Abstract
Purpose
To evaluate the outcome of trabeculectomy versus Ahmed glaucoma valve (AGV) surgery in patients with Fuchs uveitis Syndrome (FUS).
Methods
Twenty-eight eyes with uncontrolled glaucoma and at least 6 months of follow-up were enrolled. In 16 eyes trabeculectomy and in 12 eyes AGV implant were performed. The primary outcome measure was surgical success defined as 5 < intraocular pressure (IOP) ≤ 21 mmHg (criterion A) and 5 < IOP ≤ 16 mmHg (criterion B), with at least 20% reduction in IOP, either with no medication (complete success) or with no more than preoperative medications (qualified success). The sum of complete and qualified success was defined as cumulative success.
Results
The mean age of the patients in the trabeculectomy group and the AGV group was 44.92 ± 9.02 and 45.76 ± 7.10 years, respectively (P = 0.79). The mean duration of follow-up was 23.06 ± 12.03 months in the trabeculectomy group and 22.83 ± 13.63 months in the AGV group (P = 0.96). The baseline mean IOP in trabeculectomy was 26.81 ± 6.69 mmHg which decreased to 11.61 ± 4.15 mmHg at last visit (P < 0.001). In the AGV group, mean IOP was 31.41 ± 6.76 at baseline that changed to 22.41 ± 5.09 at last visit (P = 0.005). According to criterion A, cumulative success rates were 100% and 91% at 6 months and 76% and 9% at 36 months in the trabeculectomy and the AGV group, respectively. Cumulative success rates at 6 months were 93% and 58% and 65% and 7% at 36 months according to criterion B in the trabeculectomy and the AGV group, respectively.
Kaplan-Meier survival analysis revealed a significant association between surgical method and cumulative success rate over 36 months (based on criteria A: P = 0.02, and based on criteria B: P = 0.007).
Conclusion
The success rate of trabeculectomy was higher than AGV in the surgical management of glaucoma in FUS during a medium-term follow-up.
Keywords: Fuchs uveitis syndrome, Ahmed glaucoma valve, Trabeculectomy, Glaucoma
Introduction
Fuchs uveitis syndrome (FUS) or Fuchs heterochromic iridocyclitis (FHI) is a low-grade, chronic intraocular inflammatory disease which accounts for 1.5%–4.5% of all uveitis.1, 2
Most patients with FUS are diagnosed on routine ophthalmological examination in their third or fourth decade of life, without gender preference.3, 4, 5, 6 FUS usually affects one eye, causing iris heterochromia without ocular discomfort or pain.7, 8, 9, 10
Cataract and glaucoma are the major complications of FUS; however, because of the favorable results of cataract surgery, glaucoma is considered the most serious complication.11, 12
The intraocular pressure (IOP) elevation in these patients is often intermittent and sub-acute in the early stages and may respond to the short-term use of topical corticosteroids.13 Several possible causes have been proposed for the rise of IOP such as recurrent hyphema, neovascularization of the angle, peripheral anterior synechiae, trabeculitis, trabecular sclerosis, the collapse of the Schlemm's canal, corticosteroid treatment, and cataract extraction, but usually, no single and apparent reason can be found.5, 8, 14, 15, 16 Few studies have explained the management of glaucoma in FUS and reported that medical management was not a sufficient treatment.13, 17, 18, 19 For uveitic patients with uncontrolled glaucoma, several procedures have been performed, such as trabeculectomy, glaucoma drainage implants, ciliary body destructive procedures, and angle surgeries.20, 21, 22, 23 However, little information is available in the literature about the surgical outcome of glaucoma in FUS patients.24, 25, 26
In this study, we aimed to compare the outcome of two different types of glaucoma surgery, trabeculectomy and Ahmed glaucoma valve (AGV), in patients with FUS.
Methods
In this retrospective cohort study, we reviewed the charts of all FUS patients with secondary glaucoma who underwent glaucoma surgery from 2010 to 2015. The study design was approved by the Ethics Committee of the Iran University of Medical Sciences, and all patients signed the inform consent.
