Abstract
The intracellular tyrosine kinase Pyk2 (PTK2B) is related to focal adhesion kinase and localizes to postsynaptic sites in brain. Pyk2 genetic variation contributes to late onset Alzheimer's disease (AD) risk. We recently observed that Pyk2 is required for synapse loss and for learning deficits in a transgenic mouse model of AD. Here, we explore the cellular and biochemical basis for the action of Pyk2 tyrosine kinase in amyloid-β oligomer (Aβo)-induced dendritic spine loss. Overexpression of Pyk2 reduces dendritic spine density of hippocampal neurons by a kinase-dependent mechanism. Biochemical isolation of Pyk2-interacting proteins from brain identifies Graf1c, a RhoA GTPase-activating protein inhibited by Pyk2. Aβo-induced reductions in dendritic spine motility and chronic spine loss require both Pyk2 kinase and RhoA activation. Thus, Pyk2 functions at postsynaptic sites to modulate F-actin control by RhoA and regulate synapse maintenance of relevance to AD risk.
SIGNIFICANCE STATEMENT Genetic variation at the Pyk2 locus is a risk for Alzheimer's disease. We have observed that Pyk2 is required for AD transgenic synapse loss and memory dysfunction. However, the cellular and biochemical basis for Pyk2 function related to AD is not defined. Here, we show that brain Pyk2 interacts with the RhoGAP protein Graf1 to alter dendritic spine stability via RhoA GTPase. Amyloid-β oligomer-induced dendritic spine loss requires the Pyk2/Graf1 pathway.
Keywords: actin, Alzheimer, Graf1, PTK2B, Rho, synapse loss
Introduction
Synaptic maintenance and dendritic spine stability are crucial for brain function, such that dysregulation is thought to contribute to neurodegenerative disease. Therefore, intracellular signal networks regulating dendritic spine morphology are of high interest. Pyk2 (PTK2B, FAK2) is a tyrosine kinase closely similar to FAK (focal adhesion kinase; Mitra et al., 2005). FAK and Pyk2 are expressed in neurons with Pyk2 being most prominent in the brain (Zhang et al., 1994; Menegon et al., 1999). Pyk2 regulates synaptic plasticity, in particular long-term depression (Huang et al., 2001; Bartos et al., 2010; Hsin et al., 2010; Giralt et al., 2017; Salazar et al., 2018). The kinase activity of Pyk2 is activated by increased intracellular calcium, whereas another tyrosine kinase, Fyn, phosphorylates Pyk2 to achieve its full activation (Dikic et al., 1996; Qian et al., 1997; Andreev et al., 2001; H. J. Park et al., 2004; Collins et al., 2010a,b). Fyn and Pyk2 cross-activate one another, and function in a bidirectional synergistic manner (Dikic et al., 1996; Qian et al., 1997; Andreev et al., 2001; S. Y. Park et al., 2004; Collins et al., 2010a,b; Kaufman et al., 2015). Pyk2 gene-deletion studies have not assessed dendritic spine stability or synaptic maintenance.
Alzheimer's disease (AD) creates a massive human health burden, with >5 million individuals afflicted in the United States alone (Alzheimer's Association, 2012). Pyk2 (PTK2B) is a late onset AD (LOAD) risk gene (Lambert et al., 2013). Although Pyk2 gene alters AD risk, the mechanism(s) relevant to AD accumulation of either amyloid-β (Aβ) or Tau proteins has not been defined. A Drosophila Pyk2 homolog contributes to neurodegeneration driven by mutant Tau protein (Dourlen et al., 2017), and Pyk2 binds to Tau (Li and Götz, 2018). With regard to Aβ pathology in AD, our studies indicate that Pyk2 is activated after Aβ oligomer (Aβo) binding to PrPC, which engages mGluR5 signaling to activate Fyn kinase and Pyk2 kinase (Laurén et al., 2009; Gimbel et al., 2010; Um et al., 2012, 2013; Kaufman et al., 2015; Haas et al., 2016; Kostylev et al., 2018). Although this pathway is not essential in certain experimental Alzheimer models, the role of PrPC, mGluR5, and Fyn is required for AD-related phenotypes in multiple studies using both pharmacological and genetic tools (for review, see Salazar and Strittmatter, 2017; Purro et al., 2018). The Pyk2 homolog FAK is also activated by soluble Aβ assemblies (Zhang et al., 1994). Transgenic AD mice with Aβ accumulation exhibit Pyk2 activation. Furthermore, the elevated Pyk2 activity is normalized by PrPC deletion, by mGluR5 deletion or inhibition, or by Fyn inhibition, and this correction is coincident with restoration of synapse density (Kaufman et al., 2015; Haas and Strittmatter, 2016; Haas et al., 2016, 2017). We recently showed that Pyk2 is required for Aβo-induced suppression of hippocampal long-term potentiation, and for APPswe/PS1ΔE9 transgenic synapse loss and memory impairment (Salazar et al., 2018). However, the cellular and biochemical basis for Pyk2 mediation of these AD phenotypes is not known.
Here, we sought to determine how Pyk2 might control synapse maintenance of relevance to AD. We find that Pyk2 activation reduces dendritic spine number. In brain, a major partner of Pyk2 is GTPase regulator associated with focal adhesion kinase-1 (Graf1), a RhoA GTPase activating protein (GAP) inhibited by Pyk2. The ability of Aβo to reduce dendritic spine motility, and to cause spine loss requires Pyk2 expression. Thus, the LOAD risk gene Pyk2 is coupled to an Aβo signaling pathway can function as a proximal mediator of synapse loss.
Materials and Methods
Animals
All mice were cared for by the Yale Animal Resource Center. Yale's institutional animal care and use committee approved all experiments. The APPswe/PSEN1ΔE9 mice on a C57BL/6J background were purchased from The Jackson Laboratory (RRID:MMRRC_034832-JAX; Jankowsky et al., 2003). Pyk2−/− mice (Okigaki et al., 2003; RRID:MGI:3584536) on the C57BL6J background after 10 backcrosses were generously provided by Dr. David Schlaepfer (UCSD). All experiments used littermate control mice with no preference for male or female mice.
Plasmid DNA constructs
Full-length wild-type (WT) Pyk2, K457A, PXXP1mut, PXXP2mut, ΔPRD, and PRD mutants were subcloned into AAV-CAG-GFP vector (gift from K. Svoboda, Janelia Research Campus; Addgene, plasmid #28014; RRID:Addgene_28014) for GFP tagging on N-terminus, AAV-CAG-tagRFP vector, modified from AAV-CAG-GFP by replacing the GFP with tagRFP for tagRFP tagging on N-terminus, or pcDNA3 with or without HA tag. Human Graf1a and Graf1c isoforms were generated from Graf1b isoform (DNASU plasmid repository, clone ID HsCD00639889) by PCR, subcloned into AAV-CAG-tagRFP, pcDNA3, or pGEX6P-1. pRK5-Myc-RhoA-wt and pRK5-Myc-RhoA-T19N were gift from Gary Bokoch (Addgene, plasmid #12962 and #12963; RRID:Addgene_12962 and RRID:Addgene_12963). GFP and tagRFP expression plasmids were generated from AAV-CAG-GFP and AAV-CAG-tagRFP vector by the insertion of stop codon after GFP or tagRFP open reading frame (ORF). The myristoyl-GFP plasmid has been described previously (Um et al., 2012). Graf1 shRNA was designed from mouse Graf1 sequence targeting 5′-atgatgtaccagtttcaaa (1392–1441) site and cloned into the pAAV-U6-GFP vector (Cell Biolabs).
Culture and transfection of mouse hippocampus neurons
Cultured hippocampal neurons were prepared from embryonic day 17 fetal C57BL/6J mice. Briefly, dissected hippocampi were dissociated with papain and plated on poly-d-lysine-coated 18 mm glass coverslips or culture plates with plating medium (Neurobasal-A medium supplemented with 2% B-27, 2% FBS, 1% GlutaMax, and 1 mm sodium pyruvate; all from ThermoFisher Scientific). Four hours after plating, all medium was replaced with FBS free culture medium (Neurobasal-A medium supplemented with 2% B-27, 1% GlutaMax, and 1 mm sodium pyruvate) and then 50% replacement every 7 d. Neurons were transfected before plating by Amaxa Nucleofector (shRNA efficiency test) or at 17 DIV by modified calcium-phosphate method (Chang and De Camilli, 2001). Briefly, 7 μg of plasmid DNA and 9 μl of 2 m CaCl2 were mixed in distilled water to a total volume of 75 μl, and the same volume of 2× BBS (in mm: 50 BES, 280 NaCl, and 1.5 Na2HPO4, pH 7.1) was added. Original cultured medium was completely replaced by transfection medium (MEM, 1% GlutaMax, 1 mm sodium pyruvate, 0.6% glucose, and 10 mm HEPES, pH 7.65), and plasmid DNA mixture was added to the neurons in transfection medium. After incubation for 90 min in 5% CO2 incubator, washed twice for 20 min with transfection medium, pH 7.35, and then returned to the original culture medium.
Hek293T cell culture and transfection
Human embryonic kidney 293T (Hek293T; RRID:CVCL_QW54) cells were cultured in DMEM containing 10% FBS at 5% CO2 and 37°C incubator and transfected using Lipofectamine 3000 reagent (ThermoFisher Scientific).
Immunoprecipitation
Lysates from transfected Hek293T cells or mouse forebrains were extracted with modified radio immune precipitation assay (RIPA) buffer (1% Triton X-100, 50 mm Tris, 150 mm NaCl, 1 mm EDTA, protease inhibitor cocktail, Roche, and phosphatase inhibitor cocktail, Roche) and quantitated the protein concentration by BCA assay kit (ThermoFisher Scientific). One milligram of lysates and 1 μg of appropriate antibody (anti-GFP antibody, ChromoTek, gta-20; RRID:AB_2631357; anti-Pyk2 antibody, Santa Cruz Biotechnology sc1515; RRID:AB_632286, anti-HA antibody, Covance, MMS-101P; RRID:AB_2314672) mixtures were incubated for 2 h at 4°C, 20 μl protein A/G agarose beads (ThermoFisher Scientific) was added, and incubation continued for 1 h on rotator. The immunoprecipitated complexes were washed three times with RIPA buffer, eluted in SDS-PAGE sample-loading buffer from beads, and then resolved by SDS-PAGE and immunoblotted.
