Background
Vogt–Koyanagi–Harada (VKH) disease is a bilateral, chronic granulomatous panuveitis associated with central nervous system, auditory, and integumentary manifestations. Classically, the disease begins with a prodromal phase of neuroauditory symptoms, followed by an acute uveitis phase, and finally, the chronic stage manifestations. However, it is not uncommon for patients with initial-onset VKH disease to present with the isolated ocular disease, without associated neuroauditory symptoms.1 Any delay in establishing the correct diagnosis of initial-onset VKH disease and in initiating adequate treatment may result in higher risks of chronicity, complications, and visual impairment.2, 3 Therefore, accurate recognition of the distinctive ocular features associated with the initial-onset VKH will help to establish an early definitive diagnosis, with prompt initiation of appropriate treatment.
Several criteria have been proposed to clarify the diagnostic approach for VKH disease. In 1978, Sugiura suggested a set of criteria for the diagnosis of VKH disease. Bilateral ocular inflammation, especially with posterior manifestations, along with typical fluorescein angiography findings and pleocytosis of the cerebrospinal fluid were required criteria for the diagnosis.4 Although this was an immense step in outlining the features of VKH, his criteria were not sensitive enough to diagnose patients after the acute presentation. Moreover, the necessity of obtaining cerebrospinal fluid limited its application in many countries. Two years later, the American Society of Uveitis (AUS) redefined Sugiura’s criteria by detailing the posterior uveitis manifestations and extending the items of neuroauditory signs.5 The AUS criteria did not include pleocytosis as a required criterion for diagnosis but considered it part of the neuroauditory signs. Because sympathetic ophthalmia was not clinically distinguishable from VKH, “lack of history of trauma or surgery” was set as a prerequisite for diagnosis. However, the fluorescein angiography findings, which were an important clue during the acute stage of disease, failed to be mentioned. Similar to Sugiura’s criteria, the AUS criteria failed to differentiate between acute and chronic stages of the disease.
In 1999, the first VKH International Workshop group proposed the revised diagnostic criteria for VKH disease. It aimed to overcome the shortcomings of previous criteria, in particular, boosting up the sensitivity and specificity.6 The ocular manifestations were described in more detail and fluorescein angiography, and ultrasonography findings were adopted as useful ancillary tests for the diagnosis. Moreover, it categorized the VKH patients into three groups: (1) complete, (2) incomplete, and (3) probable based on the presence of ocular and extraocular manifestations.
Our purpose here is to explain, based on recent data, why the revised diagnostic criteria for VKH disease failed to improve management and should be reconsidered from the perspective of reaching a rapid diagnosis of initial-onset disease. Limitations of the revised criteria currently include: (1) not clearly differentiating initial-onset disease from chronic disease, which has a crucial impact on management; (2) division into three groups, which have very little clinical relevance; and (3) not considering highly sensitive investigations such as indocyanine green angiography (ICGA), spectral domain optical coherence tomography (SD-OCT), and high-penetration imaging techniques of OCT [enhanced depth imaging optical coherence tomography (EDI-OCT) and swept source OCT].
The different evolutional stages of VKH disease are not segregated properly
There is a growing body of evidence that underlines the importance of segregating the different evolutional stages of disease into initial-onset and chronic stages with varying intrinsic behaviors, courses, and prognoses. Identifying initial-onset ocular disease with or without associated neuroauditory signs is crucial, as it is a potentially curable condition when treated aggressively with dual steroidal and non-steroidal immunosuppressive therapy.7, 8, 9 If the patient is treated properly at the early stage of disease onset, he or she may never reach the chronic stages, and chronic ocular and integumentary signs may never occur.2, 3 On the other hand, the chronic disease that is associated with chronic ocular signs and integumentary manifestations results from late or insufficient treatment of initial-onset disease. Acute signs are no longer present at this stage. The disease will also have a different clinical course in the chronic stages where it is more vulnerable to recurrences and more resistant to treatment.10, 11, 12 Although the revised criteria partially addressed the acute and chronic manifestations, the importance of such a distinction was not put forward, and they did not discriminate between the different natural behaviors and clinical courses and, hence, did not contribute fundamentally to a better appraisal of VKH disease.