Twenty-eight eyes of 28 patients with at least 6 months of follow-up who met the inclusion criteria were enrolled in this study. Inclusion criteria were patients with FUS that required glaucoma surgery due to the insufficiency of medical treatment to achieve the target IOP or low compliance of patients for medical therapy. Exclusion criterion was previous intraocular surgeries except history of uncomplicated cataract surgery more than 6 months before glaucoma surgery and any other ocular diseases that could influence the results, such as history or presence of diabetic retinopathy or retinal vascular diseases. FUS diagnosis was according to the clinical features described by Kimura et al., in 1955 and La Hey et al. in 1991, which included: characteristic diffusely spread stellate shape keratic precipitates (KPs), low-grade anterior chamber reaction, iris atrophy and depigmentation with or without heterochromia, absence of posterior synechiae, open angle in gonioscopy and lack of acute symptoms of severe pain, redness, and photophobia during follow-up visits.5, 26 Glaucoma was defined as the presence of glaucomatous optic neuropathy (neuroretinal rim loss, vertical cup: disc ratio ≥0.7, asymmetric cup: disc ratio >0.2 between two eyes and nerve fiber loss in the fundoscopic examination) and corresponding glaucomatous visual field defect.27 Demographic data and preoperative findings such as best corrected visual acuity (BCVA), IOP (measured by calibrated Goldmann applanation tonometry), slit-lamp biomicroscopy fundoscopy (using 78 diopter lens), gonioscopy, number of glaucoma medications, mean deviation in Humphrey visual field test as well as corresponding postoperative values at each follow-up visit were collected. Betamethasone eye drop, 4 times a day, was started from 7 days before the date of the surgery for all patients.
Since there was no strong evidence regarding the better outcome of either of these surgeries in comparison to other one, patients were allocated to each group according to the socioeconomic condition, compliance for the possible strict postoperative visits, and preference of the patients based on the information given by the surgeon about the possibility of complications and reoperation following each surgery.
The primary outcome measure was defined as the surgical success based on two criteria: 5 < IOP ≤ 21 mmHg (criterion A) and 5 < IOP ≤ 16 mmHg (criterion B) with at least 20% reduction in IOP either with no medication (complete success) or no more than preoperative medications (qualified success). Cumulative success was defined as the sum of qualified and complete success. Subjects with IOP outside the range of success on 2 consecutive visits after month 3 or those who needed repeated glaucoma surgery were classified as a failure. Needling of the bleb behind the slit-lamp was not considered a failure.
Surgical technique
Surgeries were performed under local or general anesthesia by a single surgeon (N.N.) by considering the age or systemic condition of the patient. In the trabeculectomy group, each patient underwent a fornix-based trabeculectomy by making a conjunctival flap in the supranasal quadrant followed by a half thickness trapezoidal scleral flap (3 × 2 mm). After creating the scleral flap, mitomycin C (MMC) 0.02% was applied using multiple thin sponges under the scleral flap and between the sclera and tenon capsule for 3 min. The sponges then were removed, and the surgical field was irrigated with copious amounts of balanced salt solution. Sclerectomy was performed with a Kelly-Descemet punch, and a peripheral iridectomy was created with Vannas scissors. The scleral flap was secured with two 10-0 nylon sutures using the releasable technique. At the end of the surgery, the conjunctiva was closed with 10-0 nylon sutures, and subtenon long-acting betamethasone was injected in the inferotemporal quadrant.
In the shunt group, fornix-based peritomy was performed in the superotemporal quadrant, and MMC 0.02% was applied for 2 min and then irrigated with 50 CC balanced salt solution. After priming of AGV model FP7 (New World Medical, Rancho Cucamonga, LA) with a balanced salt solution, it was secured to the sclera with two 8-0 nylon sutures 10–11 mm behind the limbus. The tube was trimmed beveled up and then inserted into the anterior chamber through the tunnel made by a 23 gauge needle at the posterior limbus. The tube was then secured to the sclera with a 10-0 nylon suture and covered with a donor sclera. Conjunctiva was closed with 10 vicryl suture in a continuous fashion. At the end of the surgery subtenon, long-acting betamethasone was injected in the inferotemporal quadrant.
In both groups, postoperative medications included ciprofloxacin eye drops every 6 h for approximately 1–2 weeks and betamethasone eye drops every 2 h for approximately 2 weeks which was tapered gradually to 2 times a day over the next 8–12 weeks and then was switched to fluorometholone twice a day for the next 1–2 months. Patients were examined on postoperative day 1 and then at least weekly for 4 weeks and then every 1–3 months based on the clinical judgment. IOP lowering medication was started after the surgery according to the stages of glaucoma, target IOP, and discretion of the surgeon.
