Abstract
Background
The outcome of status epilepticus (SE) can be improved by facilitating early recognition and treatment with antiepileptic drugs. The purpose of this study was to analyze the treatment delay of SE in a prospectively recruited patient cohort. Improvements to the treatment process are suggested.
Methods
Consecutive adult patients with SE were recruited in the emergency department of Kuopio University Hospital (KUH) between March 23 and December 31, 2015. SE was defined as a prolonged (> 5 min) epileptic seizure or recurrent tonic-clonic seizures (≥ 3 seizures within any 24 h). Diagnostic and treatment delays and the features of SE were subject to statistical analysis.
Results
We recorded 151 cases of SE during the study period. First-line treatment was initiated outside of hospital in 79 cases (52.3%), with a significantly shorter median delay compared to intrahospital initiation (28 min vs. 2 h 5 min, p < 0.001). Forty-six episodes of SE (30.5%) were not recognized during the prehospital phase. The median delay in recognition of tonic-clonic SE (23 min) was significantly shorter than in focal aware (2 h 0 min, p = 0.045) or focal impaired awareness SE (2 h 25 min, p < 0.001). Second-line treatment was used in 91 cases (60.3%), with a median delay of 2 h 42 min. Anesthesia was used in seven cases (4.6%) with refractory SE, with a median delay of 6 h 40 min.
Conclusions
SE is often not recognized during the prehospital phase of treatment, which delays the initiation of first-line treatment. Intrahospital delay could be reduced by streamlining patient transition between the three lines of treatment.
Keywords: Status epilepticus, Seizure, Treatment, Delay, Emergency, Paramedic
Background
Status epilepticus (SE) is an abnormally prolonged epileptic seizure that may cause long-term neurologic complications [1]. The International League Against Epilepsy (ILAE) has defined two critical time points during seizure; seizures that continue beyond time point t1 are prolonged and seldom cease spontaneously. After time point t2, neuronal death or injury may occur [1]. The case fatality of SE is 7.6–22%, as reported in several studies [2].
The pathophysiology of SE involves the failure of endogenous seizure inhibition or the initiation of a mechanism that leads to an abnormally prolonged seizure [1]. The seizure triggers molecular mechanisms that promote receptor trafficking and altered neuropeptide expression, which cause sustained hyperexcitation in the affected neuronal network [3]. As the seizure duration grows, GABAergic anticonvulsants lose their potency [4] and the risk of refractory seizure [5] and adverse outcome increases [6].
Emergency treatment of SE should be started at time point t1, which is at 5 min in tonic-clonic seizures, 10 min in focal impaired awareness seizures, and 10–15 min in absence seizures [1]. The recommended first-line treatment is benzodiazepine administration [7]. Other antiepileptic drugs are frequently needed as second-line treatment. In refractory SE, the patient is usually treated in the intensive care unit (ICU) with general anesthesia as third-line treatment [8]. The one-year mortality in ICU-treated refractory SE is 23%, and an additional 29% show neurologic deficits [9].
Seizure duration is the only modifiable prognostic factor in SE [10]. The patient outcome can be improved by facilitating early and effective treatment with antiepileptic drugs [10]. Early treatment shortens hospital stay and reduces the risk of being admitted to the ICU with refractory SE [11].
Hill et al. recently reviewed 17 observational studies on treatment delay in SE [12]. They noted that 17–64% of patients have a longer than 30-min delay to first-line treatment and only 31–54% receive drug treatment before arrival at the hospital. Most studies on treatment delay of SE are performed retrospectively, which makes it difficult to accurately assess seizure duration and to analyze root causes of delay [12].
Study objective
The objective of this study was to measure and analyze treatment delay of SE in patients admitted to the emergency department of a major academic Finnish hospital. The study design was prospective to ensure high data quality. Causes of delayed treatment were analyzed and improvements to the treatment process were suggested.