Mixing up the different evolutional stages of the disease also affects the result of studies on VKH. Remarkable dissimilarities exist in reports on VKH patients with regard to complications, visual outcomes, and proportions of disease subcategories.13, 14, 15, 16 Although these dissimilarities could be the result of diverse ethnical characteristics or different therapeutic approaches, it is also probable that they arise from inaccurate recognition and allocation of patients to initial-onset and chronic stages. These two conditions should not be included in the same study and should be the object of segregated studies.
Very recently, a new study on diagnostic criteria for VKH was published by a Chinese group that segregated initial-onset versus chronic disease in a very detailed fashion corresponding to what occurs in real clinical situations.17 This article contributed to make it clear in the mind of the clinician that early-onset and late diseases have to be differentiated and are quasi-different diseases as far as behavior and management are concerned.
Categorization of VKH patients as “complete, incomplete, and probable” has limited clinical, therapeutic, and prognostic implications
The usefulness of dividing the VKH patients into the three groups has been challenged in previous studies. In the Singaporean population, Chee et al. did not find any association between the clinical course and the final disease category of VKH patients.18 In another retrospective study on 67 VKH patients in Brazil, no association was found between the disease severity parameters (relapses, the presence of ocular complications, and the use of immunosuppressive therapy) and disease categories, and hence, the authors doubted the clinical and prognostic implications of such categorization.19
Early diagnosis and aggressive treatment have a significant impact on the course of VKH disease. A patient with the initial-onset disease will probably never reach the chronic phase if treatment is started properly, and the patient may never show the integumentary signs fulfilling the “complete” criteria.3 Therefore, having a specific type of VKH (complete, incomplete, or probable) is more influenced by treatment strategy and timing, rather than the real nature of disease per se.
The signs and symptoms of VKH evolve over time. Patients in the early phase of VKH disease rarely present with the complete form because it includes both acute and chronic signs, which obviously do not occur concomitantly. Rao and associates applied the diagnostic criteria to 116 well-documented patients with VKH.20 Although all the patients fulfilled the criteria, none of the 22 patients in the early phase group (symptoms lasting <4 weeks) was classified as complete VKH. In another retrospective study on a Japanese population of 41 VKH patients with the acute presentation, none of the patients would be classified as having “complete” VKH, had the revised diagnostic criteria been applied at two weeks after disease onset.21
On the other hand, only a minor fraction of patients seen after initial-onset disease could be categorized as “complete”. In Rao’s study, only 22% of 88 patients in the late phase fulfilled the criteria to be classified as “complete”.20 In another study in Iran, in spite of rather a long follow-up time (median 28 months, range 6–228 months), only 4.5% of VKH patients were classified as having the “complete” type of the disease.22 Similar observations were reported in other studies from around the world when trying to classify VKH according to the revised criteria.16, 22, 23, 24 The reasons for the low proportion of the “complete” VKH type are:
-
(1)
As discussed earlier, prompt diagnosis and proper treatment at disease onset may prevent the patient from passing into chronic stages, and the patient may never manifest integumentary signs to fulfill the “complete” criteria.
-
(2)
Establishing the “complete” type mandates to obtain in past disease history that there is evidence of acute presentation, which is not always feasible especially in those patients who were first seen in chronic stages and were initially treated by other primary care physicians during the acute presentation. In a Japanese study, the revised VKH criteria could not be applied in 5 out of 169 patients, who had previously been diagnosed with VKH disease (based on Sugiura’s criteria) and had an obvious sunset glow fundus, as they were unable to obtain enough evidence that could be used to document early manifestations.24
After publication of the revised criteria, dozens of studies were devoted to subdividing VKH series into the three categories, a theoretical exercise to no avail that did not contribute to a better appraisal and management of the disease.
Failure to refer to and include imaging techniques that precisely characterize stromal choroiditis
Imaging modalities, such as ICGA and to a lesser extent EDI-OCT, give precise and very sensitive information on choroiditis.25 Although ICGA was already well characterized in VKH and routinely used in many countries at the time the revised diagnostic criteria were generated, it was not included in the revised criteria.26 Several studies demonstrated that ICGA findings are positive in close to 100% of cases25, 27 and hence, should be considered a disease-defining criterion.