Serious intraoperative complications were defined as supra choroidal hemorrhage, vitreous loss, vitreous hemorrhage, malignant glaucoma, and scleral flap tear or dehiscence. Postoperative complications were defined as extensive or kissing choroidal detachment, malignant glaucoma, suprachoroidal hemorrhage, retinal vascular accident, blebitis, or endophthalmitis.
The hypertensive phase (HP) was defined as IOP measurement >21 mmHg during the first 3 months after AGV surgery (with or without medications), after reduction of IOP to less than 21 mmHg during the first postoperative week, and not as a result of tube obstruction, retraction, or valve malfunctions.28
Statistical analysis
Data analysis was performed using SPSS software version 16 (SPSS for Windows, Chicago, IL). Data were reported as mean ± SD and frequency (relative frequency). Chi-square and student T-test were used to compare proportion and means, respectively. The cumulative probability of success was analyzed by Kaplan–Meier life table analysis. P-value of <0.05 was considered significant.
Results
A total of 28 eyes with the diagnosis of FUS and glaucoma with no history of previous glaucoma surgery were enrolled in the study. Sixteen patients (53.5%) were in the trabeculectomy group, and 12 patients (46.5%) were in the shunt group. Eight patients (46.6%) in the trabeculectomy and 7 patients (58.30%) in the AGV group were men. Table 1 shows the baseline characteristics of the patients in trabeculectomy and shunt groups. All cases with pseudophakia had a previous history of uncomplicated temporal clear cornea phaco surgery. The conjunctiva was completely intact at the superior part in these cases.
Table 1.
Baseline characteristics of patients with Fuchs uveitis Syndrome (FUS) treated with trabeculectomy and Ahmed glaucoma valve (AGV).
| Trabeculectomy | Ahmed glaucoma valve (AGV) | Independent t-test and Chi-square Test | |
|---|---|---|---|
| Age, year (mean ± SD) | 44.92 ± 9.02 | 45.76 ± 7.1 | P = 0.79 |
| Number of cases (male) | 16 (8) | 12 (7) | P = 0.54 |
| Preoperative IOP (mean ± SD) mmHg (range) 95% confidence interval (lower-upper) |
26.81 ± 6.69 (18–38) (23.50–27.75) |
31.41 ± 6.76 (22–40) (27.60–35.33) |
P = 0.08 |
| Preoperative number of anti-glaucoma medications (mean ± SD), (range) 95% confidence interval (lower-upper) |
2.62 ± 0.80 (1–4) (2.21–3.00) |
3.00 ± 0.95 (1–4) (2.42–3.57) |
P = 0.40 |
| Preoperative visual acuity (mean ± SD) logMAR | 0.4 ± 0.3 | 0.4 ± 0.26 | P = 1.00 |
| Preoperative mean deviation in visual field (mean ± SD) Decibel | −10.40 ± 15.42 | −13.97 ± 11.23 | P = 0.43 |
| Lens status phakic/pseudophakic | 4/12 | 3/9 | P = 0.83 |
IOP: Intraocular pressure.
Mean ± SD of the follow-up period was 23.06 ± 12.03 (range, 6–36) and 22.83 ± 13.63 months (range, 6–36) in the trabeculectomy and AGV group, respectively (P = 0.96).