Methods
Study design and setting
Our prospectively recruited study cohort consists of consecutive adult patients admitted to the emergency department (ED) of Kuopio University Hospital (KUH) due to prolonged or recurrent epileptic seizures, between March 23 and December 31, 2015. The study was observational and did not modify the management of patients. An investigator (JS) actively searched the patient lists of the ED for eligible study patients. Study data were obtained from hospital records and paper forms used by the emergency medical services (EMS). The use of medical records was authorized by the hospital district in accordance with Finnish legislation. The Committee on Research Ethics of North Savo Hospital District approved the study design.
The study hospital provides the only 24/7 emergency neurology service for a population of 248,000 in the North Savo region of Eastern Finland. The North Savo region has an area of 20,367 km2, and travel time to the hospital from the most distant areas served can be up to two hours by car. There are two small regional hospitals in the region and a network of publicly run community health centers. The regional hospitals and health centers provide a 24/7 availability of general practitioners (GPs) but limited diagnostic and treatment options in neurologic emergencies. SE patients are generally referred to KUH, unless do-not-hospitalize (DNH) orders or other special circumstances exist.
The EMS are arranged by KUH for the whole North Savo region with 24 ambulances and a physician-staffed emergency medical helicopter. The ambulance units consist of seven basic (BLS) and seventeen advanced life support (ALS) units. SE is classified as a high-priority mission and the closest unit is dispatched. According to the regional treatment guideline of SE, any ambulance unit can start the treatment of SE with buccal midazolam. After venous access has been obtained, an ALS unit may use intravenous diazepam. Benzodiazepine treatment can be started based on clinical judgement of the paramedics before consulting a physician. In the case of benzodiazepine-resistant seizure with long distance to hospital, a physician-staffed unit will be dispatched with the capability to administer second-line drugs.
Participants
Study participants were adults (≥ 16 years of age) who fulfilled the operational definition of SE presented in the Finnish Current Care Guideline [13]: a prolonged (> 5 min) epileptic seizure, a seizure cluster (≥ 2 discrete seizures) with no complete interictal recovery, or a recurrent tonic-clonic seizure with three or more isolated seizures within any 24-h period. Patients with postanoxic seizures were excluded. The seizures were recognized clinically, and the diagnosis was always verified by a physician. Electroencephalogram (EEG) confirmation was required for nonconvulsive seizures. Cases of SE were registered independently; we did not exclude cases in which the same study patient was readmitted for SE and again fulfilled the inclusion criteria.
Measurements
An investigator (JS) collected the data from medical records and structured forms filled by EMS personnel on a preformatted form. The data were stored as a SPSS 24 (IBM Corp, New York) dataset. Treatment delay was measured as time medians from the onset of SE to specific points of the treatment process, except that if the onset of SE was not witnessed, the delays were measured from the moment the patient was discovered. In a recurrent tonic-clonic seizure, the delays were measured from the onset of third seizure, which by operational definition marks the beginning of SE. The time of SE recognition was the moment when a person capable of treating SE (a paramedic, a physician or a caretaker) correctly recognized SE or when seizure activity was found on EEG.
Analysis
SPSS 24 was used in data analysis. Descriptive statistics were used to report the basic features of the study cohort. The cases were grouped according to seizure type and prehospital or intrahospital initiation of drug treatment. In subgroup analyses, the Mann-Whitney test and the Kruskal-Wallis test were used to find statistically significant differences in treatment delay between the patient groups. Dunn’s test was used in post-hoc analyses and the Bonferroni correction was used to adjust p-values. Two-tailed tests were used, and the level of statistical significance was set at p < 0.05. In our previous studies, the ratio of tonic-clonic SE to other types of SE has been 60:40, so we estimated that a sample size of 150 would be sufficient to compare the clinically meaningful treatment delays in main seizure types.
Results
Characteristics of study cohort
A total of 151 cases of SE in 137 individual patients (mean age: 59.5 years) were recorded. Half of the 137 patients (49.6%) had a prior diagnosis of epilepsy. Cerebrovascular disease (29.9%) and dementia of any etiology (22.6%) were common comorbidities, as well as psychiatric illness with ongoing drug treatment or psychotherapy (34.3%). There was a clinically relevant history of alcohol abuse in one of every three (33.6%) patients. The majority (80.3%) of patients lived independently at home.