Due to less apparent choroidal signs during the very early stage of choroiditis, the clinical diagnosis may be missed or delayed when the revised diagnostic criteria are followed. The inflammation in VKH starts from the choroidal stroma and subsequently spreads towards the adjacent retina, optic nerve, and vitreous body. In very early disease, the inflamed choroid may not produce clinically detectable changes in the retina (e.g., exudative retinal detachment) and remain occult.28 Using ICGA and EDI-OCT, early choroidal involvement can be detected, contributing to early diagnosis and treatment.29, 30
In some patients, optic disc swelling is the initial ocular presentation of VKH and serous retinal detachment is absent or less remarkable.31 In a study by Okunuki, 14 out of 96 patients with acute VKH presented with optic disc swelling while there was minimal or no serous retinal detachment.32 Interestingly, in six patients, there was no detectable serous retinal detachment even on OCT, and only early manifestations of choroiditis on ICGA enabled a diagnosis of VKH disease. Kitumore acknowledged a similar limitation when trying to diagnose VKH patients based on revised criteria.24
Furthermore, there are a few reports of VKH disease that remained unilateral after a long follow-up. However, a subclinical disease in the fellow eye was not excluded in these patients by ICGA or B-scan ultrasonography before initiating systemic corticosteroids.33, 34, 35 Again, using ICGA and/or EDI-OCT and, when not available, B-scan ultrasonography, subclinical involvement in the other eye can be detected in the early stages of the disease and not be overlooked. Thanks to these extremely sensitive techniques, it can be said that there is no such thing as unilateral VKH. Such published cases are simply insufficiently investigated cases where the subclinical disease in the contralateral eye was missed.
The recently reported concept of “therapeutic window of opportunity” indicated that the earlier therapy is given (within the therapeutic window of opportunity), the higher the chances of not only to achieve successful therapy but even to cure the disease.3 Using the new sensitive diagnostic tools will contribute to catch more cases within this therapeutic window of opportunity. In the Chinese study cited above, the non-inclusion of ICGA in their criteria is difficult to understand, explained by its inaccessibility in China and its cost. However, the results of this study clearly showed that ICGA was most valuable for detecting choroidal inflammation in acute VKH disease. Except for bilaterality, a positive ICGA was their most frequent finding. ICGA also had high sensitivity and specificity of 80% and the best combination of positive predictive value and negative predictive value. Therefore, the ICGA findings should have been included in the diagnostic criteria, an important element for rapid diagnosis especially of early-onset disease, where rapid therapeutic intervention is critical.
OCT is another modality very helpful in the diagnosis of acute VKH disease. Beside fine characterization of signs, it accelerates the diagnosis of initial-onset disease by potentially showing subretinal fluid not detected clinically and choroidal thickening thanks to EDI-OCT or swept-source OCT.
Considering the arguments put forward in these lines, the approach for the diagnosis of VKH should be reconsidered and sharpened. Based on our experience and evidence-based medicine, we recommend to go beyond these criteria that have neglected the crucial distinction between initial-onset and chronic disease, and to include new and extremely sensitive diagnostic modalities, such as ICGA, spectral or swept-source OCT and EDI-OCT, that allow a quick diagnosis of early-onset disease when quick, aggressive, and sustained treatment especially matters. Adopting such an approach will lead to successful treatment of initial-onset disease and prevention of chronic evolution, as shown in the illustrative case below (Fig. 1, Fig. 2, Fig. 3).