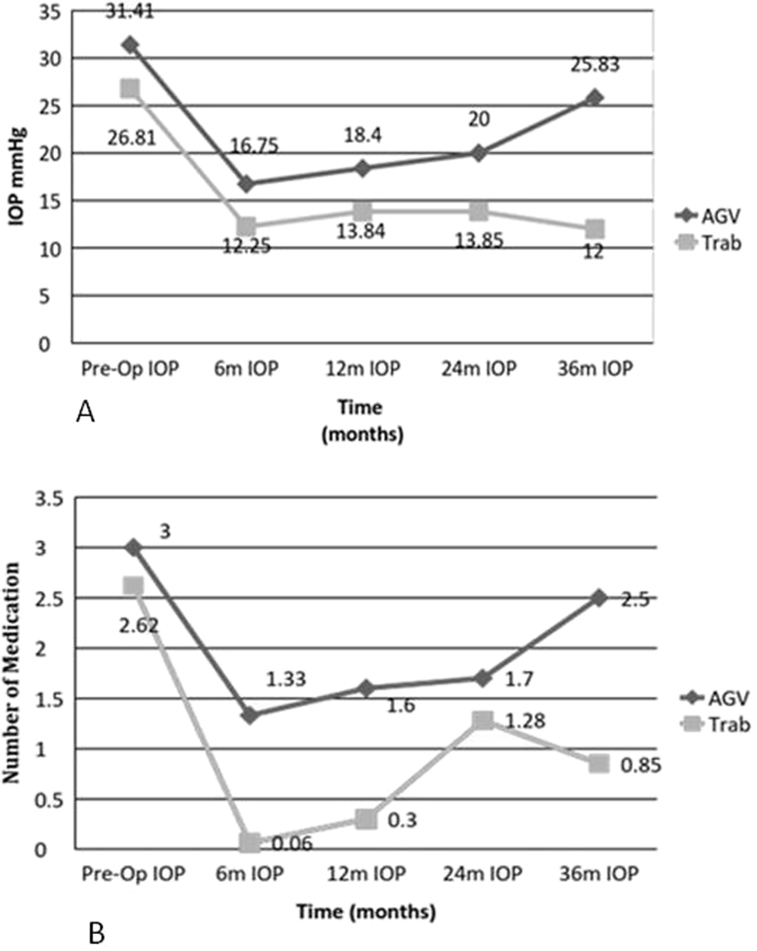
At last visit, IOP was significantly lower compared with baseline IOP in both groups, but the number of medication was lowered only in the trabeculectomy group compared with baseline (Table 2). Fig. 1A,B show the mean IOP and number of medications during the follow-up period in each group. The IOP changes between baseline, 6 months, one year, and last visit values were not significantly different between the two groups (All P > 0.05). The mean percent of IOP changes in the 6th month, and 1 year from baseline values were not significantly different between the two groups (P > 0.05), but it was significantly higher in the trabeculectomy group at last visit (P = 0.02).
Table 2.
Comparison of intraocular pressure (IOP) and number of anti-glaucoma medication between baseline and last postoperative visits in each group.
| Type of surgery | Variable | Baseline visit | Last visit | P value |
|---|---|---|---|---|
| Trabeculectomy | IOP mmHg (mean ± SD) | 26.81 ± 6.69 (18–38) | 12.31 ± 4.98 | <0.001 |
| (range) | (23.50–27.75) | (5−21) | ||
| 95% confidence interval (lower-upper) | (9.93–14.68) | |||
| Anti-glaucoma | 2.62 ± 0.80 | 0.68 ± 1.19 | <0.001 | |
| Medication (mean ± SD) | ||||
| (range) | (1–4) | (0–3) | ||
| 95% confidence interval (lower-upper) | (2.18–3.00) | (0.18–1.33) | ||
| Ahmed glaucoma valve (AGV) | IOP mmHg (mean ± SD) | 31.41 ± 6.76 | 21.83 ± 7.05 | 0.002 |
| (range) | (22–40) | (13–40) | ||
| 95% confidence interval (lower-upper) | (27.60–35.33) | (18.25–26.33) | ||
| Anti-glaucoma medication (mean ± SD) | 3.00 ± 0.95 (1–4) | 2.16 ± 0.93 | 0.010 | |
| (range) | (1–4) | (1–4) | ||
| 95% confidence interval (lower-upper) | (2.5–3.5) | (1.66–2.66) |
IOP: Intraocular pressure.
Fig. 1.
A: Pre-operation and Post-operation intraocular pressure (IOP) measures in two surgical groups. B: Pre-operation and post-operation number of medications in two surgical groups.
Complete success was achieved in 75% and 50% of trabeculectomy cases based on criterion A and B, respectively. In the AGV group, all cases received medication, thus, complete success was not achieved in this group.
Table 3 shows the cumulative success rate during the follow-up period in two groups based on two criteria. Overall, the failure occurred in 7 (43.75%) patients of the AGV group and 2 (16.66%) patients of the trabeculectomy group based on criterion A, and in 10 (62.5%) patients of the AGV group and 4 (33.33%) patients of the trabeculectomy group based on criterion B.
Table 3.