Clinical features of the 151 SE cases are summarized in Table 1. Tonic-clonic seizures were the most common (69.5%), followed by focal impaired awareness seizures (14.6%) and focal aware seizures (9.9%). Myoclonic and absence seizures were rare. The most common etiology of a seizure was unknown (25.8%), either with or without diagnosis of pre-existing epilepsy. The second most common etiology was alcohol withdrawal (17.2%) followed by past cerebrovascular accident (15.9%). The usual scene of onset was at home, either with someone else (41.7%) or alone (7.9%). A large proportion of seizures began in a healthcare unit (20.5%) or a nursing home (13.2%). The onset of seizure was not witnessed in 27 cases (17.9%). Forty-seven (31.1%) of the SE episodes had an intermittent course either de novo or because of partial treatment response.
Table 1.
Clinical features of the 151 SE cases
| N | % | |
|---|---|---|
| Scene of SE onset | ||
| Home, with someone else | 63 | 41.7 |
| Healthcare unit | 31 | 20.5 |
| Public place | 23 | 15.2 |
| Nursing home | 20 | 13.2 |
| Home, alone | 12 | 7.9 |
| Prison | 2 | 1.3 |
| Seizure etiology | ||
| Acute symptomatic | 57 | 37.7 |
| Alcohol withdrawal | 26 | 17.2 |
| Drug withdrawal | 18 | 11.9 |
| Cerebrovascular accident | 6 | 4.0 |
| CNS infection | 3 | 2.0 |
| Drug toxicity | 2 | 1.3 |
| Metabolic insult | 1 | 0.7 |
| Head trauma | 1 | 0.7 |
| Unknown | 39 | 25.8 |
| Epilepsy with unknown or genetic etiology | 24 | 15.9 |
| Unknown | 15 | 9.9 |
| Remote symptomatic | 38 | 25.2 |
| Previous cerebrovascular accident | 24 | 15.9 |
| Previous brain injury | 6 | 4.0 |
| CNS anomaly | 4 | 2.6 |
| Previous brain surgery | 2 | 1.3 |
| Previous CNS infection | 2 | 1.3 |
| Progressive symptomatic | 17 | 11.3 |
| Degenerative brain disease | 9 | 6.0 |
| Brain tumor | 8 | 5.3 |
| Seizure type before treatment | ||
| Tonic-clonic | 105 | 69.5 |
| Focal, impaired awareness | 22 | 14.6 |
| Focal, aware | 15 | 9.9 |
| Nonconvulsive comatose | 5 | 3.3 |
| Absence | 2 | 1.3 |
| Myoclonic | 2 | 1.3 |
| SE recognition | ||
| In prehospital setting | 90 | 59.6 |
| Paramedic | 68 | 45.0 |
| GP | 11 | 7.3 |
| Caretaker | 6 | 4.0 |
| EMS physician | 5 | 3.3 |
| In hospital | 61 | 40.4 |
| ED neurologist | 56 | 37.1 |
| ED neurologist/clinical neurophysiologist (EEG diagnosis) | 5 | 3.3 |
| Means of transportation after onset of SE | ||
| Ambulance | 133 | 88.1 |
| Already admitted to ED | 10 | 6.6 |
| Private car | 5 | 3.3 |
| Helicopter | 3 | 2.0 |
| Health care unit where first treated | ||
| Kuopio University Hospital | 124 | 82.1 |
| Community health center (no neurologist on call) | 21 | 13.9 |
| Regional hospital (no neurologist on call) | 6 | 4.0 |
SE Status epilepticus, CNS Central nervous system, GP General practitioner, EMS Emergency medical services, ED Emergency department, EEG Electroencephalogram. Transport by helicopter is arranged by the Helicopter Emergency Medical Service that has a landing pad at Kuopio University Hospital
The onset of SE was outside of KUH in 141 cases (93.4%). A caretaker, paramedic, GP or an EMS physician recognized SE in 90 cases during the prehospital treatment. In forty-six cases, SE was initially not recognized, and in five cases a private car was used to get to hospital. Ten episodes of SE (6.6%) began in the ED. The diagnosis of SE was made by an ED neurologist in 56 cases. In five cases, the diagnosis was made by either an ED neurologist or a clinical neurophysiologist after an acute EEG study was conducted.