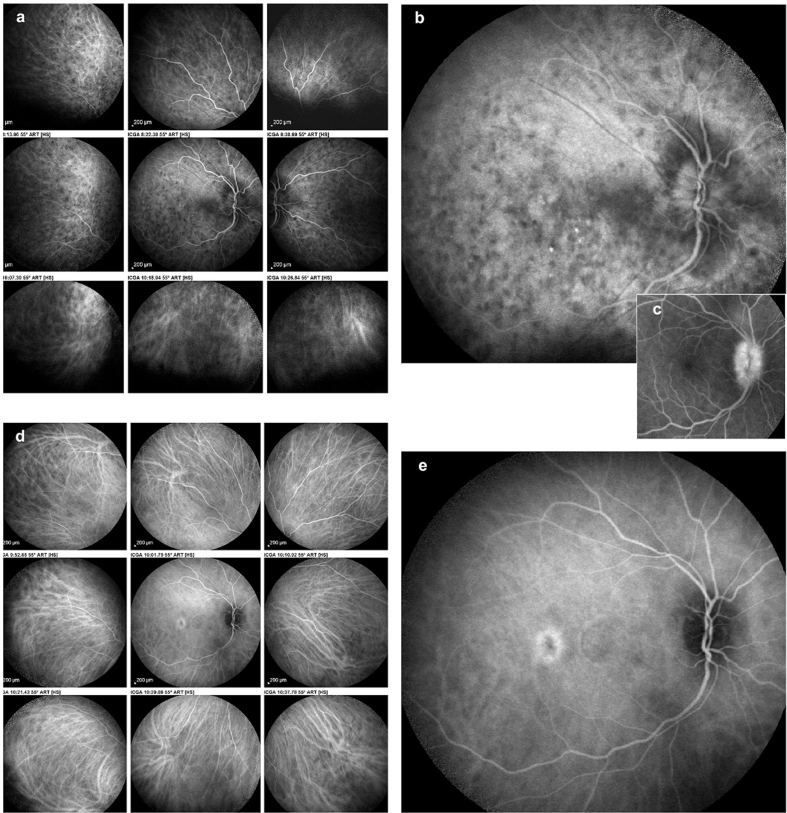
Fig. 1.
Case of initial-onset Vogt–Koyanagi–Harada (VKH) disease at presentation. Indocyanine green angiography (ICGA) performed about 10 days after the onset of symptoms showing numerous hypofluorescent dark dots (HDDs) and severe choroidal vasculitis (fuzzy unrecognizable choroidal vessels) (ICGA score = 31)36(1a). Many posterior pole HDDs and ICGA disc hyperfluorescence indicating severe choroidal inflammation (1b). Fluorescein angiography disc hyperfluorescence (1c). Three weeks after combined systemic corticosteroid and cyclosporine therapy, choroidal vessels have a normal pattern, and HDDs have decreased substantially (ICGA score = 6) (1d). In the posterior pole, HDDs are only faintly noted, and disc ICGA hyperfluorescence has disappeared (1e).
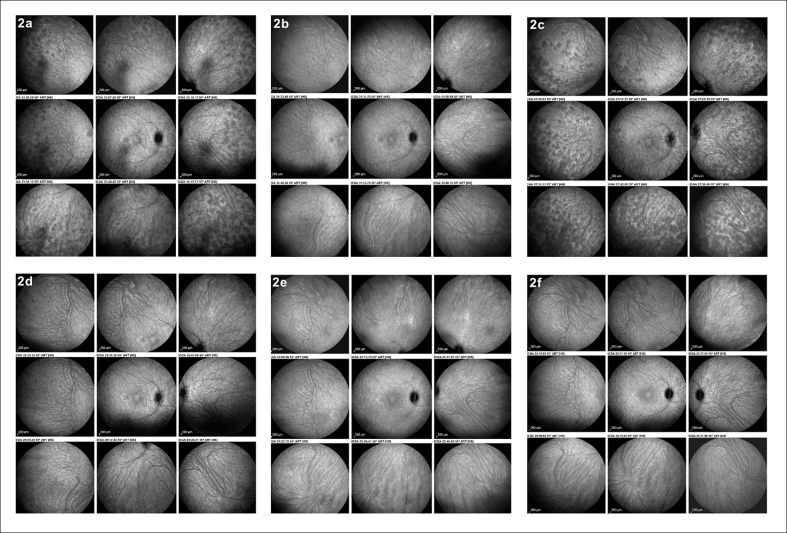
Fig. 2.
Follow-up of a case of initial-onset Vogt–Koyanagi–Harada (VKH) disease monitored by Indocyanine green angiography (ICGA). Three weeks after tapering of corticosteroids to 30 mg, there is subclinical recurrence with numerous HDDs and choroidal vasculitis (ICGA score = 17) (2a). Resolution of choroiditis after the introduction of mycophenolic acid (ICGA score = 0) (2b). After discontinuation of corticosteroids because of suspected central serous chorioretinopathy, a new subclinical recurrence of choroiditis occurred with many HDDs and choroidal vasculitis (ICGA score = 20) (2c). Complete resolution of choroiditis after the introduction of infliximab (2d). No recurrence after discontinuation of cyclosporine (2e) and discontinuation of mycophenolic acid (2f).
