had a microvascular complication: 18.5% had neuropathy, 4.0% retinopathy, 12.4% microalbuminuria and 1.3% had macroalbuminuria. Twenty-three per cent of patients had a history of macrovascular complications. Considering treatment, 19% of patients were treated with dietary measures alone, while 78% received OBGLD and 13% (also) received insulin therapy. Antihypertensive or lipid lowering medication was prescribed to 85% and 80% of patients respectively, while 18% used thrombocyte aggregation inhibitors. All patients with macrovascular complications used platelet aggregation inhibitors.
Table 1.
Baseline patient characteristics expressed as tertiles of serum R-SH concentration.
| Overall N = 943 | Tertile 1 N = 310 | Tertile 2 N = 315 | Tertile 3 N = 318 | P-value | |
|---|---|---|---|---|---|
| R-SH, µM | 202 ± 48 | <181 (149 ± 25) | 181–227 (202 ± 13) | >227 (255 ± 21) | |
| Demographics | |||||
| Age, years | 65.0 ± 10.0 | 66.9 ± 9.6 | 64.9 ± 10.4 | 63.4 ± 9.8 | <0.001 |
| Diabetes duration, years | 6.5 [2.9–10.1] | 5.9 [2.9–9.8] | 6.4 [2.6–9.8] | 6.7 [3.4–10.6] | 0.543 |
| Male gender, n (%) | 517 (54.7) | 142 (45.8) | 179 (56.8) | 195 (61.3) | <0.001 |
| Current smoker, n (%) | 158 (16.7) | 53 (17.1) | 57 (18.1) | 48 (15.1) | 0.578 |
| BMI, kg/m2 | 29.3 [26.8–33.0] | 29.7 [27.1–33.9] | 29.7 [26.8–33.5] | 28.7 [26.2–32.0] | 0.001 |
| Systolic blood pressure, mm Hg | 135.6 [125.0–144.0] | 136.0 [128.0–144.0] | 134.0 [124.0–145.0] | 135.0 [125.0–142.0] | 0.488 |
| Diastolic blood pressure, mm Hg | 80.0 [71.0–83.0] | 80.0 [72.0–84.0] | 80.0 [70.0–82.0] | 80.0 [70.0–82.0] | 0.492 |
| Complications | |||||
| Microvascular complications, n (%) | 275 (29.2) | 101 (32.6) | 98 (31.1) | 76 (23.9) | 0.037 |
| Micro-albuminuria, n (%) | 117 (12.4) | 38 (12.3) | 40 (12.7) | 39 (12.3) | 0.982 |
| Macro-albuminuria, n (%) | 11 (1.3) | 5 (1.6) | 5 (1.6) | 2 (0.6) | 0.453 |
| Retinopathy, n (%) | 38 (4.0) | 19 (6.1) | 15 (4.8) | 4 (1.3) | 0.006 |
| Neuropathy, n (%) | 175 (18.5) | 66 (21.3) | 62 (19.7) | 47 (14.8) | 0.091 |
| Macrovascular complications, n (%) | 215 (22.7) | 77 (24.8) | 65 (20.6) | 72 (22.6) | 0.589 |
| Angina pectoris, n (%) | 81 (8.6) | 26 (8.4) | 29 (9.2) | 26 (8.2) | 0.593 |
| Myocardial infarction, n (%) | 82 (8.7) | 32 (10.3) | 26 (8.3) | 23 (7.2) | 0.330 |
| PTCA, n (%) | 50 (5.3) | 15 (4.8) | 18 (5.7) | 17 (5.3) | 0.729 |
| CABG, n (%) | 45 (4.8) | 19 (6.1) | 12 (3.8) | 14 (4.4) | 0.424 |
| TIA, n (%) | 29 (3.1) | 9 (2.9) | 12 (3.8) | 8 (2.5) | 0.470 |
| CVA, n (%) | 38 (4.0) | 15 (4.8) | 7 (2.2) | 16 (5.0) | 0.214 |
| Heart failure, n (%) | 25 (2.6) | 11 (3.5) | 10 (3.2) | 4 (1.3) | 0.115 |
| Laboratory Measurements | |||||
| HbA1c, % (mmol/mol) | 6.7 ± 0.7 (50 ± 9) | 6.8 ± 0.9 (51 ± 9) | 6.7 ± 0.8 (50 ± 9) | 6.5 ± 0.7 (48 ± 8) | <0.001 |