Immunoblotting
Proteins were resolved using precast 4–20% tris-glycine gels (Bio-Rad) and transferred to nitrocellulose membrane by iBlot Gel Transfer Device (ThermoFisher Scientific). The membranes were blocked in blocking buffer (Rockland, MB-070-010) for fluorescent immunoblot for 1 h at room temperature (RT) and incubated overnight in primary antibodies at 4°C. The following antibodies were used: rabbit anti-Pyk2 (Abcam, ab32571, 1:1000; RRID:AB_777566), mouse anti-Pyk2 (Santa Cruz Biotechnology, SC130077, 1:1000; RRID:AB_2174109), anti-phospho-Pyk2 (Y402; Abcam ab131543, 1:1000; RRID:AB_11157717), anti-GFP (Abcam ab13970, 1:5000; RRID:AB_300798), anti-Fyn (Santa Cruz Biotechnology, sc71133, 1:1000; RRID:AB_1123049), anti-phospho-SFK (Cell Signaling Technology, 6943, 1:1000; RRID:AB_10013641), anti-flag (Sigma-Aldrich, F3165, 1:1000; RRID:AB_259529), anti-Graf1 (Abcam, ab137085, 1:1000), anti-PSD-95 (Synaptic Systems, 124 002, 1:2000; RRID:AB_887760), anti-Actin (Sigma-Aldrich, A2066, 1:2000; RRID:AB_476693), anti-Myc (Cell Signaling Technology, 2276, 1:5000; RRID:AB_331783), anti-RhoA (Cytoskeleton ARH04, 1:1000; RRID:AB_2718698), and anti-pY (Cell Signaling Technology, 9411, 1:1000; RRID:AB_331228). After primary antibody incubation, the membranes were washed and applied appropriate secondary antibodies (Odyssey donkey anti-rabbit, anti-mouse, or anti-chicken IRDye 680 or 800 conjugates, LI-COR Biosciences) for 1 h at RT. The proteins were visualized using a LI-COR Odyssey infrared imaging system and quantified with Image Studio Lite software (RRID:SCR_013715).
Immunocytochemistry
Cultured neurons were fixed in 4% paraformaldehyde/4% sucrose/PBS for 15 min, permeabilized for 5 min in 0.25% Triton X-100/Tyrode's solution (in mm: 136 NaCl, 2.5 KCl, 2 CaCl2, 1.3 MgCl2, 10 Na-HEPES, 10 d-glucose, pH 7.3) and then incubated in 10% BSA for 30 min at 37°C for blocking. Primary antibodies (anti-PSD-95, Millipore, MAB1596, 1:1000; RRID:AB_2092365; anti-Pyk2, Abcam, ab32571, 1:1000; anti-Myc Cell Signaling Technology, 2276, 1:2000) diluted in 3% BSA/Tyrode's solution were incubated for 2 h at 37°C. Then, appropriate secondary antibodies (AlexaFluor 488 or AlexaFluor 568-conjugated donkey anti-mouse IgG or anti-rabbit-IgG, ThermoFisher Scientific, 1:1000) were diluted in 3% BSA/Tyrode's solution and applied for 45 min at 37°C.
Pyk2 binding-protein identification
Anti-GFP antibody captured immunoprecipitates from 1 mg protein from GFP or GFP-Pyk2 expressed Hek293T cell lysates with or without 5 mg mouse brain lysate mixtures were separated by SDS-PAGE and silver-stained using silver-staining kit (Pierce, 24600). Pyk2-specific binding proteins in mouse brain were excised from the stained gel and tryptic peptides were identified by LC-MS/MS analysis with Keck Proteomics Facility (Yale University).
Aβ1–42 preparation
Synthesized Aβ1–42 peptide was obtained from Keck large scale Peptide Synthesis Facility (Yale University). Aβ1–42 oligomers (Aβo) were prepared in specially formulated glutamate-free F-12 to avoid direct stimulation of cultured neurons as described previously (Laurén et al., 2009). Concentration of Aβo are expressed in monomer equivalents, with 1 μm total Aβ1–42 peptide corresponding to ∼10 nm oligomeric species (Laurén et al., 2009). Each new preparation of Aβo was confirmed to be >95% high molecular weight soluble oligomers by size exclusion chromatography as described previously (Laurén et al., 2009; Um et al., 2012; Kaufman et al., 2015).
Mouse brain tissue collection for PSD fractionation and biochemistry
After rapid decapitation, whole forebrain or cortex and hippocampus were quickly dissected and homogenized in ice-cold Syn-PER reagent (ThermoFisher Scientific) containing complete protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Roche) with Teflon homogenizer. Homogenized brain extract was spun at 1000 × g for 10 min to remove pelleted nuclear fraction (P1). Supernatant (S1) was centrifuged at 15,000 × g for 15 min to collect the crude membrane pellet (P2). P2 was resuspended in HEPES-buffered sucrose [0.32 m sucrose, 4 mm HEPES, pH 7.4, complete protease inhibitor cocktail (Roche), phosphatase inhibitor cocktail (Roche)], and then spun at 15,000 × g for 15 min to yield the washed crude synaptosomal fraction (P2′). Lysed resulting pellet by hypo-osmotic shock in cold H2O with protease and phosphatase cocktail inhibitor and homogenized again with Teflon homogenizer then rapidly adjust to 4 mm HEPES. After hypo-osmotic lysis, samples were spun at 25,000 × g for 20 min to separate the supernatant (S3) and pellet (P3). The P3 suspension in HEPES-buffered sucrose was loaded onto a discontinuous sucrose gradient (0.8–1–1.2 m sucrose solution in 4 mm HEPES, pH 7.4), followed by centrifugation for 2 h at 150,000 × g. The synaptosome fraction between 1 m and 1.2 sucrose layer was collected and adjusted to 4 ml with 4 mm HEPES pH7.4, spun at 150,000 × g for 30 min to yield the pellet (SPM). SPM was resuspended in lysis buffer (50 mm HEPES, 2 mm EDTA, 1% Triton X-100, protease inhibitor cocktail, and phosphatase inhibitor cocktail) and incubated for 15 min. The suspension was spun at 32,000 × g for 20 min. The resulting pellet was extracted again with 0.5% Triton X-100 lysis buffer for 15 min, and spun again at 200,000 × g for 1 h. Resulting pellet (PSD) was analyzed by immunoblot.
RBD pull-down assay
GST tagged Rhotekin RBD (GST-RBD) expression plasmid was transformed into BL-21 (DE3) Escherichia coli (Agilent) and the cells were cultured in 2× YT medium with ampicillin until OD600 reached 0.6 at 37°C, and then protein expression was induced with 0.5 mm IPTG for 6 h at 25°C. Protein expression-induced cells were lysed in lysis buffer (1% Triton X-100, 150 mm NaCl, 20 mm Tris pH7.4, 1 mm MgCl2, 1 mm EGTA, 1 mm PMSF) with sonication and centrifuged at 20,000 × g for 15 min at 4°C. Supernatant was incubated with Glutathione Sepharose 4B beads (GE Healthcare) for 1 h. GST-RBD immobilized beads were applied to lysates from transfected Hek293T cells or mouse brain lysates (lysis buffer: 1% Triton X-100, 150 mm NaCl, 50 mm Tris pH7.4, 10 mm MgCl2, protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktail (Roche), incubated for 1 h at 4°C and then analyzed by SDS-PAGE and immunoblotting.
Image acquisition and analysis of dendritic spine density
GFP, GFP-Pyk2, and GFP-K457A transfected neurons were fixed at 21 DIV. In some experiments, GFP-Pyk2 expressed neurons were incubated immediately in 1 μm PF-719 after transfection. For Aβo-induced spine loss experiments, Aβo (1 μm monomer, 10 nm oligomer estimate) or Vehicle (Veh) were applied to GFP, GFP-Pyk2-PRD, and GFP with Myc-RhoA-T19N expressed neurons at 17 DIV and replaced 50% culture medium with fresh Aβo or Veh included conditioned culture medium every 24 h for 4 d. For 6 h incubation, Aβo or Veh were applied to 21 DIV neurons without media change. Neurons were fixed and imaged with a 40× objective oil lens on a Nikon Eclipse Ti Spinning Disk Confocal Microscope driven by Volocity software (PerkinElmer). Images were obtained as a 1 μm Z-stack with 0.5 μm spacing using a 488 laser. All imaging and analyses were completed by an observer unaware of genotype or treatment group.
Spine density was analyzed by an automated method using Imaris (Bitplane; RRID:SCR_007370) software in maximum intensity projected 2D images. In Imaris Surpass mode, a new filament was created using the AutoPath mode of the FilamentTracer to define the dendrite with minor modifications from previous studies (Swanger et al., 2011). All filaments counted were devoid of crossing neurites or additional dendritic branch points between defined start and end points. For the automatic spine detection, the minimum spine end diameter and maximum spine length were set at 0.3 and 4 μm. Automatic thresholds were used for generating spine seed points and surface rendering. After generating the trace, a filter was applied to ensure all dendritic protrusions <3 μm2 were defined as spines. For each condition, 3 dendrites were measured for each neuron and 5–7 neurons were assessed per coverslip. Myc-RhoA-T19N and flag-Graf1c expression was confirmed by immunostaining with anti-Myc and anti-Flag antibodies for imaged dendrites. All imaging and analyses were completed by an observer unaware of Pyk2 genotype or Aβo treatment group.
Image acquisition and analysis of Pyk2 localization in immunostained neurons
After Aβo (1 μm monomer, 10 nm oligomer estimate) or Veh treatment for 1 h, 6 h, and 4 d, 21 DIV low-density cultured neurons were immunostained with appropriate antibodies and imaged with a 60× oil-immersion lens on a same microscope as for dendritic spine imaging. Postsynaptic area was selected from PSD-95 images by a predefined computer script using a constant threshold value and then the average fluorescence intensity was measured for Pyk2 within each PSD-95-positive area using Volocity software. All imaging and analyses were completed by an observer unaware of genotype or treatment group.
Image acquisition and analysis of live neurons
To measure the dendritic spine motility, myristoylated-GFP-expressing 21 DIV neurons from WT or Pyk2−/− mice were incubated with Aβo (1 μm monomer, 10 nm oligomer estimate) or Veh for 24 h and mounted in a magnetic chamber (Live Cell Instrument, CM-B18-1) on the stage of a Nikon Eclipse Ti Spinning Disk Confocal Microscope. Time-lapse images were acquired for 5 min with 10 s intervals in Tyrode's solution using 60× objective lens driven Volocity software. Time-lapse images (31 time frames over 5 min) were subjected to SD projection to visualize spine motility in single image and colorized with thermal scale using ImageJ software (NIH; RRID:SCR_002074). Quantitative measurements of spine motility by calculating the changed area at individual dendritic spines were conducted by using ImageJ software by an observer unaware of treatment group. Spine motility was monitored as changed area by subtracting thresholded, binarized individual images from the merged area for every 30 s time frame over 5 min. In some experiments, 1 μm PF-719 or 10 μm Y-27632 were applied with or without Aβo for 24 h.
Brain tissue collection
Mice were killed and brains were dissected, divided at the midline into two hemispheres where one hemisphere was drop-fixed in 4% paraformaldehyde (PFA) for 24 h. The other hemisphere was flash frozen and stored at −80°C. Following PFA fixation, brains were stored in PBS with 0.05% sodium azide. For immunostaining, brains were cut into 40 μm coronal sections using a Leica WT1000S Vibratome. Sections were stored in PBS with 0.05% sodium azide at 4°C until staining. Dentate gyrus PSD-95-immunoreactive area was measured as described previously (Gimbel et al., 2010; Um et al., 2013; Salazar et al., 2018). Thioflavin-S-positive plaque burden was assessed in sections as percentage area occupied, and TBS-soluble Aβo was measured using immobilized PrPC capture (Kostylev et al., 2015).
PF-719 synthesis
The previously described PF-719 (Tse et al., 2012) small molecule was synthesized by Chinglu Pharmaceutical Research. They provided mass spectroscopy data with appropriate parent ions and NMR spectroscopy data confirming the structure of the compound with ∼95% purity. This was validated independently by our laboratory and confirmed the presence of the major peak with an appropriate m/z.