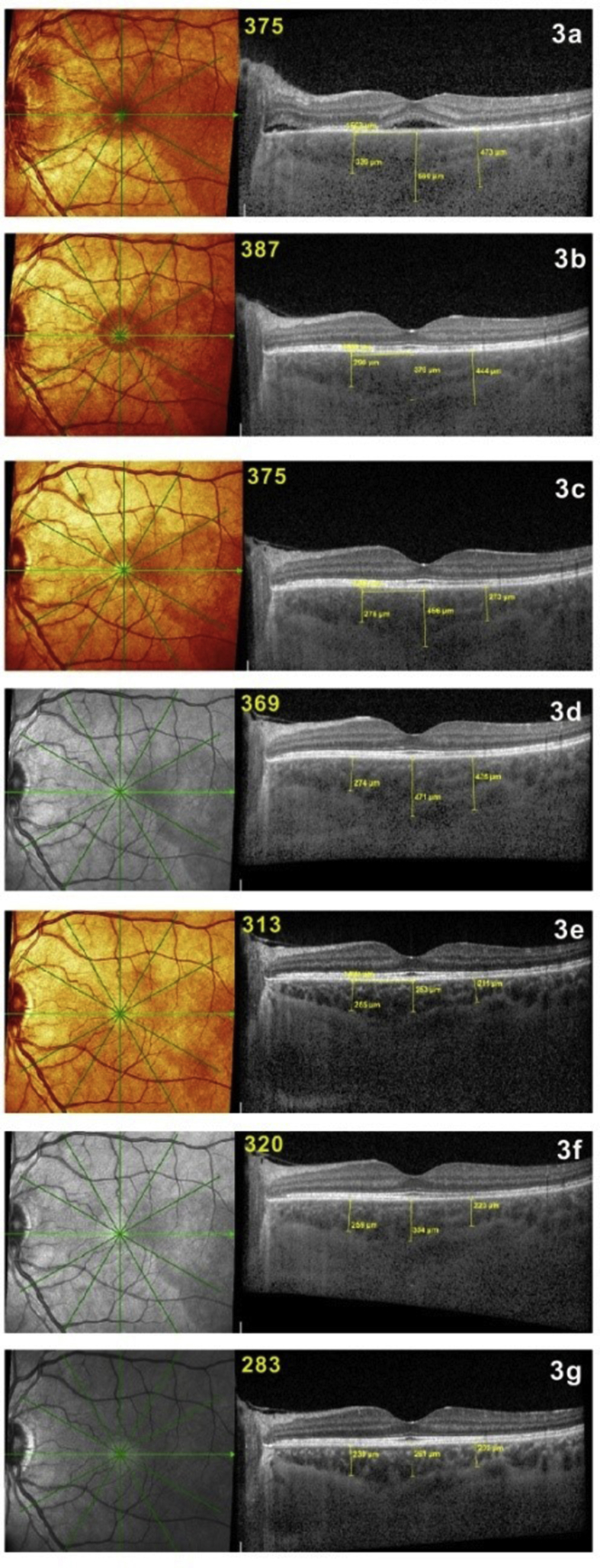
Fig. 3.
Follow-up of a case of initial-onset Vogt–Koyanagi–Harada (VKH) disease monitored by enhanced depth imaging optical coherence tomography (EDI-OCT) (average thickness indicated in yellow on scans). Three weeks after aggressive corticosteroid and cyclosporine therapy, there was a decrease of choroidal thickness from 550 to 375 μm with remaining pouches of subretinal fluid (3a). After that, EDI-OCT was unable to detect subclinical recurrences and their resolution seen by Indocyanine green angiography (ICGA) (3b-3f), although the choroidal thickness is gradually decreasing over time to 283 μm (3g).