Main results
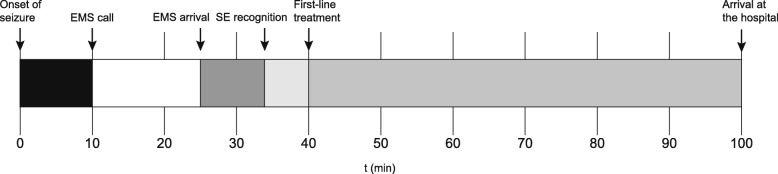
The measured delay components in the treatment of SE are shown in Table 2. The median delay to EMS call was 10 min, followed by EMS arrival at 25 min. In 14 cases (9.3%), an EMS physician met the patient outside of hospital with a median delay of 48 min. The time of initial SE recognition could be verified in 148 cases (98.0%), and the median SE recognition delay was 34 min. Fig. 1 shows the reported delays on a timeline.
Table 2.
Treatment delays of SE
| Delay component | Median | Range | N | % |
|---|---|---|---|---|
| EMS call | 10 min | 0–12 h 4 min | 117 | 77.5 |
| EMS arrival | 25 min | 0–12 h 31 min | 116 | 76.8 |
| EMS physician arrival | 48 min | 5 min – 1 h 33 min | 14 | 9.3 |
| SE recognition | 34 min | 0–67 h 29 min | 148 | 98.0 |
| First-line treatment | 40 min | 0–48 h 44 min | 121 | 80.1 |
| Arrival at the hospital | 1 h 40 min | 0–51 h 23 min | 150 | 99.3 |
| Second-line treatment | 2 h 42 min | 10 min – 71 h 30 min | 91 | 60.3 |
| EEG initiation | 5 h 11 min | 1 h 41 min – 67 h 29 min | 44 | 29.1 |
| Onset of anesthesia | 6 h 40 min | 3 h 48 min – 7 h 30 min | 7 | 4.6 |
| BS pattern on EEG | 8 h 20 min | 5 h 35 min – 9 h 20 min | 7 | 4.6 |
SE Status epilepticus, EMS Emergency medical services, ED Emergency department, EEG electroencephalogram, BS burst suppression
Time parameters are counted from the onset of SE. In cases where the onset of SE was not witnessed, the parameters are counted from when the patient was discovered
Fig. 1.
Delay components in the prehospital treatment of status epilepticus. EMS, emergency medical services; SE, status epilepticus. Delay components are shown where their median value (min) falls on the timeline
First-line treatment was given in 121 cases (80.1%), with a median delay of 40 min. Reasons why first-line treatment was not given include (1) spontaneous recovery in 17 cases, (2) the decision to proceed straight to second-line treatment in 11 cases and (3) consistency issues with the treatment guideline in two cases: one patient was given intravenous midazolam, and one patient received oral diazepam mixture to treat alcohol withdrawal symptoms and prevent the recurrence of a tonic-clonic seizure.
Of all 121 first-line treatments given, 79 (65.3%) were initiated outside of hospital. Prehospital initiation of drug treatment was associated with significantly shorter first-line treatment delay compared to intrahospital initiation (28 min vs. 2 h 5 min, p < 0.001).
The median delay to arrival at the hospital was 1 h 40 min. Second-line treatment was given in 91 cases (60.3%), with a median delay of 2 h 42 min. An acute EEG study was conducted on 44 subjects (29.1%) at the median time point of 5 h 11 min. Seven patients (4.6%) received third-line treatment for refractory SE, and the median delay to anesthesia was 6 h 40 min.