| eGFR (MDRD), ml/min/1.73 m2 | 73.0 [61.0–86.0] | 69.0 [60.0–81.0] | 73.0 [60.0–86.0] | 77.0 [66.0–90.0] | 0.528 |
| Total cholesterol, mmol/L | 4.3 [3.7–5.0] | 4.3 [3.6–4.9] | 4.4 [3.7–5.1] | 4.2 [3.7–4.8] | 0.138 |
| HDL cholesterol, mmol/L | 1.2 [1.0–1.5] | 1.2 [1.0–1.5] | 1.2 [1.0–1.5] | 1.3 [1.1–1.5] | 0.158 |
| Total cholesterol:HDL ratio | 3.4 [2.8–4.2] | 3.3 [2.8–4.3] | 3.6 [2.9–4.3] | 3.3 [2.7–3.9] | 0.002 |
| LDL cholesterol, mmol/L | 2.3 [1.8–2.9] | 2.2 [1.8–2.8] | 2.4 [1.9–3.0] | 2.2 [1.8–2.8] | 0.023 |
| Triglycerides, mmol/L | 1.5 [1.1–2.1] | 1.5 [1.1–2.2] | 1.6 [1.1–2.3] | 1.4 [1.0–1.9] | 0.060 |
| Albumine:creatinine ratio, mg/mmol | 0.8 [0.4–1.5] | 0.8 [0.4–1.5] | 1.0 [0.4–1.5] | 0.6 [0.2–1.4] | 0.104 |
| Medication | |||||
| Dietary measures only, n (%) | 182 (19.2) | 69 (21.9) | 67 (21.3) | 47 (14.8) | 0.042 |
| Oral blood glucose lowering drugs, n (%) | 741 (78.3) | 228 (73.5) | 245 (77.8) | 265 (83.3) | 0.012 |
| Metformin, n (%) | 707 (74.7) | 217 (70.0) | 230 (73.0) | 257 (80.8) | 0.006 |
| Sulfonylurea derivatives, n (%) | 261 (27.6) | 84 (27.1) | 86 (27.3) | 91 (28.6) | 0.899 |
| Thiazolinediones, n (%) | 10 (1.1) | 4 (1.3) | 5 (1.6) | 1 (0.3) | 0.263 |
| DDP4 inhibitors, n (%) | 35 (3.7) | 10 (3.2) | 16 (5.1) | 9 (2.8) | 0.281 |
| Insulin, n (%) | 123 (13.0) | 40 (12.9) | 35 (11.1) | 48 (15.1) | 0.331 |
| Antihypertensive drugs, n (%) | 799 (84.5) | 270 (87.7) | 269 (85.4) | 258 (81.1) | 0.070 |
| Diuretics, n (%) | 349 (36.9) | 125 (40.3) | 112 (35.6) | 112 (35.2) | 0.207 |
| Beta blockers, n (%) | 354 (37.4) | 111 (35.8) | 131 (41.6) | 111 (34.9) | 0.182 |
| Calcium antagonists, n (%) | 170 (18.0) | 60 (19.4) | 54 (17.1) | 56 (17.6) | 0.657 |
| RAAS blockers, n (%) | 514 (54.3) | 170 (54.8) | 170 (54.0) | 174 (54.7) | 0.855 |
| Cholesterol lowering drugs, n (%) | 752 (79.5) | 248 (80.0) | 242 (76.8) | 260 (81.8) | 0.288 |
| Antidepressive drugs, n (%) | 44 (4.7) | 12 (3.9) | 19 (6.0) | 13 (4.1) | 0.375 |
| Thrombocyte aggregation inhibitors, n (%) | 168 (17.8) | 47 (15.2) | 59 (18.7) | 62 (19.5) | 0.340 |
Data are presented as number (%), mean (SD) or median [IQR]. Significant (p < 0.05) values are emphasized in bold. HbA1c is expressed in both IFCC derived mmol/mol as DCCT derived %. Missing values: gender n = 2, HbA1c n = 4, total cholesterol n = 4, HDL-cholesterol n = 5, smoking n = 7. eGFR-MDRD n = 7, systolic blood pressue n = 11. Abbreviations: BMI, body mass index; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass grafting; TIA, transient ischemic attack; CVA, cerebral vascular event; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, high-density lipoprotein; DDP4, dipeptidyl peptidase 4; RAAS, renin-angiotensin-aldosterone system; R-SH total, free thiol groups.