Case: the crucial role of ICGA and EDI-OCT for quick diagnosis, prompt therapy, and precise ICGA-monitored tapering of therapy in a case of initial-onset VKH disease
A middle-aged woman was seen for a panuveitis with predominant posterior involvement including bilateral papillitis with peripapillary serous detachments. Fluorescein angiography showed hyperfluorescent discs (Fig. 1c) and areas of subretinal staining/pooling in the posterior pole. On ICGA, signs characteristic of VKH were noted, such as pronounced choroidal vasculitis (fuzzy vessels) and multiple, round, hypofluorescent dark dots (HDDs) in the posterior pole and evenly scattered in the periphery (Fig. 1a). Additionally, ICGA showed hyperfluorescent discs, a finding only rarely seen and present in pronounced choroidal inflammation (Fig. 1b). The mean EDI-OCT thickness was difficult to measure and was rated >550 μm, allowing a prompt diagnosis of VKH disease. Dual inflammation suppressive treatment (IST) was given, including IV methylprednisolone (1000 mg daily for 3 days), followed by oral prednisone (50 mg/day) together with cyclosporine (250 mg/day). After 3 weeks of this high dose IST, there was a spectacular regression of ICGA signs with a decrease in the ICGA score36 from 31 to 6, indicating that the choroiditis seemed to be under control (Fig. 1d) with the disappearance of ICGA disc hyperfluorescence (Fig. 1e). In parallel, the EDI-OCT choroidal thickness decreased from 550 to 375 μm (Fig. 3a). Upon tapering of corticosteroids to 30 mg, occult subclinical recurrence occurred (Fig. 2a) that responded to the introduction of mycophenolic acid (1400 mg/day) (Fig. 2b). Because of suspected central serous chorioretinopathy, corticosteroids were discontinued, followed by a new subclinical ICGA recurrence (Fig. 2c). EDI-OCT did not detect these subclinical recurrences and values remained between 387 and 369 μm (Fig. 3b–d), but subsequent EDI-OCT values decreased slightly (Fig. 3e–g). When infliximab (5 mg/kg) was introduced, choroiditis disappeared and never recurred, allowing discontinuation of cyclosporine and mycophenolic acid and prolonged intervals between infliximab infusions. This case shows how combined ICGA and EDI-OCT were very efficient in the rapid diagnosis of VKH disease in a case with a compatible clinical picture and how ICGA allowed the safe tapering of therapy without recurrence.
In summary, our aims here were: (1) to stress the crucial importance of distinguishing chronic VKH from initial-onset disease, as the latter can be cured when diagnosed and treated early; (2) to draw attention to the new diagnostic modalities available to clinicians, such as ICGA, OCT, and EDI-OCT, that contribute to an early diagnosis, especially of initial-onset disease and allow early and appropriate therapy.
Footnotes
The authors declare no conflict of interest.
Disclosure of funding: None.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Lavezzo M.M., Sakata V.M., Morita C. Vogt-Koyanagi-Harada disease review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. 2016;11:29. doi: 10.1186/s13023-016-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu El Asrar A.M., Dosari M., Hemachandran S., Gikandi P.W., Al-Muammar A. Mycophenolate mofetil combined with systemic corticosteroids prevents progression to chronic recurrent inflammation and development of ’sunset glow fundus’ in initial-onset acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2017;95(1):85–90. doi: 10.1111/aos.13189. [DOI] [PubMed] [Google Scholar]
- 3.Herbort C.P., Jr., Abu El Asrar A.M., Takeuchi M. Catching the therapeutic window of opportunity in early initial-onset Vogt-Koyanagi-Harada uveitis can cure the disease. Int Ophthalmol. 2018 doi: 10.1007/s10792-018-0949-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Sugiura S. Vogt–Koyanagi–Harada disease. Jpn J Ophthalmol. 1978;22:9–35. [Google Scholar]