Protein purification for in vitro direct binding test, kinase assay, and RhoGAP assay
GFP, GFP-Pyk2, GFP-K457A, and GFP-PXXP2mut purification.
Hek293T cells were transfected with GFP, GFP-Pyk2, GFP-K457A, or GFP-PXXP2mut using Lipofectamine 3000 then expressed for 48 h. Lysates from transfected Hek293T cells were incubated with anti-GFP antibody trapped agarose beads (ChromoTek, GFP-Trap_A) for 2 h at 4°C. The immunoprecipitated complexes were washed 3 times with RIPA buffer then replaced RIPA buffer with detergent-free assay buffer (50 mm Tris, 150 mm NaCl, pH 7.4). Immobilized proteins were eluted by 0.1 mm glycine-HCl, pH 2.3, for direct binding test or kept in 4°C for kinase assay buffer for RhoGAP assay. Protein concentration was examined by SDS PAGE and Coomassie blue staining with BSA standard.
Graf1c and Graf1c-SH3 domain purification.
GST, GST-Graf1c, or GST-Graf1c-SH3 domain (Graf1c, aa 558–686) expression plasmid were transformed into BL-21 (DE3) E. coli. Cells were cultured in 2× YT medium with ampicillin until OD600 reached 0.6 at 37°C, and then protein expression was induced with 0.5 mm IPTG for 6 h at 25°C. Cells were lysed in lysis buffer (1% Triton X-100, 150 mm NaCl, 20 mm Tris pH7.4, 1 mm MgCl2, 1 mm EGTA, 1 mm PMSF) with sonication and centrifuged at 20,000 × g for 15 min at 4°C. Supernatant was incubated with Glutathione Sepharose 4B beads (GE Healthcare) for 1 h. GST tagged protein immobilized beads were washed three times with lysis buffer, and then kept in 4°C for binding test or washed two times more with cleavage buffer (50 mm Tris-HCl, pH7.4, 150 mm NaCl, 1 mm EDTA) for on-column cleavage for kinase assay and RhoGAP assay. Eluted protein was concentrated using Amicon ultra-0.5 centrifugal filter (Millipore-Sigma) and quantitated by SDS-PAGE and Coomassie blue staining with BSA standard.
In vitro direct binding test
One microgram of purified GFP-Pyk2 protein incubated with GST, GST-Graf1c, or GST-Graf1c-SH3 immobilized agarose beads in lysis buffer for 1 h at 4°C. Beads and protein complexes were washed with lysis buffer three times and subjected to SDS-PAGE for Coomassie blue staining or immunoblotting with anti-GFP antibody.
In vitro Pyk2 kinase assay
One microgra of bead immobilized GFP, GFP-Pyk2, GFP-K457A, GFP-PXXP2mut were incubated with or without 1 μg Graf1c, 2 mm ATP in 10 μl kinase buffer (50 mm HEPES, 10 mm MgCl2, 1 mm DTT). Reactions were incubated at 24°C for 1 h and terminated by addition of SDS sample buffer and boiling. Proteins were subjected to SDS-PAGE and immunoblotting with indicated antibodies.
In vitro Graf1c RhoGAP assay
The RhoGAP Assay Biochem kit #BK105 (Cytoskeleton) was used to determine the relative GAP activity of Graf1c with Pyk2 WT, Pyk2-K457A, or Pyk2-PXXP2mut, or Graf1c alone. Briefly, kinase reactive samples were mixed with recombinant RhoA–His, RhoA-His alone, or RhoA-His with Graf1c. Reactions were combined with 1× reaction buffer in a 96-well plate on ice, and GTP was added to each well at a final concentration of 200 μm. The plate was shaken at 200 rpm for 5 s and then incubated at 37°C for 20 min. At the end of the reaction, 120 μl of CytoPhos reagent was added to each well. The reactions were incubated for 10 m at RT and then the absorbance was read at 650 nm to measure the level of GTP hydrolysis using FlexStation Microplate Reader (Molecular Devices). Reactions containing 1× reaction buffer + CytoPhos reagent only were used as background controls.
Quantification and statistical analysis
One-way ANOVA with post hoc Tukey pairwise comparisons, repeated-measures ANOVA, and Student's t test as specified in the figure legends were performed using GraphPad Prism v5.0d (RRID:SCR_002798) or SPSS Statistics v22 (RRID:SCR_002865). Mean ± SEM and specific n values are reported in each figure legend and in Table 1. Data are considered to be statistically significant if p ≤ 0.05. The assumption of Gaussian distribution was checked using D'Agostino-Pearson omnibus test.
Table 1.
Statistical analysis details
| Figure | Assay performed | Parameter | Comparison WT vs HT |
Descriptive Statistics |
Statistical Analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variables | n | Average | Error (SE or SD) | Statistical test | F | DF | ANOVA p values | Significance | |||
| 1C | Fluorescence imaging | GFP intensity, AFU | GFP | GFP = 7 coverslips | 7088 | 338.5 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 24.63 | 34 | p < 0.0001 | p = 0.9976 for GFP-Pyk2 vs GFP-Pyk2 & PF719 |
| GFP-Pyk2 | GFP-Pyk2 = 7 coverslips | 3814 | 299.8 (SE) | p = 0.9521 for GFP-Pyk2 vs GFP-Pyk2-K457A | |||||||
| GFP-Pyk2 & PF-719 | GFP-Pyk2 & PF-719 = 7 coverslips | 3680 | 341.4 (SE) | p = 0.0003 for GFP-Pyk2 vs GFP-Pyk2 & PF719 | |||||||
| GFP-Pyk2-K457A | GFP-Pyk2-K457A = 7 coverslips | 3515 | 163.4 (SE) | p < 0.0001 for GFP-Pyk2 vs GFP-Pyk2 & PF719 | |||||||
| GFP & Pyk2 | GFP & Pyk2 = 7 coverslips | 5104 | 305.6 (SE) | ||||||||
| 1D | Fluorescence imaging | Spine density, μm−1 | GFP | GFP = 7 coverslips | 0.445 | 0.023 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 22.4 | 34 | p < 0.0001 | p < 0.0001 for GFP vs GFP-Pyk2 |
| GFP-Pyk2 | GFP-Pyk2 = 7 coverslips | 0.219 | 0.024 (SE) | p = 0.5611 for GFP vs GFP-Pyk2 & PF719 | |||||||
| GFP-Pyk2 & PF-719 | GFP-Pyk2 & PF-719 = 7 coverslips | 0.392 | 0.035 (SE) | p = 0.9978 for GFP vs GFP-Pyk2-K457A | |||||||
| GFP-Pyk2-K457A | GFP-Pyk2-K457A = 7 coverslips | 0.435 | 0.016 (SE) | p < 0.0001 for GFP vs GFP & Pyk2 | |||||||
| GFP & Pyk2 | GFP & Pyk2 = 7 coverslips | 0.202 | 0.021 (SE) | p = 0.0003 for GFP-Pyk2 vs GFP-Pyk2 & PF719 | |||||||
| p < 0.0001 for GFP-Pyk2 vs GFP-Pyk2-K457A | |||||||||||
| 3B | Fluorescence imaging and colocalization test | Merged area, % | GFP & RFP-Graf1c | GFP & RFP-Graf1c = 11 images | 20.03 | 8.255 (SD) | One-way ANOVA; Turkey's multiple-comparisons test | 64.96 | 42 | p < 0.0001 | p < 0.0001 for GFP & RFP-Graf1c vs GFP-Pyk2 & RFP-Graf1c |
| GFP-Pyk2 & RFP-Graf1c | GFP-Pyk2 & RFP-Graf1c = 12 images | 86.76 | 18.02 (SD) | p = 0.1656 for GFP & RFP-Graf1c vs GFP-PXXP2mut & RFP-Grf1c | |||||||
| GFP-PXXP2mut & RFP-Graf1c | GFP-PXXP2mut & RFP-Graf1c = 10 images | 32.81 | 11.4 (SD) | ||||||||
| GFP-PSD95 & RFP-Graf1c | GFP-PSD95 & RFP-Graf1c = 10 images | 80.99 | 15 (SD) | ||||||||
| 4B | Western blotting | Graf1c/actin ratio, normalized by control | Control | Control = 5 experiments | 1 | 0 (SD) | Student's two tailed t test | p = 0.0007 for Control vs Graf1 shRNA | |||
| Graf1 shRNA | Graf1 shRNA = 5 experiements | 0.579 | 0.1753 (SD) | ||||||||
| 4D | ICC | Pyk2 intensity in synapse, normalized to U6 Control | Control = 22 images | 1 | 0.1035 (SE) | Student's two tailed t test | p = 0.035 for Control vs Graf1 shRNA | ||||
| Graf1 shRNA = 21 images | 0.736 | 0.0597 (SE) | |||||||||
| Pyk2 intensity in ex-synapse, normalized to U6 Control | Control = 22 images | 1 | 0.0754 (SE) | Student's two tailed t test | p = 0.2593 for Control vs Graf1 shRNA | ||||||
| Graf1 shRNA = 21 images | 1.072 | 0.0781 (SE) | |||||||||
| PSD-95 puncta # in GFP area, normalized to U6 Control | Control = 22 images | 1 | 0.075 (SE) | Student's two tailed t test | p = 0.9549 for Control vs Graf1 shRNA | ||||||
| Graf1 shRNA = 21 images | 0.739 | 0.0668 (SE) | |||||||||