Treatment delay in different types of seizures
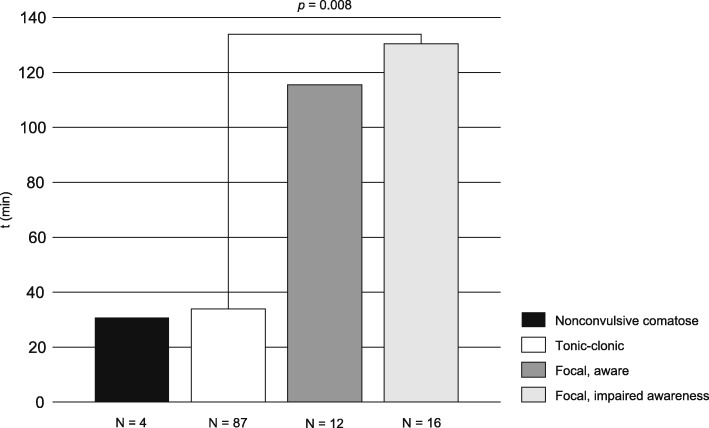
Across groups with different seizure types, a Kruskal-Wallis test revealed statistically significant differences in the following delay components: time to EMS call (p = 0.002), time to SE recognition (p < 0.001), time to first-line treatment (p < 0.001) and time to second-line treatment (p = 0.015). In pairwise analysis (Dunn-Bonferroni test), the delay to SE recognition was significantly shorter in a tonic-clonic seizure (23 min) than in a focal aware (2 h 0 min, p = 0.045) or a focal impaired awareness seizure (2 h 25 min, p < 0.001). Figure 2 illustrates how the first-line treatment time in a tonic-clonic seizure (32 min) was significantly shorter than in a focal impaired awareness seizure (2 h 10 min, p = 0.008). The time to EMS call was significantly shorter in a comatose patient with a nonconvulsive seizure (2 min) than in a patient with a focal impaired awareness seizure (28 min, p = 0.042). Other pairwise analyses yielded statistically insignificant results.
Fig. 2.
Comparison of median first-line treatment delay in the four most common seizure types. In pairwise analysis (Dunn-Bonferroni test), the treatment delay was significantly shorter in tonic-clonic seizures compared to focal impaired awareness seizures (32 min vs. 2 h 10 min, p = 0.008)
Discussion
In this study, treatment delays of SE were investigated. Long delays were found in both the prehospital and intrahospital phases of the treatment process. Our measurements of first-line, second-line and third-line treatment delay are in line with those summarized earlier in the review by Hill et al. [12] except that the delay to third-line treatment, 6 h 40 min, is noticeably longer in our study. There is considerable room for improvement in earlier recognition and treatment of SE, especially in nonconvulsive seizures.
Timely administration of first-line drugs can stop the seizure before it becomes refractory to treatment [3] and before neurologic damage occurs [7]. The t2 points of tonic-clonic SE and focal SE with impaired awareness are 30 min and > 60 min, respectively [1]. Such a short time window usually requires initiation of drug treatment outside of hospital, either by EMS personnel or, in some cases with refractory epilepsy, the patient’s caretaker. Home prescriptions of rescue medication are of limited help because (1) only half of the patients with SE have a prior diagnosis of epilepsy, (2) not all patients with epilepsy have a predictable need for rescue medication and (3) not all patients with refractory epilepsy can be prescribed rescue medication.
The prehospital phase of treatment in our cohort was time-consuming: its median duration was 1 h 40 min. Two earlier Finnish studies reported a median prehospital delay of 1 h 45 min [14] and 2 h 4 min [15]. The median EMS call delay (10 min) was short, so most of the overall delay was due to movement of the EMS unit, treatment given on-site and patient transport. Over half of our cases (52%) received first-line treatment outside of hospital with the median delay of 28 min, which is within the t2 of tonic-clonic SE. In contrast, if the first-line treatment was initiated only after arrival at hospital, its median delay was 2 h 5 min. This highlights the role of EMS personnel and caretakers in successful symptom recognition and rapid treatment initiation.