- 5.Snyder D.A., Tessler H.H. Vogt–Koyanagi–Harada syndrome. Am J Ophthalmol. 1980;90(1):69–75. [PubMed] [Google Scholar]
- 6.Read R.W., Holland G.N., Rao N.A. Revised criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131(5):647–652. doi: 10.1016/s0002-9394(01)00925-4. [DOI] [PubMed] [Google Scholar]
- 7.Herbort C.P., Jr., Abu El Asrar A.M., Yamamoto J.H. Reappraisal of the management of Vogt-Koyanagi-Harada disease: sunset glow fundus is no more a fatality. Int Ophthalmol. 2017;37(6):1383–1395. doi: 10.1007/s10792-016-0395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu El-Asrar A.M., Hemachandran S., Al-Mezaine H.S., Kangave D., Al-Muammar A.M. The outcomes of mycophenolate mofetil therapy combined with systemic corticosteroids in acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2012;90(8):e603–e608. doi: 10.1111/j.1755-3768.2012.02498.x. [DOI] [PubMed] [Google Scholar]
- 9.Bouchenaki N., Herbort C.P. Indocyanine green angiography guided management of Vogt-Koyanagi-Harada disease. J Ophthalmic Vis Res. 2011;6(4):241–248. [PMC free article] [PubMed] [Google Scholar]
- 10.Abu El-Asrar A.M., Al Mudhaiyan T., Al Najashi A.A. Chronic recurrent Vogt-Koyanagi-Harada disease and development of “sunset glow fundus” predict a worse retinal sensitivity. Ocul Immunol Inflamm 2015. 2017;25(4):475–485. doi: 10.3109/09273948.2016.1139730. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi T., Horie S., Bouchenaki N., Ohno-Matsui K., Mochizuki M., Herbort C.P. Suboptimal therapy controls clinically apparent disease but not subclinical progression of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2010;30(1):41–50. doi: 10.1007/s10792-008-9288-1. [DOI] [PubMed] [Google Scholar]
- 12.Abu El-Asrar A.M., Al Tamimi M., Hemachandran S., Al-Mezaine H.S., Al-Muammar A., Kangave D. Prognostic factors for clinical outcomes in patients with Vogt-Koyanagi-Harada disease treated with high-dose corticosteroids. Acta Ophthalmol. 2013;91(6):e486–e493. doi: 10.1111/aos.12127. [DOI] [PubMed] [Google Scholar]
- 13.Murthy S.I., Moreker M.R., Sangwan V.S., Khanna R.C., Tejwani S. The spectrum of Vogt-Koyanagi-Harada disease in South India. Int Ophthalmol. 2007;27(2-3):131–136. doi: 10.1007/s10792-007-9046-9. [DOI] [PubMed] [Google Scholar]
- 14.Ozdal P., Ozdamar Y., Yazici A., Teke M.Y., Ozturk F. Vogt-Koyanagi-Harada disease: clinical and demographic characteristics of patients in a specialized eye hospital in Turkey. Ocul Immunol Inflamm. 2014;22(4):277–286. doi: 10.3109/09273948.2013.856448. [DOI] [PubMed] [Google Scholar]
- 15.Yodmuang T., Rothova A., Kunavisarut P., Pathanapitoon K. Vogt-Koyanagi-Harada disease in Thailand. Ocul Immunol Inflamm. 2012;20(6):419–422. doi: 10.3109/09273948.2012.723780. [DOI] [PubMed] [Google Scholar]
- 16.Khairallah M., Zaouali S., Messaoud R. The spectrum of vogt-koyanagi-harada. Disease in Tunisia, north Africa. Int Ophthalmol. 2007;27(2-3):125–130. doi: 10.1007/s10792-006-9013-x. [DOI] [PubMed] [Google Scholar]
- 17.Yang P., Zhong Y., Du L. Development and evaluation of diagnostic criteria for vogt-koyanagi-harada disease. JAMA Ophthalmol. 2018 Jul 5;136(9):1025–1031. doi: 10.1001/jamaophthalmol.2018.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chee S.P., Jap A., Bacsal K. Spectrum of vogt-koyanagi-harada disease in Singapore. Int Ophthalmol. 2007;27(2-3):137–142. doi: 10.1007/s10792-006-9009-6. [DOI] [PubMed] [Google Scholar]
- 19.da Silva F.T., Damico F.M., Marin M.L. Revised diagnostic criteria for vogt-koyanagi-harada disease: considerations on the different disease categories. Am J Ophthalmol. 2009;147(2):339–345. doi: 10.1016/j.ajo.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Rao N.A., Sukavatcharin S., Tsai J.H. Vogt-Koyanagi-Harada disease diagnostic criteria. Int Ophthalmol. 2007;27(2-3):195–199. doi: 10.1007/s10792-006-9021-x. [DOI] [PubMed] [Google Scholar]