Among all patients, R-SH concentrations were normally distributed, with a mean concentration of 203 [170–239] μM (see Fig. 1). Concentrations of R-SH were significantly higher in men: 210 (±47) versus 194 (±47) μM in women, respectively (P < 0.001). When comparing the different tertiles of R-SH, patients within the highest tertile (R-SH concentration of >227 μM) were significantly younger, more frequently male, had a lower BMI, less microvascular complications, a lower HbA1c, and a more favourable lipid profile. Moreover, these individuals were more often treated with diet alone or metformin as compared to those in the other tertiles. According to the multivariate model (see Table 2), the presence of macrovascular complications was inversely related to concentrations of R-SH. In addition, for each unit increase in age (year), BMI (kg/m2), diastolic blood pressure (mmHg) and HbA1c (mmol/mol) the concentrations of R-SH decreased. Male gender, diabetes duration (per year) and the use of platelet aggregation inhibitors were positively associated with R-SH concentration.
Fig. 1.
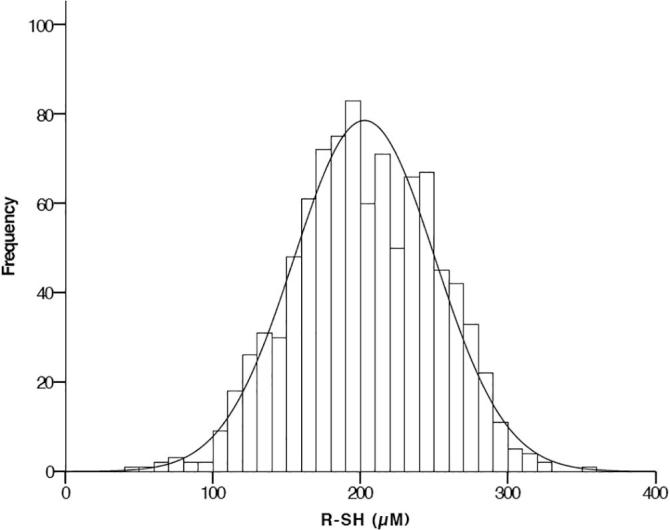
Histogram of serum R-SH concentrations in type 2 diabetes.
Table 2.
Univariable and multivariable associations of serum R-SH concentrations with clinical parameters in type 2 diabetes.