| 4G | ICC | Pyk2 intensity in PSD-95 area, normalized to GFP Control | GFP = 19 images | 1 | 0.1209 (SE) | Student's two tailed t test | p = 0.0478 for GFP vs GFP-PRD | ||||
| GFP-PRD = 14 images | 0.689 | 0.0607 (SE) | |||||||||
| 5B | ICC | Pyk2 intensity in PSD-95 puncta, normalized by Veh | 1 h | Veh = 11 coverslips | 1 | 0.1133 (SE) | Student's two tailed t test | p = 0.7810 for Veh vs Aβo | |||
| Aβo = 10 coverslips | 1.042 | 0.0958 (SE) | |||||||||
| 6 h | Veh = 10 coverslips | 1 | 0.0798 (SE) | Student's two tailed t test | p = 0.3157 for Veh vs Aβo | ||||||
| Aβo = 9 coverslips | 1.153 | 0.1286 (SE) | |||||||||
| 24 h | Veh = 9 coverslips | 1 | 0.1118 (SE) | Student's two tailed t test | p = 0.0352 for Veh vs Aβo | ||||||
| Aβo = 10 coverslips | 1.284 | 0.0612 (SE) | |||||||||
| 5C | ICC | PSD-95 puncta # /Pyk2 intensity, normalized by Veh | 1 h | Veh = 11 coverslips | 1 | 0.0553 (SE) | Student's two tailed t test | p = 0.7969 for Veh vs Aβo | |||
| Aβo = 10 coverslips | 1.031 | 0.1098 (SE) | |||||||||
| 6 h | Veh = 10 coverslips | 1 | 0.0533 (SE) | Student's two tailed t test | p = 0.4203 for Veh vs Aβo | ||||||
| Aβo = 9 coverslips | 0.901 | 0.1107 (SE) | |||||||||
| 24 h | Veh = 9 coverslips | 1 | 0.0704 (SE) | Student's two tailed t test | p = 0.0352 for Veh vs Aβo | ||||||
| Aβo = 10 coverslips | 0.815 | 0.0493 (SE) | |||||||||
| 5E | Western blotting | Protein / PSD-95 ratio at 6 mo, normalized by WT | Graf1c/ PSD-95 | WT = 6 mice | 1 | 0.0977 (SD) | Student's two tailed t test | p = 0.0281 for WT vs APP/PS1 | |||
| APP/PS1 = 6 mice | 1.168 | 0.1276 (SD) | |||||||||
| Pyk2/PSD-95 | WT = 6 mice | 1 | 0.2251 (SD) | Student's two tailed t test | p = 0.9793 for WT vs APP/PS1 | ||||||
| APP/PS1 = 6 mice | 1.003 | 0.1081 (SD) | |||||||||
| Fyn/PSD-95 | WT = 6 mice | 1 | 0.2381 (SD) | Student's two tailed t test | p = 0.7231 for WT vs APP/PS1 | ||||||
| APP/PS1 = 6 mice | 0.9489 | 0.2479 (SD) | |||||||||
| 5G | Western blotting | Protein/PSD-95 ratio at 9 mo, normalized by WT | Graf1c/ PSD-95 | Total_WT = 5 mice | 1 | 0.1085 (SD) | |||||
| Total_APP/PS1 = 5 mice | 1.033 | 0.0789 (SD) | |||||||||
| PSD_WT = 5 mice | 1 | 0.0595 (SD) | Student's two tailed t test | p = 0.0004 for WT vs APP/PS1 | |||||||
| PSD_APP/PS1 = 5 mice | 1.197 | 0.0458 (SD) | |||||||||
| Pyk2/PSD-95 | Total_WT = 5 mice | 1 | 0.1698 (SD) | ||||||||
| Total_APP/PS1 = 5 mice | 0.984 | 0.0794 (SD) | |||||||||
| PSD_WT = 5 mice | 1 | 0.2054 (SD) | Student's two tailed t test | p = 0.0022 for WT vs APP/PS1 | |||||||
| PSD_APP/PS1 = 5 mice | 1.565 | 0.1971 (SD) | |||||||||
| Fyn / PSD-95 | Total_WT = 5 mice | 1 | 0.1317 (SD) | ||||||||
| Total_APP/PS1 = 5 mice | 1.071 | 0.0856 (SD) | |||||||||
| PSD_WT = 5 mice | 1 | 0.1182 (SD) | Student's two tailed t test | p = 0.5068 for WT vs APP/PS1 | |||||||
| PSD_APP/PS1 = 5 mice | 1.071 | 0.1965 (SD) | |||||||||
| 5I | Western Blotting | Protein/PSD-95 ratio at 13 mo, normalized by WT | Graf1c / PSD-95 | PSD_WT = 6 mice | 1 | 0.0419 (SD) | Student's two tailed t test | p = 0.0411 for WT vs APP/PS1 | |||
| PSD_APP/PS1 = 6 mice | 1.143 | 0.0445(SD) | |||||||||
| Pyk2 / PSD-95 | PSD_WT = 6 mice | 1 | 0.0442 (SD) | Student's two tailed t test | p = 0.0128 for WT vs APP/PS1 | ||||||
| PSD_APP/PS1 = 6 mice | 1.326 | 0.0981 (SD) | |||||||||
| Fyn / PSD-95 | PSD_WT = 6 mice | 1 | 0.0489 (SD) | Student's two tailed t test | p = 0.9337for WT vs APP/PS1 | ||||||
| PSD_APP/PS1 = 6 mice | 1.009 | 0.088 (SD) | |||||||||
| 5J | PLISA | ng Aβo, ng/tissue, g | WT, 3 mo | WT = 6 mice | 0.135 | 0.1343 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 19.23 | 35 | p < 0.0001 | p < 0.0001 for APP/PS1 3 mo vs APP/PS1 12 mo |
| APP/PS1, 3 mo | APP/PS1 = 6 mice | 0.4194 | 0.2721 (SE) | p < 0.0001 for APP/PS1 6 mo vs APP/PS1 12 mo | |||||||
| APP/PS1, 6 mo | APP/PS1 = 6 mice | 1.237 | 0.6306 (SE) | p < 0.0001 for APP/PS1 9 mo vs APP/PS1 12 mo | |||||||
| APP/PS1, 9 mo | APP/PS1 = 6 mice | 6.008 | 2.126 (SE) | ||||||||
| WT, 12 mo | APP/PS1 = 6 mice | 0.354 | 0.0897 (SE) | ||||||||
| APP/PS1, 12 mo | APP/PS1 = 6 mice | 35.43 | 7.478 (SE) | ||||||||
| 5K | IHC | Thioflavin S, % area | WT, 3 mo | WT = 8 mice | 0.0001279 | 8.331e−005 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 34.01 | 46 | p < 0.0001 | p < 0.0001 for APP/PS1 3 mo vs APP/PS1 12 mo |
| APP/PS1, 3 mo mo | APP/PS1 = 8 mice | 0.0005608 | 0.0002712 (SE) | p < 0.0001 for APP/PS1 6 mo vs APP/PS1 12 mo | |||||||
| APP/PS1, 6 mo | APP/PS1 = 8 mice | 0.07512 | 0.02726 (SE) | p < 0.0001 for APP/PS1 9 mo vs APP/PS1 12 mo | |||||||
| APP/PS1, 9 mo | APP/PS1 = 8 mice | 0.573 | 0.1102 (SE) | ||||||||
| WT, 12 mo | APP/PS1 = 7 mice | 0.003523 | 0.00183 (SE) | ||||||||
| APP/PS1, 12 mo | APP/PS1 = 8 mice | 1.436 | 0.2099 (SE) | ||||||||
| 5L | IHC | PSD-95, % area | 12 mo | WT = 7 mice | 4.19 | 0.5074 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 1.666 | 62 | p = 0.1368 | p = 0.0389 for WT vs APP/PS1 |
| APP/PS1 = 8 mice | 1.331 | 0.3533 (SE) | |||||||||
| 6B | In vitro GTP hydrolysis assay | GTP hydrolysis, normalized by RhoA only | Rho | RhoA = 8 experiments | 1 | 0 (SD) | One-way ANOVA; Turkey's multiple-comparisons test | 11.92 | 39 | p < 0.0001 | p = 0.0028 for RhoA & Graf1c vs RhoA, Graf1c & GFP-Pyk2 |
| RhoA, Graf1c | RhoA, Graf1c = 8 experiments | 2.134 | 0.5146 (SD) | p = 0.3415 for RhoA & Graf1c vs RhoA, Graf1c & GFP-K457A | |||||||
| RhoA, GFP-Pyk2, Graf1c | RhoA, GFP-Pyk2, Graf1c = 8 experiments | 1.411 | 0.3605 (SD) | p = 0.6208 for RhoA & Graf1c vs RhoA, Graf1c & GFP-PXXP2mut | |||||||
| RhoA, GFP-K457A, Graf1c | RhoA, GFP-K457A, Graf1c = 8 experiments | 1.791 | 0.2888 (SD) | ||||||||
| RhoA, GFP-PXXP2mut, Graf1c | RhoA, GFP-PXXP2mut, Graf1c = 8 experiments | 1.877 | 0.4246 (SD) | ||||||||
| 6D | RBD pull-down assay | RhoA-GTP/ total RhoA, normalized by WT | RhoA | RhoA = 3 experiments | 1 | 0 (SD) | One-way ANOVA; Turkey's multiple- comparisons test | 23.59 | 17 | p < 0.0001 | p = 0.0089 RhoA vs RhoA & GFP-Pyk2 |
| RhoA & GFP-Pyk2 | RhoA & GFP-Pyk2 = 3 experiments | 1.476 | 0.1363 (SD) | p = 0.0155 RhoA vs RhoA & Graf1c | |||||||
| RhoA & Graf1c | RhoA & Graf1c = 3 experiments | 0.559 | 0.0917 (SD) | p = 0.0018 RhoA & Graf1c vs RhoA, GFP-Pyk2, Graf1c | |||||||
| RhoA, GFP-Pyk2, Graf1c | RhoA, GFP-Pyk2, Graf1c = 3 experiments | 1.142 | 0.1307 (SD) | p > 0.0001 RhoA & Graf1c vs RhoA, GFP-K457A, Graf1c | |||||||
| RhoA, GFP-K457A, Graf1c | RhoA, GFP-K457A, Graf1c = 3 experiments | 0.563 | 0.1812 (SD) | p = 0.9703 RhoA & Graf1c vs RhoA, GFP-PXXP2mut, Graf1c | |||||||
| RhoA, GFP-PXXP2mut, Graf1c | RhoA, GFP-PXXP2mut, Graf1c = 3 experiments | 0.641 | 0.1713 (SD) | ||||||||
| 7B | RBD pull-down assay | RhoA-GTP/ total RhoA, normalized by WT | WT | Veh = 4 experiments | 1 | 0.1474 (SD) | One-way ANOVA; Turkey's multiple- comparisons test | 15.53 | 15 | p = 0.0002 | p = 0.0075 WT veh vs WT Aβo |
| Aβo = 4 experiments | 1.651 | 0.3115 (SD) | |||||||||
| Pyk2−/− | Veh = 4 experiments | 0.715 | 0.2195 (SD) | p = 0.9985 Pyk2−/− veh vs Pyk2−/− Aβo | |||||||
| Aβo = 4 experiments | 0.689 | 0.1985 (SD) | |||||||||
| 7D | RBD pull-down assay | RhoA-GTP/ total RhoA, normalized by WT | 6 mo | WT = 6 mice | 1 | 0.5115 (SD) | Student's two tailed t test | p = 0.9534 for WT vs APP/PS1 | |||
| APP/PS1 = 6 mice | 0.986 | 0.1899 (SD) | |||||||||