Cumulative success rate in different months in trabeculectomy versus Ahmed glaucoma valve (AGV) group.
| Survival group | 6 months |
12 months |
24 months |
36 months |
||||
|---|---|---|---|---|---|---|---|---|
| Criterion A | Criterion B | Criterion A | Criterion B | Criterion A | Criterion B | Criterion A | Criterion B | |
| Trabeculectomy | 100% (na = 16) | 93% (n = 16) | 100% (n = 13) | 75% (n = 13) | 76% (n = 7) | 65% (n = 7) | 76% (n = 7) | 65% (n = 7) |
| Ahmed glaucoma valve (AGV) | 91% (n = 12) | 58% (n = 12) | 91% (n = 10) | 49% (n = 10) | 61% (n = 7) | 20% (n = 7) | 9% (n = 6) | 7% (n = 6) |
Since our study was a longitudinal study, we had some patients with loss to follow-up during time which is common in these kinds of study.
Number of patients in each group in the specific time period.
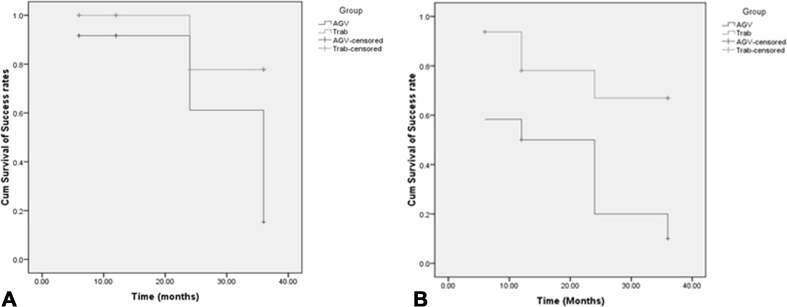
Kaplan-Meier analysis revealed a significant association between surgical method and total success rate over 36 months (based on criteria A: P = 0.02, and based on criteria B: P = 0.007) in favor of trabeculectomy (Fig. 2A, B).
Fig. 2.
A: Comparison of the cumulative success rate of Ahmed glaucoma valve (AGV) surgery and trabeculectomy based on criterion A in patients with Fuchs uveitis Syndrome (FUS). B: Comparison of the cumulative success rate of AGV surgery and trabeculectomy based on criterion B in patients with FUS.
In the AGV group, the HP was detected in 4 patients (30%). In both groups, there were no serious intraoperative or postoperative complications. In the AGV group, one eye had postoperative hyphema, and in the trabeculectomy group, two eyes had hypotonic maculopathy which all resolved spontaneously without any surgical intervention. In the trabeculectomy group, one case needed bleb needling and subconjunctival injection of MMC in weeks 3, 5, and 7 after surgery.
Discussion
In the current study, we found that the complete and cumulative success rates were significantly higher in the trabeculectomy group in comparison with the AGV group during 36 months of follow-up. Although the changes in the IOP from baseline values in different time points between the two groups were not significant, based on our definition of success rate, better control of IOP was found in the trabeculectomy group. According to our findings, it seems that AGV works well during the first year after the surgery, but the effects decline with a steep slope over the following years. This should be considered especially for those patients with advanced glaucoma and those with poor compliance for using medications.
Secondary glaucoma is the most troublesome complication in FUS and can lead to a permanent visual loss. The etiology of glaucoma in FUS is poorly understood. Huber considered the main cause of ocular hypertension to be an increase in the outflow resistance at the site of the trabecular meshwork.29 Chandler and Grant stated that rubeosis of the iris in the anterior chamber angle induces the development of chronic secondary glaucoma.30
Previous studies reported a prevalence of 6.5%–59% for secondary glaucoma in FUS.8 This large variation may be because of different definitions of glaucoma, follow-up time, and different manifestations of FUS. Arrelanes-Garcia et al. noticed elevated IOP at some stage of FUS in 30.66% of their patients, but the diagnosis of glaucoma was made only in 4%.31 In the cohort of Norrsell and Sjödell, these percentages were 24% and 11%, respectively.10 Jones and Liesegang, in the retrospective studies, found the risk of glaucoma development 4% and 0.5% per each year of follow-up, respectively.8, 13
As earlier studies showed, usually glaucoma in FUS becomes refractory to medical treatment13, 17, 18; therefore, surgical intervention is needed to control the IOP. Liesegang reported sufficient IOP control following one surgery (usual trabeculectomy without MMC) in 12 of the 21 (57%) patients.13 La Hey and colleagues reported that surgical intervention (mostly trabeculectomies) was able to control IOP in 72% of patients after a mean follow-up of 26 months.19
In a retrospective study conducted by Iverson et al. patients with uncontrolled glaucoma due to uveitis (only 1 case of FUS in each group) that had undergone trabeculectomy with anti-fibrotic therapy or Baerveldt shunt implantation were enrolled. At the end of the follow-up mean IOP, the number of anti-glaucoma medications and also complications were similar between the two groups. But the cumulative probability of failure after 5 years of follow-up was significantly greater in trabeculectomy eyes (62%) compared with glaucoma drainage device eyes (25%), and they concluded that non-valved shunt surgery is more likely to maintain IOP control in long-term.32 Kwon and colleagues33 in a recent retrospective study on patients with uveitis reported that the rate of success in the trabeculectomy and glaucoma device implant was not significantly different.