| Univariable |
Multivariable |
||||
|---|---|---|---|---|---|
| St. Beta | P-value | St. Beta | P-value | Part correlation | |
| Demographics | |||||
| Age, years | −0.176 | <0.001 | −0.190 | <0.001 | −0.148 |
| Diabetes duration, years | 0.012 | 0.355 | 0.115 | 0.006 | 0.098 |
| Male gender (male = 1) | −0.162 | <0.001 | 0.219 | <0.001 | 0.138 |
| Current smoker | −0.012 | 0.363 | −0.060 | 0.119 | −0.056 |
| BMI, kg/m2 | −0.171 | <0.001 | −0.164 | <0.001 | −0.149 |
| Systolic blood pressure, mm Hg | −0.051 | <0.001 | 0.016 | 0.714 | 0.013 |
| Diastolic blood pressure, mm Hg | −0.054 | <0.001 | −0.093 | 0.033 | −0.076 |
| Complications | |||||
| Microvascular complications | −0.070 | <0.001 | −0.088 | 0.282 | −0.039 |
| Micro-albuminuria | 0.000 | 0.986 | 0.046 | 0.499 | 0.024 |
| Macro-albuminuria | −0.064 | <0.001 | 0.080 | 0.298 | 0.037 |
| Retinopathy | −0.091 | <0.001 | −0.035 | 0.490 | −0.027 |
| Neuropathy | −0.065 | <0.001 | −0.024 | 0.685 | −0.015 |
| Macrovascular complications | −0.054 | <0.001 | −0.127 | 0.024 | −0.081 |
| Angina pectoris | 0.001 | 0.935 | 0.068 | 0.089 | 0.061 |
| Myocardial infarction | −0.057 | <0.001 | −0.013 | 0.791 | 0.009 |
| PTCA | −0.026 | 0.049 | 0.000 | 0.994 | 0.000 |
| CABG | −0.056 | <0.001 | −0.036 | 0.368 | −0.032 |
| TIA | 0.055 | <0.001 | 0.068 | 0.086 | 0.062 |
| CVA | 0.048 | <0.001 | 0.036 | 0.336 | 0.034 |
| Heart failure | −0.074 | <0.001 | −0.065 | 0.087 | −0.061 |
| Laboratory Measurements | |||||
| HbA1c, mmol/mol | −0.135 | <0.001 | −0.204 | <0.001 | −0.182 |
| Creatinine , µmol/L | −0.123 | <0.001 | −0.123 | 0.110 | −0.057 |
| eGFR (MDRD), ml/min/1.73 m2 | 0.036 | 0.007 | 0.097 | 0.167 | 0.050 |
| Total cholesterol, mmol/L | −0.030 | 0.025 | 0.226 | 0.279 | 0.039 |
| Total cholesterol:HDL ratio | −0.060 | <0.001 | −0.193 | 0.129 | −0.054 |
| HDL cholesterol, mmol/L | 0.022 | 0.102 | −0.169 | 0.205 | −0.045 |
| LDL cholesterol, mmol/L | −0.034 | 0.011 | −0.073 | 0.584 | −0.016 |
| Triglycerides, mmol/L | −0.055 | <0.001 | −0.018 | 0.810 | −0.009 |
| Albumin:creatinine ratio, mg/mmol | −0.089 | <0.001 | −0.059 | 0.477 | −0.025 |
| Medication | |||||
| Dietary measures only | −0.064 | <0.001 | 0.121 | 0.229 | 0.041 |
| Oral blood glucose lowering drugs | 0.087 | <0.001 | 0.025 | 0.849 | 0.007 |
| Metformin | 0.104 | <0.001 | 0.125 | 0.155 | 0.049 |
| Sulfonylurea derivatives | 0.026 | 0.053 | 0.019 | 0.632 | 0.017 |
| Thiazolinediones | −0.046 | 0.001 | −0.044 | 0.230 | −0.041 |
| DDP4 inhibitors | −0.002 | 0.872 | −0.027 | 0.447 | −0.026 |
| Insulin | −0.015 | 0.254 | 0.066 | 0.123 | 0.053 |
| Antihypertensive drugs | −0.075 | <0.001 | −0.051 | 0.232 | −0.041 |
| Diuretics | −0.050 | <0.001 | −0.031 | 0.426 | −0.027 |
| Beta blockers | −0.005 | 0.723 | 0.044 | 0.270 | 0.038 |
| Calcium antagonists | −0.035 | 0.011 | −0.031 | 0.399 | −0.029 |
| RAAS blockers | −0.012 | 0.367 | 0.043 | 0.295 | 0.036 |
| Cholesterol lowering drugs | 0.013 | 0.343 | −0.018 | 0.619 | −0.017 |
| Thrombocyte aggregation inhibitor | 0.044 | 0.001 | 0.137 | 0.001 | 0.116 |
Significant (p < 0.05) values are emphasized in bold. Abbreviations: BMI, body mass index; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass grafting; TIA, transient ischemic attack; CVA, cerebral vascular event; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, high-density lipoprotein; DDP4, dipeptidyl peptidase 4; RAAS, renin-angiotensin-aldosterone system; R-SH, total free thiol groups.