| 9 mo | WT = 6 mice | 1 | 0.1688 (SD) | Student's two tailed t test | p = 0.0027 for WT vs APP/PS1 | ||||||
| APP/PS1 = 6 mice | 2.105 | 0.6613 (SD) | |||||||||
| 13 mo | WT = 6 mice | 1 | 0.3357 (SD) | Student's two tailed t test | p < 0.0001 for WT vs APP/PS1 | ||||||
| APP/PS1 = 6 mice | 2.828 | 0.568 (SD) | |||||||||
| 8B | Fluorescence imaging and Spine density | Spine density, μm−1 | GFP | GFP = 7 coverslips | 0.483 | 0.0152 (SE) | One-way ANOVA; Turkey's multiple- comparisons test | 11.48 | 48 | p < 0.0001 | p = 0.4065for GFP vs GFP & Y27632 |
| GFP & Y27632 | GFP & Y27632 = 6 coverslips | 0.412 | 0.0253 (SE) | p = 0.6567 for GFP vs GFP & RhoA-T19N | |||||||
| GFP & RhoA-T19N | GFP & RhoA-T19N = 6 coverslips | 0.425 | 0.0311 (SE) | p < 0.0001 for GFP vs GFP-Pyk2 | |||||||
| GFP-Pyk2 | GFP-Pyk2 = 6 coverslips | 0.213 | 0.0254 (SE) | p < 0.0001 for GFP-Pyk2 vs GFP-Pyk2 & Y27632 | |||||||
| GFP-Pyk2 & Y27632 | GFP-Pyk2 & Y27632 = 7 coverslips | 0.422 | 0.0279 (SE) | p < 0.0001 for GFP-Pyk2 vs GFP-Pyk2 & RhoAT19N | |||||||
| GFP-Pyk2 & RhoA-T19N | GFP-Pyk2 & RhoA-T19N = 4 coverslips | 0.43 | 0.0302 (SE) | p < 0.0001 for GFP-Pyk2 vs GFP-Pyk2 & Graf1c | |||||||
| GFP-Pyk2 & Graf1c | GFP-Pyk2 & RhoA-T19N = 6 coverslips | 0.447 | 0.0197 (SE) | p = 0.9117 for GFP vs GFP-PXXP2mut | |||||||
| GFP-PXXP2mut | GFP-PXXP2mut = 7 coverslips | 0.423 | 0.0164 (SE) | ||||||||
| 8D | Fluorescence imaging and Spine density | Spine density, μm−1 | Control | Control = 8 coverslips | 0.466 | 0.0165 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 23.81 | 22 | p < 0.0001 | p < 0.0001 for Control vs Graf1 shRNA |
| Graf1 shRNA | Graf1 shRNA = 5 coverslips | 0.234 | 0.0223 (SE) | p = 0.0006for Graf1 shRNA vs Graf1 shRNA & Y27632 | |||||||
| Graf1 shRNA & Y27632 | Graf1 shRNA & Y27632 = 5 coverslips | 0.391 | 0.0227 (SE) | p < 0.0001 for Graf1 shRNA vs Graf1 shRNA & RhoA-T19N | |||||||
| Graf1 shRNA & RhoA-T19N | Graf1 shRNA & RhoA-T19N = 5 coverslips | 0.456 | 0.0269 (SE) | ||||||||
| 9B | Live fluorescence imaging spine motility | Changed area, min−1, % | WT | Veh = 16 coverslips | 26.81 | 0.943 (SE) | Student's two tailed t test | p < 0.0001 for Veh vs Aβo | |||
| Aβo = 14 coverslips | 20.39 | 0.9693 (SE) | |||||||||
| Pyk2−/− | Veh = 14 coverslips | 32.65 | 1.562 (SE) | Student's two tailed t test | p = 0.6913 for Veh vs Aβo | ||||||
| Aβo = 14 coverslips | 31.82 | 1.348 (SE) | |||||||||
| WT & PF-719 | Veh = 17 coverslips | 32.21 | 1.134 (SE) | Student's two tailed t test | p = 0.9965 for Veh vs Aβo | ||||||
| Aβo = 16 coverslips | 32.21 | 1.144 (SE) | |||||||||
| WT & Pyk2-PRD | Veh = 6 coverslips | 30.31 | 4.104 (SE) | Student's two tailed t test | p = 0.8334 for Veh vs Aβo | ||||||
| Aβo = 6 coverslips | 29.19 | 3.162 (SE) | |||||||||
| WT & Y27632 | Veh = 15 coverslips | 33.21 | 1.578 (SE) | Student's two tailed t test | p = 0.7427 for Veh vs Aβo | ||||||
| Aβo = 11 coverslips | 32.38 | 1.945 (SE) | |||||||||
| 9D | Live fluorescence imaging Spine motility | Changed area, min−1, % | Con | Con = 6 coverslips | 28.15 | 1.299 (SE) | Student's two tailed t test | p < 0.0001 for Con vs CytoD | |||
| CytoD | CytoD = 6 coverslips | 9.37 | 0.806 (SE) | ||||||||
| 10B | Fluorescence imaging and Spine density | Spine density, μm−1 | WT | Con = 8 coverslips | 0.447 | 0.0233(SE) | One-way ANOVA; Turkey's multiple-comparisons test | 6.43 | 37 | p = 0.0006 | p = 0.0034 for Con vs Aβo 4d |
| Veh 6 h = 7 coverslips | 0.456 | 0.0198 (SE) | p = 0.0023 for Veh 4d vs Aβo 4d | ||||||||
| Aβo 6 h = 7 coverslips | 0.402 | 0.0203 (SE) | |||||||||
| Veh 4d = 8 coverslips | 0.455 | 0.0248 (SE) | |||||||||
| Aβo 4d = 8 coverslips | 0.333 | 0.0163 (SE) | |||||||||
| 10D | Fluorescence imaging & Spine density | Spine density, μm−1 | Pyk2−/− | Control = 8 coverslips | 0.439 | 0.0119 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 0.2701 | 39 | p = 0.8952 | p = 0.997 for Veh 4d vs Aβo 4d |
| Veh 6 h = 8 coverslips | 0.456 | 0.0228 (SE) | |||||||||
| Aβo 6 h = 8 coverslips | 0.429 | 0.0211 (SE) | |||||||||
| Veh 4d = 8 coverslips | 0.454 | 0.0282 (SE) | |||||||||
| Aβo 4d = 8 coverslips | 0.44 | 0.0203 (SE) | |||||||||
| 10F | Fluorescence imaging & Spine density | Spine density, μm−1 | PF-719 | Con = 8 coverslips | 0.442 | 0.0102 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 0.3202 | 23 | p = 0.7295 | p = 0.7998 for Veh 4d vs Aβo 4d |
| Veh 4d = 8 coverslips | 0.44 | 0.0204 (SE) | |||||||||
| Aβo 4d = 8 coverslips | 0.426 | 0.0137 (SE) | |||||||||
| Pyk2-PRD | Con = 5 coverslips | 0.44 | 0.0238 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 0.6194 | 14 | p = 0.5546 | p = 0.7966 for Veh 4d vs Aβo 4d | |||
| Veh 4d = 5 coverslips | 0.427 | 0.0179 (SE) | |||||||||
| Aβo 4d = 5 coverslips | 0.409 | 0.018 (SE) | |||||||||
| RhoA-T19N | Con = 5 coverslips | 0.425 | 0.0246 (SE) | One-way ANOVA; Turkey's multiple-comparisons test | 0.4549 | 14 | p = 0.6450 | p = 0.7974 for Veh 4d vs Aβo 4d | |||
| Veh 4d = 5 coverslips | 0.458 | 0.027 (SE) | |||||||||
| Aβo 4d = 5 coverslips | 0.435 | 0.0234 (SE) | |||||||||
This table delineates the numerical values, replicates, variance, and statistical tests for the data presented in the indicated figures.
Results
Pyk2 overexpression induces spine loss in neurons
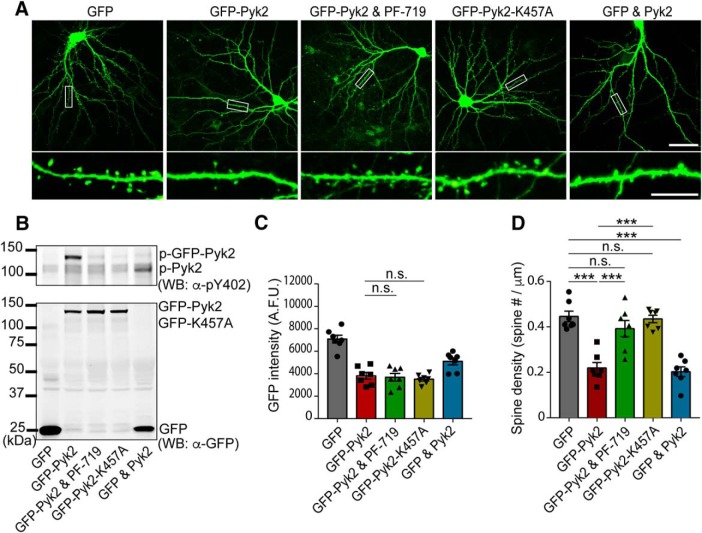
To evaluate the potential consequences of Pyk2 gain-of-function in AD, we expressed Pyk2 in primary culture of mouse hippocampal neurons. We overexpressed GFP-Pyk2, or untagged Pyk2 (GFP and Pyk2), in neurons, and observed activation by autophosphorylation on residue Y402 (H. J. Park et al., 2004) and an expression pattern throughout dendrites (Fig. 1A–C). This expression led to a decrease in spine density by 60% compared with GFP-transfected neurons using an automated quantitation method (Fig. 1A,D). To test whether the kinase activity of Pyk2 was responsible for the observed spine loss, we pretreated GFP-Pyk2-expressing neurons with a specific small molecule inhibitor of Pyk2, PF-719 (Tse et al., 2012), and observed a rescue of GFP-Pyk2-induced spine loss. Concordantly, overexpressing the kinase inactive GFP-K457A mutant of Pyk2, at a level equal to WT GFP-Pyk2 (Fig. 1C), did not alter dendritic spine density compared with GFP-only transfected control (Fig. 1D). Thus, increased Pyk2 kinase activity induces synapse loss, consistent with Pyk2 activation contributing to synapse loss in AD.
Figure 1.
Pyk2 induces dendritic spine loss. A, Representative GFP fluorescent images of cultured mouse hippocampal neurons. Neurons were transfected with GFP alone, GFP-Pyk2, GFP-Pyk2 with 1 μm PF-719, GFP-K457A, or GFP and Pyk2 (untagged) at DIV 14 and then fixed at 21 DIV. Bottom, Enlarged images of the enclosed rectangles on the top. Scale bars: low-magnification (top), 50 μm; high-magnification (bottom), 10 μm. B, Lysates from transfected neurons were subjected to Western blotting with anti-GFP and anti-p-Pyk2 Y402 antibodies. C, GFP intensity quantification from imaged neurons for spine quantitation in A. D, Quantification of dendritic spine density in the transfected neurons. Data are presented as mean ± SEM (GFP, n = 10; GFP-Pyk2, n = 11; GFP-Pyk2 and PF-719, n = 9; GFP-K457A, n = 10 coverslips from 3 different cultures). ***p < 0.001 by one-way ANOVA, Tukey's multiple-comparisons test.