In the present study, we used a valved shunt (AGV), and the study subjects were all from FUS patients who had a relatively benign course in comparison to other types of uveitis. These factors may affect the final results and differences seen in these studies. Based on criterion A, we found failure in 6 patients of the AGV group. Of them, 3 patients had IOP more than 21 mmHg, one patient needed one drug more than the preoperative number of medications, and 2 patients had both of the above criteria. In the trabeculectomy group, 2 patients experienced failure due to the higher number of medications as compared to preoperative ones. Based on criterion B, 4 and 2 cases were added to the previous failure number in the AGV and the trabeculectomy groups, respectively, due to the IOP more than 16 mmHg. In both groups, AGV was implanted for those cases with the diagnosis of failure with satisfactory IOP control at the end of the follow-up.
In a study on patients with FUS that underwent trabeculectomy with intraoperative applications of MMC (11 patients with follow-up of 25.09 ± 14.4 months), You et al. reported the total success of 90.9% at 1 year, 62.3% at 4 years, and the complete success of 63.6% and 31.8% at 1 and 4 years, respectively.25 In a retrospective study by Voykov and colleagues, the outcome of AGV implantation in FUS patients with glaucoma was evaluated. They defined two criteria: 6 mmHg ≤ IOP ≤21 mmHg (success 1), and 6 mmHg ≤ IOP ≤21 mmHg and at least a 25% reduction from baseline (success 2). They reported complete success rates of 23.5% (n = 17) after 1 year and 23% (n = 13) after 3 years and qualified success rates of 58.3% (n = 17) after 1 and 38.4% (n = 13) after 3 years for both definitions.34
In general, the main complication of filtration surgery in FUS is bleb-related failure including encapsulated bleb and bleb flattening. Both Jones and La Hey et al. recommended the use of fibrosis-inhibiting drugs (such as 5-fluorouracil or MMC) in FUS patients to inhibit this process even though its benefit has yet to be proven in FUS.8, 19 We used MMC in all trabeculectomy surgeries with no reported case of bleb failure. The level of preoperative ocular inflammation is always a concern in glaucoma surgeries and is even more prominent in uveitis patients. Although Fuchs patients are among the low-grade uveitis, as a routine, we started steroid drops a week before surgeries. Thus, on the day of the surgery, eyes were at a quiescent stage. This strategy also made both groups more uniform in terms of the preoperative level of inflammation.
The non-randomized and retrospective design of this study as well as a small number of study subjects and the short duration of follow-up were among the limitations of this study. However, to the best of our knowledge, this is the first study which compared the outcome of trabeculectomy and shunt surgery in FUS patients with secondary glaucoma. We found a higher success rate and better IOP result with trabeculectomy. The number of postoperative antiglaucoma medications was also significantly more in the AGV group, and actually, all patients in this group needed medication. This is not surprising and is in concordance with most studies about AGV and different kinds of glaucoma.
Finally, to better understand the surgical outcome of glaucoma surgery in FUS patients, randomized control trials with a longer duration of the follow-up period are recommended.
Footnotes
Financial Disclosure(s): None.