During the follow-up period of 1.2 [0.4–1.5] years, 3 (0.3%) patients died and there were 212 (22.4%) new microvascular and 51 (5.4%) macrovascular complications. Sixty-three patients (6.7%) developed neuropathy, 20 (2.1%) retinopathy, 118 (12.5%) micro-albuminuria and 33 (3.5%) macro-albuminuria. Thirty-nine (4.1%) of the patients developed angina pectoris, 1 (0.1%) myocardial infarction, 1 (0.1%) CVA, 4 (0.4%) TIA and 5 (0.5%) heart failure. One (0.1%) patient underwent a CABG and 5 patients (0.5%) a PTCA.
As presented in Table 3, the age- and sex adjusted hazard ratios (HRs) for developing microvascular, macrovascular or any complication plus death were 0.994 (95% CI 0.991, 0.997) in the lowest tertile, 0.992 (95% CI 0.985, 0.998) and 0.993 (95% CI 0.990, 0.996) in middle and highest tertile, respectively. After adjustment for potential confounders these HRs remained stable. There was no relevant difference in the Harrell's C statistics to predict macrovascular complications in the models with or without R-SH: 0.68 (95%CI 0.61, 0.76) and 0.67 (95%CI 0.58, 0.75). The Harrell’s C statistics to predict microvascular complications or any complication plus death were 0.63 (95% CI 0.58, 0.67) and 0.62 (95% CI 0.58, 0.66) in the models with R-SH and 0.59 (95% CI 0.55, 0.64) and 0.58 (95% CI 0.54, 0.63) without R-SH.
Table 3.
Hazard ratio’s and additional value of baseline R-SH concentrations in risk prediction compared to established cardiovascular risk markers.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Any complication and death | ||||
| Hazard ratio (95%CI) | 0.994 (0.991, 0.996) | 0.993 (0.990, 0.996) | 0.994 (0.991, 0.996) | NA |
| Harrell’s C (95% CI) | 0.58 (0.54, 0.63) | 0.60 (0.56, 0.64) | 0.62 (0.58, 0.66) | 0.58 (0.54, 0.63) |
| R2 | 0.05 (0.02, 0.11) | 0.06 (0.03, 0.13) | 0.06 (0.03, 0.15) | 0.01 (0.02, 0.09) |
| Grønnesby and Borgan test p-value | 0.45 | 0.58 | 0.41 | 0.58 |
| Macrovascular | ||||
| Hazard ratio (95%CI) | 0.994 (0.991, 0.997) | 0.992 (0.985, 0.998) | 0.993 (0.986, 0.999) | NA |
| Harrell’s C (95% CI) | 0.62 (0.53, 0.71) | 0.63 (0.54, 0.72) | 0.68 (0.61, 0.76) | 0.67 (0.58, 0.75) |
| R2 | 0.09 (0.00, 0.26) | 0.09 (0.00, 0.29) | 0.02 (−0.01, 0.32) | 0.03 (−0.02, 0.26) |
| Grønnesby and Borgan test p-value | 0.46 | 0.60 | 0.14 | 0.66 |
| Microvascular | ||||
| Hazard ratio (95%CI) | 0.994 (0.991, 0.997) | 0.994 (0.991, 0.997) | 0.993 (0.990, 0.996) | NA |
| Harrell’s C (95% CI) | 0.57 (0.53, 0.63) | 0.61 (0.57, 0.65) | 0.63 (0.58, 0.67) | 0.59 (0.55, 0.64) |
| R2 | 0.04 (0.01, 0.1) | 0.06 (0.03, 0.12) | 0.06 (0.03, 0.17) | 0.01 (0.00, 0.11) |
| Grønnesby and Borgan test p-value | 0.41 | 0.92 | 0.49 | 0.25 |
Model 1: crude.