Difficulty recognizing SE may delay treatment initiation to the point when benzodiazepine medication is no longer effective. Across all 151 cases, the median delay to SE recognition was 34 min, and 46 cases of SE were initially missed during the prehospital phase. We noticed a tendency towards longer delay in focal seizures. In focal aware and focal impaired awareness SE, the recognition delays were 2 h 0 min and 2 h 25 min, respectively, and the seizure symptoms were often missed before hospital arrival. Some delay could possibly have been eliminated if the patient’s history of unusual seizures had been known to the EMS responders. EMS personnel and GPs should also suspect nonconvulsive SE more readily when encountering obtunded elderly patients. In uncertain prehospital situations the correct place of follow-up care is a hospital with available acute EEG.
Second-line treatment was given in 91 cases (60.3%), and the associated delay of 2 h 42 min is alarming. Seven patients (4.6%) received third-line treatment for refractory SE, and the median time to anesthesia was 6 h 40 min. These findings suggest problems in the transition between the three lines of treatment. Variability in the dosing of benzodiazepines, such as too many treatment attempts before moving on to the next line of treatment, has been noted as a potential source of delay [12]. Sometimes the resolution of seizure remains uncertain after the administration of second-line drugs. If the patient’s ongoing seizure is not correctly recognized, the acute EEG is ordered too late and the third-line treatment of refractory SE is needlessly delayed.
The phrase “time is brain” is well-known in stroke research. The diagnostic and treatment delays of acute stroke can be greatly reduced by developing EMS-ED cooperation and streamlining intrahospital care [16]. Some measures that have proved themselves valuable in stroke should be used in the treatment of SE as well. They include an EMS pre-notice before hospital arrival and a rapid neurologic evaluation in a prearranged place, for example the resuscitation room, with a preordered laboratory test package. Ictal symptoms and the given antiepileptic drugs with appropriate time tags should be documented systematically so that the need for second- or third-line medication can be noticed earlier.
The limitations of this study include the relatively small cohort size and possible bias in recording the exact time points of seizure onset and treatment procedures. Some of our subgroup analyses lack power because there were only few cases with a rare seizure type. The data were collected during a nine-month period in a single Finnish tertiary hospital. Our cohort does not include those SE patients in our hospital district who were not treated in KUH emergency department due to special circumstances, for example do-not-hospitalize (DNH) orders. The local EMS guidelines and especially geography have influenced the measured treatment delays in this study and our results may not be readily generalized to other populations with different geographical distribution and access to care even in Finland.
Conclusion
In summary, significant delay occurs in all phases of treatment in SE. Early recognition of SE is crucial to ensure rapid treatment initiation, preferably during the prehospital phase of the treatment process. EMS personnel must be prepared to treat all types of epileptic seizures promptly with the recommended first-line drugs. EMS personnel and GPs must be educated about the possibility and clinical signs of a focal seizure. Rescue medication should be made available to any eligible patient with a caretaker and a history of uncontrolled seizures.
Acknowledgements
We thank biostatistician MSc Tuomas Selander of the Science Service Center of Kuopio University Hospital for technical assistance with the data analysis.
Funding
The research reported in this manuscript was supported by the Finnish State Research Funding, the Finnish Epilepsy Research Foundation and the Maire Taponen Foundation.
Availability of data and materials
The datasets analyzed in this study are available from the corresponding author on request.
Abbreviations
- ALS
Advanced life support
- BLS
Basic life support
- CNS
Central nervous system
- DNH
do-not-hospitalize
- ED
Emergency department
- EEG
electroencephalogram
- EMS
Emergency medical services
- GP
General practitioner
- ICU
Intensive care unit
- ILAE
International League Against Epilepsy
- KUH
Kuopio University Hospital
- SE
Status epilepticus
Authors’ contributions
JS, AMK, HH and RK designed the study. JS collected and analyzed the study data. HH and RK supervised the data collection and the conduct of the study. All authors contributed equally towards data interpretation, literature search and writing of the manuscript. We confirm that the list of authors includes all persons who fulfill the criteria for authorship. All authors have approved the final manuscript and the listed order of authors.
Ethics approval and consent to participate
The Committee on Research Ethics of North Savo Hospital District approved the study design. The use of medical records was authorized by the hospital district in accordance with Finnish legislation. Informed consent was waived because of the non-interventional nature of the study. Subject rights and confidentiality were protected by using anonymous clinical data in all analyses.