- 21.Yamaki K., Hara K., Sakuragi S. Application of revised diagnostic criteria for vogt-koyanagi-harada disease in Japanese patients. Jpn J Ophthalmol. 2005;49(2):143–148. doi: 10.1007/s10384-004-0165-9. [DOI] [PubMed] [Google Scholar]
- 22.Hedayatfar A., Hosseini S.M., Karimi N. The spectrum of Vogt-Koyanagi-Harada disease in Iran. Int Ophthalmol. 2018;38(2):443–449. doi: 10.1007/s10792-017-0478-6. [DOI] [PubMed] [Google Scholar]
- 23.Sheu S.J., Kou H.K., Chen J.F. Prognostic factors for Vogt-Koyanagi-Harada disease. J Chin Med Assoc. 2003;66(3):148–154. [PubMed] [Google Scholar]
- 24.Kitamura M., Takami K., Kitaichi N. Comparative study of two sets of criteria for the diagnosis of Vogt-Koyanagi-Harada’s disease. Am J Ophthalmol. 2005;139(6):1080–1085. doi: 10.1016/j.ajo.2005.01.046. Erratum in: Am J Ophthalmol. 2006;141(6):1179. [DOI] [PubMed] [Google Scholar]
- 25.Balci O., Gasc A., Jeanin B., Herbort C.P., Jr. Enhanced depth imaging is less suited than indocyanine green angiography for close monitoring of primary choroiditis: a pilot study. Int Ophthalmol. 2017;37(3):737–748. doi: 10.1007/s10792-016-0303-7. [DOI] [PubMed] [Google Scholar]
- 26.Bouchenaki N., Herbort C.P. The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi-Harada disease. Ophthalmology. 2001;108(1):54–64. doi: 10.1016/s0161-6420(00)00428-0. [DOI] [PubMed] [Google Scholar]
- 27.Abouammoh M.A., Gupta V., Hemachandran S., Herbort C.P., Abu El-Asrar A.M. Indocyanine green angiographic findings in initial-onset acute Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2016;94(6):573–578. doi: 10.1111/aos.12974. [DOI] [PubMed] [Google Scholar]
- 28.Attia S., Khochtali S., Kahloun R. Clinical and multimodal imaging characteristics of acute Vogt-Koyanagi-Harada disease unassociated with clinically evident exudative retinal detachment. Int Ophthalmol. 2016;36(1):37–44. doi: 10.1007/s10792-015-0073-7. [DOI] [PubMed] [Google Scholar]
- 29.Herbort C.P., Mantovani A., Bouchenaki N. Indocyanine green angiography in Vogt-Koyanagi-Harada disease: angiographic signs and utility in patient follow-up. Int Ophthalmol. 2007;27(2-3):173–182. doi: 10.1007/s10792-007-9060-y. [DOI] [PubMed] [Google Scholar]
- 30.Miyanaga M., Kawaguchi T., Miyata K., Horie S., Mochizuki M., Herbort C.P. Indocyanine green angiography findings in initial acute pretreatment Vogt-Koyanagi-Harada disease in Japanese patients. Jpn J Ophthalmol. 2010;54(5):377–382. doi: 10.1007/s10384-010-0853-6. [DOI] [PubMed] [Google Scholar]
- 31.Nakao K., Abematsu N., Mizushima Y., Sakamoto T. Optic disc swelling in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2012;53(4):1917–1922. doi: 10.1167/iovs.11-8984. [DOI] [PubMed] [Google Scholar]
- 32.Okunuki Y., Tsubota K., Kezuka T., Goto H. Differences in the clinical features of two types of Vogt-Koyanagi-Harada disease: serous retinal detachment and optic disc swelling. Jpn J Ophthalmol. 2015;59(2):103–108. doi: 10.1007/s10384-014-0367-8. [DOI] [PubMed] [Google Scholar]
- 33.Usui Y., Goto H., Sakai J., Takeuchi M., Usui M., Rao N.A. Presumed Vogt-Koyanagi-Harada disease with unilateral ocular involvement: report of three cases. Graefes Arch Clin Exp Ophthalmol. 2009;247(8):1127–1132. doi: 10.1007/s00417-009-1068-8. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal A., Biswas J. Unilateral vogt-koyanagi-harada disease: report of two cases. Middle East Afr J Ophthalmol. 2011;18(1):82–84. doi: 10.4103/0974-9233.75898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neves A., Cardoso A., Almeida M., Campos J., Campos A., Castro Sousa J.P. Unilateral vogt-koyanagi-harada disease: a clinical case report. Case Rep Ophthalmol. 2015;6(3):361–365. doi: 10.1159/000441616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tugal-Tutkun I., Herbort C.P., Khairallah M. Angiography Scoring for Uveitis Working Group (ASUWOG). Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis) Int Ophthalmol. 2010;30(5):539–552. doi: 10.1007/s10792-008-9263-x. [DOI] [PubMed] [Google Scholar]