Model 2: as model 1 and also adjusted for age and sex.
Model 3: as model 2 and also adjusted for duration of diabetes, smoking (yes/no), BMI, SBP, HbA1c, log serum creatinine , cholesterol-HDL ratio, use of blood glucose lowering drugs (yes/no), use of insulin (yes/no), and R-SH.
Model 4: as model 3 without R-SH.
Discussion
Serum free thiols, a measure of systemic redox status, are associated with HbA1c and micro- and macrovascular complications in outpatients with T2DM. Patients with higher concentrations of free thiols had better glycemic control as compared to patients with lower concentrations. Nevertheless, the additional value of R-SH for risk prediction of long-term complications was very modest during the short-term follow-up of this study. To the best of our knowledge, these data are the first to connect total serum R-SH status with clinical parameters and outcomes in a large T2DM cohort.
In this study we measured total R-SH concentrations to test the hypothesis that protein R-SH availability in human serum from T2DM patients is a relatively simple integrative marker of systemic redox status, and thereby disease status in T2DM. R-SH may serve as an important buffer for ROS and may thereby contribute to protection against the detrimental effects of reduced insulin signalling [2]. Indeed, our data demonstrate that higher concentrations of R-SH were associated with better glycemic control. The multivariable analysis revealed a strong correlation between serum R-SH and male gender. This gender difference may be related to differences in physical activity and/or muscle mass and the subsequent need for a greater buffer capacity to detoxify exercise-related free radical formation. Nevertheless, the nature of this needs to be explored in the future but is in line with our findings in a cohort of renal transplant recipients (n = 707) in which males also had higher serum R-SH concentrations [13]. Interestingly, diabetes duration was also positively association with levels of R-SH. This finding seems contra-intuitive since (increasing) diabetes duration is a well-known risk factor for the development of complications. Although speculative, it could be hypothesized that the diabetes duration of the present population (median of 6.5 years) distorted the relation with R-SH: with a high proportion of participants having a short diabetes duration, short treatment duration, suboptimal glycemic control and consequent low R-SH concentrations.
We observed that baseline serum R-SH concentrations were inversely associated with complications. This raises the question whether R-SH might be a promising therapeutic target. In renal transplant recipients we found that higher circulating R-SH concentrations were associated with a significantly lower future risk of premature death and graft failure [13]. This makes free R-SH measurements a potentially interesting and inexpensive screening tool for the early detection of a ‘high risk’ profile for future development of graft failure. The current data, however, does not support use of R-SH measurements for risk prediction in T2DM at present: there was only a very modest increase in predictive power of our model when adding R-SH to the model with known cardiovascular risk factors. However, the short follow-up duration of this study may well have influenced this finding. This may also be emphasized by the already low Harrell’s C statistics (of 0.59–0.67) in the models with known (long-term) cardiovascular risk factors and without R-SH. Therefore, studies with longer follow-up duration are warranted.
In addition to the limited follow-up duration our study has other limitations. For 45% of patients we were unable to match blood samples with clinical data. Our cohort is from a single region and comprises predominantly (98.8%) Caucasians: this limits the generalization of our data. Furthermore, this study lacks data on other plasma antioxidant species such as ascorbate, uric acid and small-molecular-weight thiols, markers of inflammation, glucose and insulin concentrations. Particular strengths of the study include the large cohort size, a considerable diversity in vintage of the duration and severity of the disease and the characterization of our patients.