Funding resource: None.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Schlaegel T.F., Jr. Differential diagnosis of uveitis. Ophthalmol Digest. 1973;35:34. [Google Scholar]
- 2.Kijlstra A., Rothova A., Baarsma G.S. Computer registration of uveitis patients. Doc Ophthalmol. 1987;67(1-2):139–143. doi: 10.1007/BF00142708. [DOI] [PubMed] [Google Scholar]
- 3.Schwab I.R. Fuchs' heterochromiciridocyclitis. Int Ophthalmol Clin. 1990;30(4):252–256. doi: 10.1097/00004397-199030040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham E.T., Jr., Baglivo E. Fuchs heterochromic iridocyclitis—syndrome, disease, or both? Am JOphthalmol. 2009;148(4):479–481. doi: 10.1016/j.ajo.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Kimura S.J., Hogan M.J., Thygeson P. Fuchs syndrome of heterochromic cyclitis. Arch Ophthalmol. 1955;54(2):179–186. doi: 10.1001/archopht.1955.00930020181003. [DOI] [PubMed] [Google Scholar]
- 6.Bonfioli A.A., Curi A.L., Orefice F. Fuchs' heterochromic cyclitis. Semin Ophthalmol. 2005;20(3):143–146. doi: 10.1080/08820530500231995. [DOI] [PubMed] [Google Scholar]
- 7.Accorinti M., Spinucci G., Pirraglia M.P., Bruschi S., Pesci F.R., Iannetti L. Fuchs' heterochromic iridocyclitis in an Italian tertiary referral centre: epidemiology, clinical features, and prognosis. J Ophthalmol. 2016;2016:1458624. doi: 10.1155/2016/1458624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones N.P. Fuchs' heterochromic uveitis: a reappraisal of the clinical spectrum. Eye. 1991;56(Pt 6):49–61. doi: 10.1038/eye.1991.121. [DOI] [PubMed] [Google Scholar]
- 9.Fearnley I.R., Rosenthal A.R. Fuchs' heterochromiciridocyclitis revisited. Acta Ophthalmol Scand. 1995;73(2):66–70. doi: 10.1111/j.1600-0420.1995.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 10.Norrsell K., Sjodell L. Fuchs' heterochromic uveitis: a longitudinal clinical study. Acta Ophthalmol. 2008;86(1):58–64. doi: 10.1111/j.1600-0420.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones N.P. Fuchs' heterochromic uveitis: an update. Surv Ophthalmol. 1993;37(4):253–272. doi: 10.1016/0039-6257(93)90009-v. [DOI] [PubMed] [Google Scholar]
- 12.La Hey E., de Jong P.T., Kijlstra A. Fuchs heterochromiccyclitis: review of the literature on the pathogenetic mechanisms. Br J Ophthalmol. 1994;78(4):307–312. doi: 10.1136/bjo.78.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liesegang T.J. Clinical features, and prognosis in Fuchs' uveitis syndrome. Arch Ophthalmol. 1982;100(10):1622–1626. doi: 10.1001/archopht.1982.01030040600009. [DOI] [PubMed] [Google Scholar]
- 14.Dernouchamps J.P. Acta Ophthalmologica; 1984. Fuchs Heterochromic Cyclitis. 49-49. [Google Scholar]
- 15.Tabbut B.R., Tessler H.H., Williams D. Fuchs' heterochromiciridocyclitis in blacks. Arch Ophthalmol. 1988;106(12):1688–1690. doi: 10.1001/archopht.1988.01060140860027. [DOI] [PubMed] [Google Scholar]
- 16.velilla S., Dios E., Herreras J.M., Calonge M. Fuchs' heterochromic iridocyclitis: a review of 26 cases. Ocul Immunol Inflamm. 2001;9(3):169–175. doi: 10.1076/ocii.9.3.169.3964. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz-Negrete Fj, Montañés J.M., Hernández-Martínez P., Rebolleda G. Current approach in the diagnosis and management of uveitic glaucoma. BioMed Res Int. 2015;2015:742792. doi: 10.1155/2015/742792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Mansour Y.S., Al-Rajhi A.A., Al-Dhibi H., Abu El-Asrar A.M. Clinical features and prognostic factors in. Fuchs' uveıtis. Int Ophthalmol. 2010;30(5):501–509. doi: 10.1007/s10792-010-9379-7. [DOI] [PubMed] [Google Scholar]