Pyk2 interacts with Graf1c and localizes to postsynaptic sites
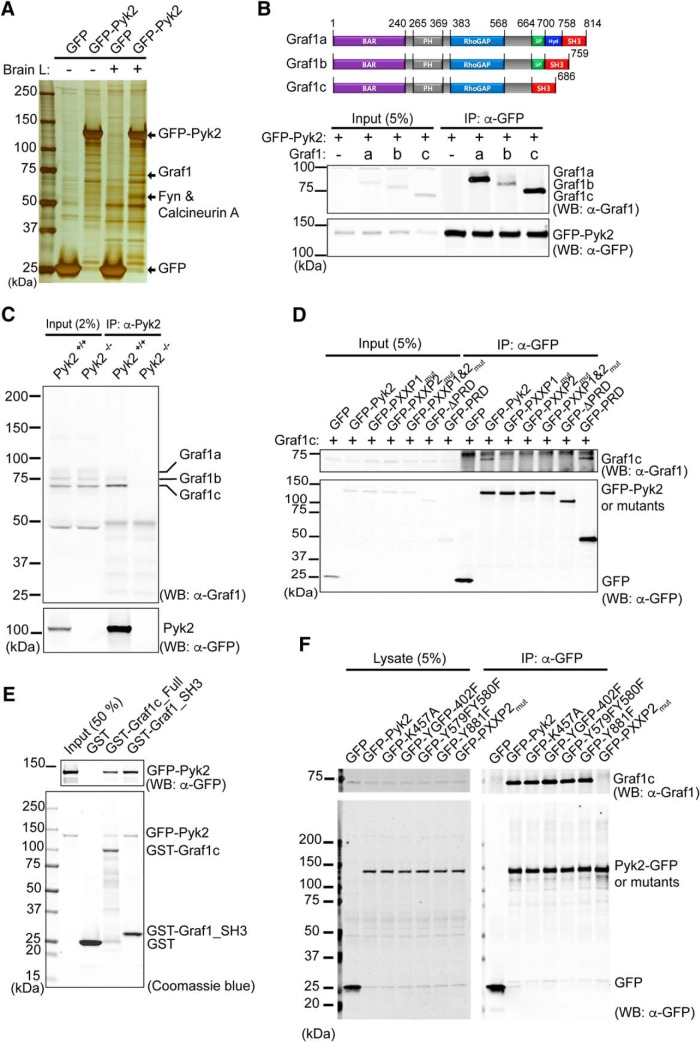
To explore the molecular basis for Pyk2 action, we examined Pyk2 immunoprecipitates for interacting proteins. We added lysate from GFP and GFP-Pyk2-expressing Hek293T cells to mouse brain lysate before capture with immobilized anti-GFP antibody (Fig. 2A). The two most prominent silver-stained protein species from the brain pull-down other than GFP-Pyk2 were excised and analyzed by LC-MS/MS. We identified Fyn and calcineurin A from one band, and Graf1 from another band as Pyk2-intearcting proteins. The Graf1 is known to exhibit GAP activity for RhoA and Cdc42, and the Graf1 BAR domain suggests a membrane sculpting function in clathrin-independent endocytosis (Hildebrand et al., 1996; Taylor et al., 1999; Lundmark et al., 2008; Doherty et al., 2011; Fig. 2B). Our identification of Graf1 confirms the results of a survey of 10 SH3 domains for Pyk2 interaction, and the coimmunoprecipitation of the two proteins (Ohba et al., 1998). Given the spine loss data described above (Fig. 1), we focused on Graf1 and the Pyk2–Graf1 interaction. Graf1 is highly expressed in the heart, brain, and lung (Taylor et al., 1998) and exists in three splice variants (Lucken-Ardjomande Häsler et al., 2014). Therefore, we sought to determine whether Pyk2 has a preference for binding among Graf1 isoforms. By immunoprecipitation of GFP-Pyk2 from Hek293T cells, we observed interaction with all three isoforms, but detectable stronger binding to isoforms a and c (Fig. 2B). Graf1c is the predominant species expressed in brain (Lucken-Ardjomande Häsler et al., 2014) and endogenous Graf1c is the predominant Graf1 isoform to coimmunoprecipitate with endogenous Pyk2 (Fig. 2C). These data suggest that Pyk2 is an endogenous binding partner of Graf1c.
Figure 2.
Graf1c interacts with Pyk2 and colocalizes to postsynaptic terminal. A, Lysates from GFP or GFP-Pyk2 transfected Hek293T cells or the same lysates mixed with mouse brain lysate (Brain L) were immunoprecipitated with anti-GFP antibody trapped agarose beads. The immunoprecipitates were separated by SDS-PAGE and silver stained to identify proteins for subsequent LC-MS/MS analysis. Three major binding proteins were identified as Graf1, Fyn, and calcineurin A. B, Graf1 isoforms domain structure diagrams and binding test in Pyk2 and Graf1 isoforms in overexpressed Hek293T cells. Pyk2 and Graf1 isoforms cotransfected Hek293T cell lysates were immunoprecipitated with anti-GFP antibody and immunoblotted with anti-Graf1 and anti-GFP antibodies. C, WT (Pyk2+/+) and Pyk2 KO (Pyk2−/−) mouse brain lysates were immunoprecipitated with anti-Pyk2 antibody and immunoblotted with anti-Graf1 and anti-Pyk2 antibodies. D, Graf1c and indicated GFP or GFP tagged Pyk2 and mutants (PXXP1mut, P714AP717A; PXXP2mut, P857AP860A; ΔPRD, aa 679–870 deleted; PRD, aa 679–870) were cotransfected in Hek293T cells and then immunoprecipitated with anti-GFP antibody and immunoblotted with anti-GFP and anti-Graf1 antibodies. E, In vitro direct binding test between purified GST, GST tagged Graf1c, or GST tagged Graf1c SH3 domain (aa 558–686) from BL21-DE3 E. coli and 1 μg of GFP-Pyk2 from Hek293T cells. Pull-downed protein complexes were separated by SDS-PAGE and stained with Coomassie blue and separately immunoblotted with anti-GFP antibody. F, Hek293T cells were cotransfected with Graf1c and indicated GFP and GFP-Pyk2 constructs. Cells were harvested and immunoprecipitated with an anti-GFP antibody. Immunoblots were probed with anti-Graf1c and anti-GFP antibodies.
Previous data showed that the proline-rich domain (PRD) of Pyk2 binds the isolated Graf1c SH3 domain (Ohba et al., 1998). To confirm this in the setting of full-length proteins, we performed an immunoprecipitation experiment with full-length Graf1c and Pyk2 along with several Pyk2-PRD mutants in Hek293T cells (Fig. 2D). Pyk2 protein contains two separate PXXP motifs, so it is possible that Pyk2 interacts with Graf1c through one, both or none of the PXXP motifs. To explore this, we immunoprecipitated GFP from Hek293T cells expressing GFP-Pyk2 and the indicated mutants (Fig. 2D). We observed coimmunoprecipitation of Graf1c with full-length GFP-Pyk2 and GFP-PRD (aa 680∼870, include both PXXP motifs), partial coimmunoprecipitation with GFP-PXXP1mut, and very little to zero coimmunoprecipitation with GFP-PXXP2mut, GFP-PXXP1&2mut, or GFP-ΔPRD (Pyk2 with both PRD regions deleted). This indicates that Graf1 interacts with both PXXP motifs but interacts more strongly with the second PXXP motif of Pyk2 (aa substitutions: P857A, P860A). To further examine the direct interaction of Pyk2 and Graf1c, we performed an in vitro binding assay with purified recombinant protein (Fig. 2E). We used a GST fusion protein with GST-Graf1c_Full, containing full-length Graf1c protein, and GST-Graf1_SH3 domain, containing only the Graf1 SH3 domain. We performed a GST pull-down with purified GFP-Pyk2 and GST-Graf1c_Full or GFP-Pyk2 and GST-Graf1_SH3 and observed a prominent band for GFP-Pyk2 by Coomassie stain or immunoblotting with anti GFP antibody. These data demonstrate a direct and specific interaction between Pyk2 and Graf1c the SH3 domains. In addition to the PRD regions of Pyk2, we sought to determine the ability of Graf1c to bind Pyk2 under different phosphorylation states (Fig. 2F). We found that Pyk2 is able to bind Graf1c independently of Pyk2 phosphorylation status. Thus, Graf1c is an endogenous binding partner of Pyk2 and their interaction is mediated by the Pyk2 PRD region and the Graf1c SH3 domain.
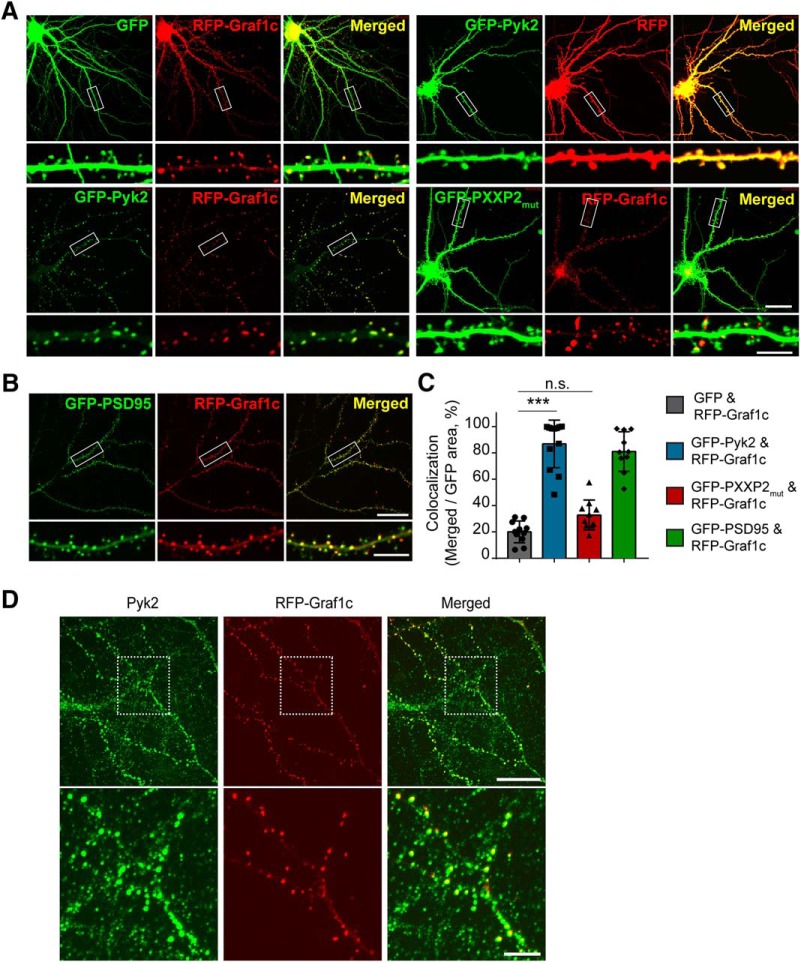
To define the potential function of the Pyk2-Graf1c interaction, we first assessed their relative subcellular localization in neurons. We expressed XFP-tagged Pyk2 and Graf1c constructs in primary mouse hippocampal neurons (Fig. 3A–C). RFP-Graf1c coexpressed together with GFP (to visualize neuronal morphology) generates RFP-Graf1c specific puncta with a synapse-like morphology. Coexpression of GFP-PSD-95 and RFP-Graf1c confirmed that RFP-Graf1c puncta are indeed in dendritic spines (Fig. 3B,C). In contrast, overexpressed GFP-Pyk2 displays a diffuse cellular pattern similar to that of RFP alone. The endogenous Pyk2 expression pattern is more punctate and not as diffuse as GFP-Pyk2, such that the RFP-Graf1c plus endogenous Pyk2 colocalization pattern is very similar to the expression pattern of GFP-Pyk2 and RFP-Graf1c (Fig. 3D). Quantitatively, coexpression of GFP-Pyk2 and RFP-Graf1c yields near complete colocalization of the two proteins (Fig. 3A,C). Furthermore, this colocalization depends on the PRD region of Pyk2 because the colocalization is abolished with the Pyk2 PRD region mutant GFP-PXXP2mut. Together, these data suggest that Pyk2 and Graf1c associate and colocalize in the dendritic spines of neurons.