Taken together, these data are the first to link total serum R-SH status to clinical parameters, including HbA1c, and outcomes in a large cohort of patients with T2DM. High serum R-SH concentrations seem to correlate with a better disease profile. However, the additional value of R-SH for risk prediction of complications was not relevant during the short-term follow-up in the present study. Further studies are warranted to explore the potential of R-SH as a modifiable marker for whole-body redox status in T2DM.
Declarations of interest
None.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2019.100182.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kaneto H., Katakami N., Matsuhisa M., Matsuoka T. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 3.Rochette L., Zeller M., Cottin Y., Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Fatehi-Hassanabad Z., Chan C.B., Furman B.L. Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol. 2010;636:8–17. doi: 10.1016/j.ejphar.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S., Gambhir J.K., Kalra O., Gautam A., Shukla K., Mehndiratta M. Association of biomarkers of inflammation and oxidative stress with the risk of chronic kidney disease in Type 2 diabetes mellitus in North Indian population. J Diabetes Complications. 2013;27:548–552. doi: 10.1016/j.jdiacomp.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Asmat U., Abad K., Ismail K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray P.D., Huang B.-W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones D.P., Sies H. The redox code. Antioxid Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turell L., Radi R., Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med. 2013;65:244–253. doi: 10.1016/j.freeradbiomed.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koning A.M., Meijers W.C., Pasch A., Leuvenink H.G.D., Frenay A.-R.S., Dekker M.M. Serum free thiols in chronic heart failure. Pharmacol Res. 2016;111:452–458. doi: 10.1016/j.phrs.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenay A.-R.S., de Borst M.H., Bachtler M., Tschopp N., Keyzer C.A., van den Berg E. Serum free sulfhydryl status is associated with patient and graft survival in renal transplant recipients. Free Radic Biol Med. 2016;99:345–351. doi: 10.1016/j.freeradbiomed.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Pasaoglu H., Sancak B., Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2004;203:211–218. doi: 10.1620/tjem.203.211. [DOI] [PubMed] [Google Scholar]
- 15.Srivatsan R., Das S., Gadde R., Manoj-Kumar K., Taduri S., Rao N. Antioxidants and lipid peroxidation status in diabetic patients with and without complications. Arch Iran Med. 2009;12:121–127. [PubMed] [Google Scholar]
- 16.Panigrahy S.K., Bhatt R., Kumar A. Reactive oxygen species: sources, consequences and targeted therapy in type 2 diabetes. J Drug Target. 2017;25:93–101. doi: 10.1080/1061186X.2016.1207650. [DOI] [PubMed] [Google Scholar]
- 17.Roelofsen Y., Hendriks S.H., Sieverink F., van Vugt M., van Hateren K.J., Snoek F.J. Design of the e-Vita diabetes mellitus study: effects and use of an interactive online care platform in patients with type 2 diabetes (e-VitaDM-1/ZODIAC-40) BMC Endocr Disord. 2014;14:22. doi: 10.1186/1472-6823-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks S.H., van Soldt E.G.W., van Vugt M., Groenier K.H., Roelofsen Y., Maas A.H.E.M. Lifestyle and emotional well-being in men and women with type 2 diabetes (e-VitaDM-4; ZODIAC-48) Eur J Gen Pract. 2017;23:83–90. doi: 10.1080/13814788.2017.1292348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014;104:1–52. doi:10.1016/j.diabres.2012.10.001. [DOI] [PubMed]
- 20.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Hu M.L., Louie S., Cross C.E., Motchnik P., Halliwell B. Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med. 1993;121:257–262. [PubMed] [Google Scholar]
- 22.Royston P., Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23:723–748. doi: 10.1002/sim.1621. [DOI] [PubMed] [Google Scholar]
- 23.May S., Hosmer D.W. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4:109–120. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