Consent for publication
Not applicable.
Competing interests
JS has received grants from the Finnish Epilepsy Research Foundation and the Maire Taponen Foundation and institutional funding from the Finnish State Research Funding via Neurocenter, Kuopio University Hospital. AMK has received a grant from the Finnish Cultural Foundation and speaker’s honoraria from Orion, Boehringer Ingelheim, MSD, BMS and a travel grant from Sanofi. HH has received speaker’s honoraria from Orion and Boehringer Ingelheim and honoraria for the membership of advisory board from MSD. RK has received grants from the Academy of Finland and the Saastamoinen Foundation, speaker’s honoraria from Eisai, UCV and Orion and honoraria for the membership of advisory board from Eisai, Fennomedical, GW Pharmaceuticals, Sage Therapeutics, Takeda and UCB.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joni J. Sairanen, Phone: +358445123111, Phone: +35817173005, Email: joni.sairanen@fimnet.fi
Anne-Mari Kantanen, Phone: +35817173005, Email: anne-mari.kantanen@kuh.fi.
Harri T. Hyppölä, Phone: +35817173005, Email: harri.hyppola@kuh.fi
Reetta K. Kälviäinen, Phone: +35817173005, Email: reetta.kalviainen@kuh.fi
References
- 1.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnas S, et al. A definition and classification of status epilepticus – report of the ILAE task force on classification of status epilepticus. Epilepsy. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 2.Logroscino G, Hesdorffer DC, Cascino G, Hauser WA, Coeytaux A, Galobardes B, et al. Mortality after a first episode of status epilepticus in the United States and Europe. Epilepsia. 2005;46:46–48. doi: 10.1111/j.1528-1167.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- 3.Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14:615–624. doi: 10.1016/S1474-4422(15)00042-3. [DOI] [PubMed] [Google Scholar]
- 4.Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–185. doi: 10.1016/S0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson K, Metsäranta P, Huhtala H, Auvinen A, Kuusela AL, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65:1316–1318. doi: 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JY. Latency to treatment of status epilepticus is associated with mortality and functional status. J Neurol Sci. 2016;370:290–295. doi: 10.1016/j.jns.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Trinka E, Kälviäinen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73. doi: 10.1016/j.seizure.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135:2314–2328. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 9.Kantanen AM, Reinikainen M, Parviainen I, Kälviäinen R. Long-term outcome of refractory status epilepticus in adults: a retrospective population-based study. Epilepsy Res. 2017;133:13–21. doi: 10.1016/j.eplepsyres.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Sutter R, Kaplan PW, Rüegg S. Outcome predictors for status epilepticus - what really counts. Nat Rev Neurol. 2013;9:525–534. doi: 10.1038/nrneurol.2013.154. [DOI] [PubMed] [Google Scholar]
- 11.Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia. 2010;51:2159–2167. doi: 10.1111/j.1528-1167.2010.02688.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill CE, Parikh AO, Ellis C, Myers JS, Litt B. Timing is everything: where status epilepticus treatment fails. Ann Neurol. 2017;82:155–165. doi: 10.1002/ana.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kälviäinen R, Bendel S, Jonsson T, Keränen T, Kurola J, Salmi T, et al. Status epilepticus (30.5.2016). Current Care Guidelines. The working group set up by the Finnish Medical Society Duodecim, the Finnish Paediatric Neurology Society and the Finnish Neurological Society. Available online at: www.kaypahoito.fi
- 14.Hillman J, Lehtimäki K, Peltola J, Liimatainen S. Clinical significance of treatment delay in status epilepticus. Int J Emerg Med. 2013;6:6. doi: 10.1186/1865-1380-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kämppi L, Mustonen H, Soinila S. Analysis of the delay components in the treatment of status epilepticus. Neurocrit Care. 2013;19:10–18. doi: 10.1007/s12028-013-9862-x. [DOI] [PubMed] [Google Scholar]
- 16.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author on request.