Figure 3.
Graf1c colocalizes with Pyk2 to postsynaptic terminal. A, Cultured hippocampal neurons were transfected with GFP and RFP-Graf1c, GFP-Pyk2 and RFP, GFP-Pyk2 and RFP-Graf1c, or GFP-PXXP2mut and RFP-Graf1c at 14 DIV and imaged at 19 DIV without fixation. Bottom, High-magnification images are enlarged views of the rectangle regions on the top. Scale bars: low-magnification, 25 μm; high-magnification, 10 μm. B, Cultured hippocampal neurons expressing GFP-PSD95 and RFP-Graf1c were imaged at 19 DIV. Scale bars: low-magnification, 25 μm; high-magnification, 10 μm. C, Quantitation of colocalization in A. B, Green and Red merged area was divided by total green area. Data are graphed as mean ± SD (n = 11, 12, 10, 10 images). ***p < 0.0001 by one-way ANOVA, Tukey's multiple-comparisons test. D, Immunofluorescence images of endogenous Pyk2 in RFP-Graf1c expressed neuron. Scale bars: low-magnification, 20 μm; high-magnification, 5 μm.
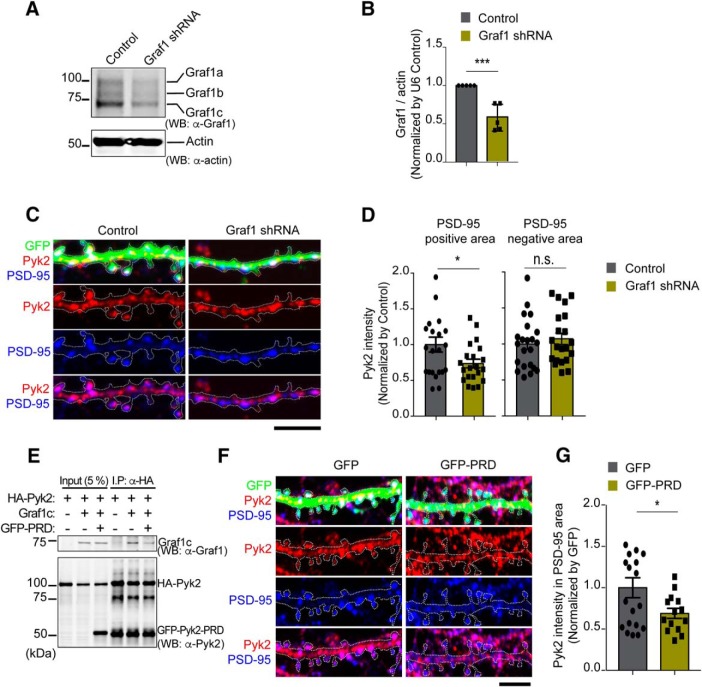
Given the dendritic spine colocalization of overexpressed Pyk2 and Graf1c, we sought to determine whether endogenous Pyk2 localization to synaptic structures requires Graf1c. We suppressed Graf1 expression by expressing a Graf1-targeting shRNA targeting all Graf1 isoforms (Fig. 4A,B). Primary mouse hippocampal neurons were visualized by coexpressed GFP (Fig. 4C). Colocalization of endogenous Pyk2 immunoreactivity within PSD-95-immunoreactive puncta significantly decreases in cells expressing Graf1 shRNA (Fig. 4C,D). This is consistent with Graf1c mediating Pyk2 colocalization at the synapse. To confirm this, we interrupted endogenous Pyk2 interaction with Graf1c by expression of a truncated GFP-PRD consisting of the isolated PRD region of Pyk2 (Fig. 4E–G). To verify the predicted dominant-negative function of the truncated fragment, we first coexpressed HA-Pyk2 together with Graf1c in Hek293T cells and immunoprecipitated HA-Pyk2 in the presence or absence of GFP-PRD. In a competitive manner, GFP-PRD blocks the HA-Pyk2 interaction with Graf1c (Fig. 4E). When overexpressed in neurons, dominant-negative GFP-PRD significantly decreases the colocalization of endogenous Pyk2 (detected by an antibody recognizing a distinct epitope) with PSD-95 (Fig. 4F,G). Together, these data demonstrate that endogenous Pyk2 localization to PSD-95-positive dendritic spines requires the interaction of its PRD domain with Graf1c.
Figure 4.
Graf1 regulates Pyk2 localization in postsynaptic terminals in cultured neurons. A, Cultured hippocampal neurons were transfected with either U6 vector (U6 Control) or Graf1-specific shRNA by electroporation before plating. Lysates from 21 DIV transfected neurons were immunoblotted with anti-Graf1 and anti-Actin antibodies. B, Densitometric analysis of Graf1 immunoreactivity from A was quantified and normalized to U6 control. Data are graphed as mean ± SEM (n = 5 wells). ***p < 0.001 by Student's two-tailed t test. C, Immunofluorescence of Pyk2 (red) and PSD-95 (blue) in 21 DIV U6 Control vector or Graf1 shRNA transfected neurons. Transfected neurites were marked with white dashed line based on GFP fluorescence. Scale bar, 10 μm. D, Quantification of Pyk2 levels in synaptic (PSD-95 plus GFP-positive) and extra-synaptic (PSD-95-negative and GFP-positive) areas. Mean ± SEM (U6 Control, n = 22; Graf1 shRNA, n = 21; n = separate neuron). *p < 0.05 by Student's two-tailed t test. E, Pyk2-PRD competes with Pyk2 and inhibits Graf1c interaction in overexpressed Hek293T cells. Lysates from indicated plasmids transfected Hek293T cells were immunoprecipitated with anti-HA antibodies. The input lysates (5%) and precipitates were subjected to Western blotting with anti-Graf1 and Pyk2 PRD region-specific anti-Pyk2 antibodies. F, GFP- or GFP-Pyk2-PRD- (GFP-PRD) expressed neurons were costained with Pyk2 N-terminal-specific (aa 1∼100) anti-Pyk2 antibody (red) and PSD-95 antibody (blue). Transfected neurites were marked with white dashed line based on GFP fluorescence. Scale bar, 10 μm. G, Quantification of postsynaptic terminal localized Pyk2 levels with same method as in C. Mean ± SEM (GFP, n = 19; GFP-PRD, n = 14 neurons). *p < 0.05 by Student's two-tailed t test.
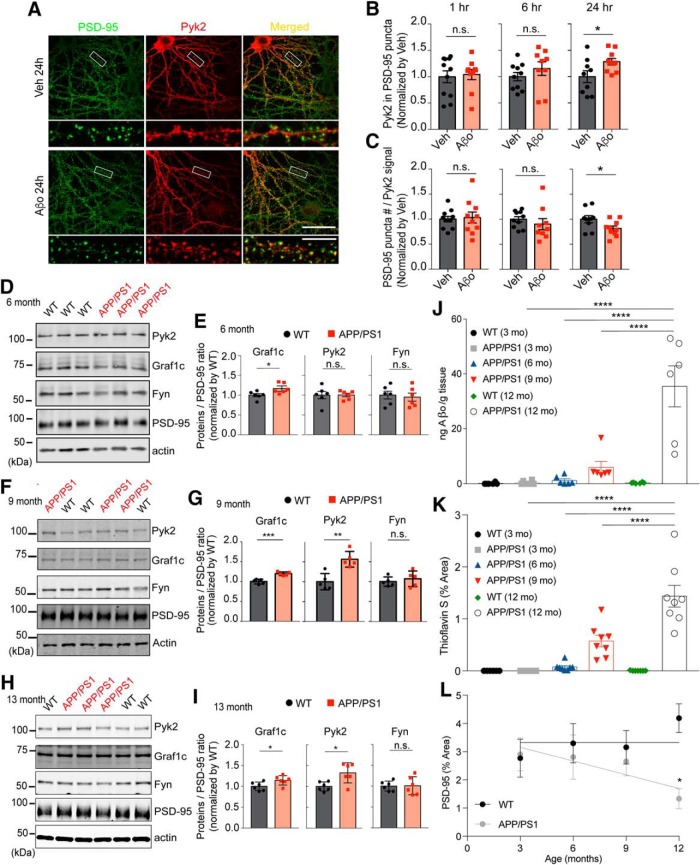
Pyk2 enrichment at synapses is increased by Aβo signaling in neurons and APP/PS1 mice
We hypothesized that AD pathology and Aβo signaling might regulate the localization of Pyk2 at postsynaptic sites. We exposed primary hippocampal neurons to Aβo and determined the extent of endogenous Pyk2 immunoreactivity colocalizing with PSD-95 puncta. Treatment with Aβo, but not vehicle, for 6 h induces a nonsignificant trend to increase Pyk2-PSD-95 colocalization, whereas 24 h exposure to Aβo significantly increases intensity of Pyk2 immunoreactivity within PSD-95-immunoreactive puncta (Fig. 5A,B). This increase is not because of greater PSD-95 area, because the Aβo treatment decreases dendritic spine density (Um et al., 2012, 2013; Heiss et al., 2017; see three sections below), and the PSD-95 puncta count decreases in the same time period (Fig. 5C).
Figure 5.
Postsynaptic Pyk2 localization is increased in Aβo-treated cultured neurons and APP/PS1 mice. A, Hippocampal neurons were incubated for 24 h with Veh or Aβo (1 μm monomer, 10 nm oligomer estimate) in culture media then fixed and stained with anti-Pyk2 and anti-PSD-95 antibodies. Bottom, Enlarged images. Scale bars: top, 40 μm; bottom, 10 μm. B, C, Quantification of Pyk2 immunofluorescence intensity within PSD-95-positive postsynaptic area (B) and PSD-95 puncta number within neuronal area defined by Pyk2 (C). Data are graphed as mean ± SEM (Veh 1 h: n = 11; Aβo 1 h: n = 10; Veh 6 h: n = 10; Aβo 6 h: n = 9; Veh 24 h: n = 10; Aβo 24 h: n = 9 coverslips from 3 different cultures). *p < 0.05 by Student's two-tailed t test. D, PSD fractions (20 μg proteins) from 6 month WT or APP/PS1 forebrain were subjected to Western blotting with anti-Pyk2, anti-Graf1, anti-Fyn, anti-PSD95, and actin antibodies. E, Quantification of Graf1c, Pyk2, and Fyn levels in D normalized to PSD-95. Mean ± SEM (n = 6 mice). F, G, Same as D and E but for samples from 9-month-old mice. **p < 0.01, ***p < 0.001 by Student's two-tailed t test. H, I, Same as D and E but for samples from 13-month-old mice. *p < 0.05 by Student's two-tailed t test. J, K, Quantification of PrPC-interacting Aβo from TBS-soluble cortical lysates or Thioflavin-positive plaque burden in sections from mice of the indicated ages. Mean ± SEM (n = 7 mice for WT, n = 8 for all other groups). ****p < 0.0001 by one-way ANOVA with Tukey's multiple comparison test. L, Quantification of synaptic density from sections of dentate gyrus measured as anti-PSD-95-immunoreactive area. Mean ± SEM (n = 8 mice for all groups). *p < 0.05 by one-way ANOVA with Tukey's multiple-comparison test.