- 19.La Hey E., de Vries J., Langerhorst C.T., Baarsma G.S., Kijlstra A. Treatment and prognosis of secondary glaucoma in Fuchs' heterochromic iridocyclitis. Am J Ophthalmol. 1993;116(3):327–340. doi: 10.1016/s0002-9394(14)71350-9. [DOI] [PubMed] [Google Scholar]
- 20.Ceballos E.M., Parrish R.K., Schiffman J.C. Outcome of Baerveldt glaucoma drainage implants for the treatment of uveitic glaucoma. Ophthalmology. 2002;109(12):2256–2260. doi: 10.1016/s0161-6420(02)01294-0. [DOI] [PubMed] [Google Scholar]
- 21.Schlote T., Derse M., Zierhut M. Transscleral diode laser cyclophotocoagulation for the treatment of refractory glaucoma secondary to inflammatory eye diseases. Br J Ophthalmol. 2001;84(9):999–1003. doi: 10.1136/bjo.84.9.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho C.L., Walton D.S. Goniosurgery for glaucoma secondary to chronic anterior uveitis: prognostic factors and surgical technique. J Glaucoma. 2004;13(6):445–449. doi: 10.1097/01.ijg.0000141368.96950.3c. [DOI] [PubMed] [Google Scholar]
- 23.Miserocchi E., Carassa R.G., Bettin P., Brancato R. Viscocanalostomy in patients with glaucoma secondary to uveitis: a preliminary report. Cataract Refract Surg. 2004;30(3):566–657. doi: 10.1016/j.jcrs.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Regenbogen L.S., NavehFloman N. Glaucoma in Fuchs' heterochromiccyclitis associated with congenital Horner's syndrome. Br J Ophthalmol. 1987;71(11):844–849. doi: 10.1136/bjo.71.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You Y.A., Wu Y., Hu S. Surgical management of secondary glaucoma in Fuchs' heterochromic iridocyclitis. Graefes Arch ClinExpOphthalmol. 2013;251(7):1785–1790. doi: 10.1007/s00417-013-2312-9. [DOI] [PubMed] [Google Scholar]
- 26.La Hey E., Baarsma G.S., De Vries J., Kijlstra A. Clinical analysis of Fuchs' heterochromiccyclitis. Documentaophthalmologica. Adv Ophthalmol. 1991;78(3-4):225–235. doi: 10.1007/BF00165685. [DOI] [PubMed] [Google Scholar]
- 27.Foster P.J., Buhrmann R., Quigley H.A., Johnson G.J. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung K.I., Park C.K. Risk factors for the hypertensive phase after implantation of a glaucoma drainage device. Acta Ophthalmol. 2016;94(5):e260–e267. doi: 10.1111/aos.12916. [DOI] [PubMed] [Google Scholar]
- 29.Huber A. Glaucoma in complicated heterochromia of Fuchs. Ophthalmologica. 1961;141:122–135. doi: 10.1159/000304049. [DOI] [PubMed] [Google Scholar]
- 30.Chandler P.A., Grant W.M. Heterochromic cyclitis. Glaucoma due to intraocular inflammation. In: Epstein D.L., editor. Glaucoma. Lea & Febiger; Philadelphia: 1986. pp. 365–368. [Google Scholar]
- 31.Arellanes-Garcia L., del Carmen Preciado-Delgadillo M., Recillas-Gispert C. Fuchs' heterochromiciridocyclitis: clinical manifestations in dark-eyed Mexican patients. Ocul Immunol Inflamm. 2002;10(2):125–131. doi: 10.1076/ocii.10.2.125.13976. [DOI] [PubMed] [Google Scholar]
- 32.Iverson S.M., Bhardwaj N., Shi W. Surgical outcomes of inflammatory glaucoma: a comparison of trabeculectomy and glaucoma-drainage-device implantation. Jpn J Ophthalmol. 2015;59(3):179–186. doi: 10.1007/s10384-015-0372-6. [DOI] [PubMed] [Google Scholar]
- 33.Kwon H.J., Kong Y.X., Tao L.W. Surgical outcomes of trabeculectomy and glaucoma drainage implant for uveitic glaucoma and relationship with uveitis activity. Clin Exp Ophthalmol. 2017;45(5):472–480. doi: 10.1111/ceo.12916. [DOI] [PubMed] [Google Scholar]
- 34.Voykov B., Doycheva D., Deuter C., Leitritz M.A., Dimopoulos S., William A. Outcomes of ahmed glaucoma valve implantation for glaucoma secondary to Fuchs uveitis syndrome. Ocul Immunol Inflamm. 2017;25(6):760–766. doi: 10.3109/09273948.2016.1168454. [DOI] [PubMed] [Google Scholar]