To assess the synaptic enrichment of Pyk2 in vivo, we measured both protein levels in postsynaptic density (PSD) preparations from WT and APPswe/PSEN1ΔE9 (hereafter referred to as APP/PS1; Jankowsky et al., 2004) mice at 6, 9, and 13 months of age (Fig. 5D–I). At 6 months of age, this strain has low levels of Aβ accumulation and no behavioral deficits or synaptic loss, whereas at 9 months of age, Aβo levels rise (Fig. 5J), plaque accumulates (Fig. 5K), synapses are lost (Fig. 5L), and memory function is impaired (Jankowsky et al., 2004; Janus et al., 2015; Hong et al., 2016). Pyk2 concentration in the PSDs of APP/PS1 mice is equal to that in WT samples at 6 months but is significantly increased by ∼50% during disease progression at 9 and 13 months (Fig. 5F,G,I). In contrast, Fyn enrichment in the PSD is not altered in the APP/PS1 mice, and Graf1c enrichment shows changes of 15% or less at all ages. Although Pyk2 is increased in the PSD at 9 months, there is no change of Pyk2 in total S1 fraction (data not shown). Thus, Pyk2 is enriched at synapses in an Aβo/APP transgene-dependent manner that correlates with phenotypic progression.
Pyk2 inhibits Graf1c to yield increased RhoA activation
We considered how Pyk2 redistribution and interaction with Graf1c might contribute to synaptic loss of relevance to AD. Although Graf1c provides a postsynaptic binding site for Pyk2 enrichment, we hypothesized that Pyk2 in turn regulates the RhoGAP activity of Graf1c to alter spine morphology. RhoA signaling is a well known modulator of F-actin and dendritic spine dynamics (Tashiro et al., 2000; Petratos et al., 2008; Huesa et al., 2010; Bolognin et al., 2014; Newell-Litwa et al., 2015) and has been implicated in AD (Petratos et al., 2008; Herskowitz et al., 2013; Yang et al., 2013; Chang et al., 2015; Henderson et al., 2016). Thus, Aβo signaling might enhance Pyk2-Graf1c interaction at the synapse, and this interaction might then impair the physiological Graf1c-RhoA regulation leading to a net local decrease in Graf1c-mediated inhibition of RhoA in the spine head, and driving net dendritic spine retraction.
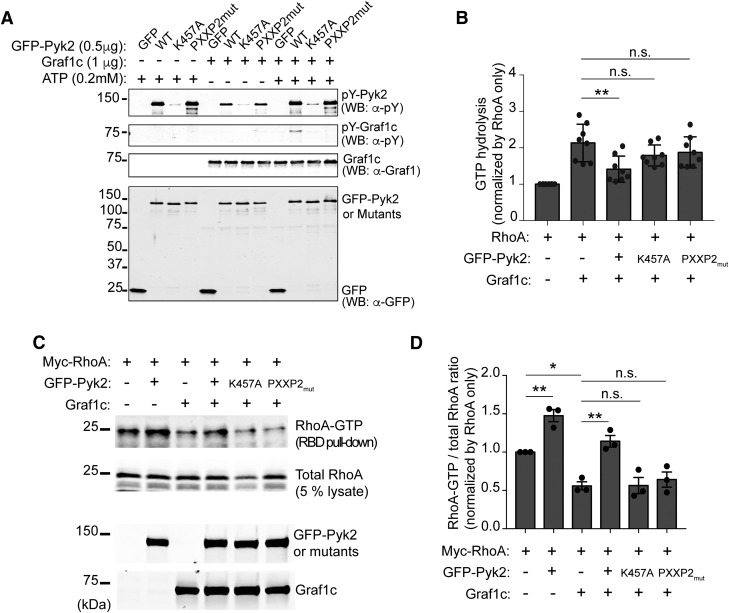
Given the kinase function of Pyk2, we first tested whether Pyk2 might phosphorylate Graf1c. We used an in vitro kinase assay with purified recombinant proteins (Fig. 6A). An antibody against all pY epitopes present detected phosphorylation of Graf1c in the presence of Pyk2 and ATP, and no phosphorylation in the absence of ATP. There was a basal level of Pyk2 phosphorylation in the absence of ATP which we attribute to basal autophosphorylation during overexpression in HEK cells. Additionally, kinase-dead Pyk2 (K457A) and the PRD mutant of Pyk2 (PXXP2mut) were unable to phosphorylate Graf1c, suggesting that both domains are necessary for the phosphorylation by Pyk2. To further test the ability of Pyk2 to modulate Graf1c function, we used an in vitro RhoGAP assay with Pyk2, Graf1c, and RhoA, and assessed GTP hydrolysis as a measure of Graf1c GAP activity. We observed a significant increase in GTP hydrolysis with RhoA and Graf1c alone, and a significant decrease with RhoA, Graf1c, and GFP-Pyk2 (Fig. 6B). Consistent with our hypothesis, Pyk2 mutants K457A and PXXP2mut were not able to significantly reduce GTP hydrolysis compared with RhoA and Graf1c alone. These data suggest that both kinase and PRD regions of Pyk2 are necessary to modulate Graf1c GAP activity. These in vitro assays tested the ability of Pyk2-Graf1c-RhoA to interact directly, but we also sought to test the ability of Pyk2 to modulate Graf1c in cells. We used a GST-RBD (Rhotektin RhoA-binding domain) pull-down assay to measure activated RhoA-GTP in Hek293T cells overexpressing Myc-RhoA, Graf1c, GFP-Pyk2, catalytically inactive Pyk2 (K457A), and Pyk2 PRD mutant (PXXP2mut; Fig. 6C,D). The observed ability of Graf1c overexpression alone to reduce RhoA-GTP activity below baseline is consistent with its known GAP activity for RhoA. Coexpression of GFP-Pyk2 together with Graf1c lead to an increase in active RhoA-GTP compared with Graf1c alone, consistent with Pyk2 inhibiting Graf1c-GAP function. The Pyk2-dependent increase of RhoA-GTP with Graf1c present does not occur with the Pyk2 mutants, K457A or PXXP2mut. Overexpression of GFP-Pyk2 alone significantly increased RhoA-GTP levels, and this is consistent with inhibition of the low level of endogenous Graf1c present in Hek293T cells (Fig. 6D). Overall, inhibition of Graf1c to increase RhoA-GTP requires both the kinase and PRD regions of Pyk2.
Figure 6.
Pyk2 induces RhoA activation through Graf1c interaction and phosphorylation. A, In vitro Pyk2 kinase assay with recombinant Graf1c as a substrate. Purified Graf1c from bacteria and GFP or GFP tagged Pyk2, K457A, and PXXP2mut from Hek293T cells were incubated in the absence or presence of ATP for 30 min at room temperature. Proteins were separated by SDS-PAGE and immunoblotted with indicated antibodies. B, In vitro RhoGAP assay with recombinant RhoA protein. The phosphate generated by the hydrolysis of GTP by RhoA GTPase is measured to determine the Graf1c GAP activity. The y-axis represents the relative ratio in indicated reactions compared with in RhoA only. Data are graphed as mean ± SEM (n = 8 experiments). **p < 0.01 by one-way ANOVA, Tukey's multiple-comparisons test. C, GST-RBD pull-down assay from Hek293T cell lysate from cells transfected with the indicated expression plasmids. The proteins retained by GST-RBD-immobilized beads and lysates were subjected to immunoblotting with anti-Myc, anti-Pyk2, anti-Graf1 antibodies. D, Levels of RhoA-GTP (RBD pull-down) and RhoA-total (5% lysate) were quantified by densitometric measurement. The y-axis represents the relative ratio in indicated cells compared with in Myc-RhoA only expressing cells. Data are graphed as mean ± SEM (n = 3 experiments). *p < 0.05, **p < 0.01 by one-way ANOVA, Tukey's multiple-comparisons test.
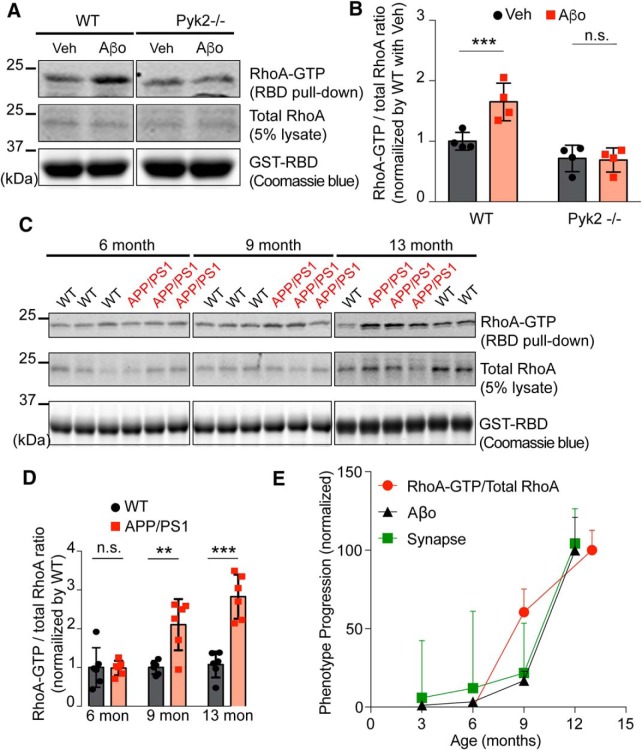
Aβo and AD transgene effects on RhoA activity are mediated by Pyk2
Based on these protein interactions, we hypothesized that Aβo drives Pyk2 recruitment to postsynaptic sites to impair local Graf1c regulation of RhoA and F-actin. We examined this possibility by performing the RBD pull down assay on acute brain slices treated with Aβo or vehicle from WT and Pyk2−/− mice (Fig. 7A,B). There was a significant Aβo-induced increase in activated RhoA in WT mice, and this increase was not observed in Pyk2−/− slices. To assess the in vivo AD relevance of this pathway, we examined the RBD pull-down from aged WT and APP/PS1 forebrain lysates. In the APP/PS1 strain, Aβo levels rise, synapses are lost, and memory is impaired progressively from 6 through 13 months of age (Fig. 5J–L; Jankowsky et al., 2004; Janus et al., 2015; Hong et al., 2016). Paralleling the temporal pattern of these phenotypes, there is a significant increase of RhoA-GTP levels in APP/PS1 mice at 9 and 13 months of age compared with WT mice or APP/PS1 mice at 6 months of age (Fig. 7C–E). These data show that RhoA activity increases with phenotype progression including synapse loss and Aβo-dependent synaptic dysfunction, in a Pyk2-dependent manner.
Figure 7.
Pyk2 is required for RhoA activation by Aβo in neurons and RhoA is activated in AD transgenic mice. A, GST-RBD pull-down assay in the Veh or Aβo-treated acute brain slice from WT or Pyk2−/− mice. The pull-down proteins and lysates were subjected to immunoblotting with anti-RhoA antibody. The bait proteins amount was examined by Coomassie blue staining from 50% of pull-down samples. B, Quantification of RhoA-GTP/total RhoA ratio by densitometric analysis. The ratio was normalized to Veh-treated WT. Data are graphed as mean ± SEM (n = 4 separate experiments). ***p < 0.001 by Student's two-tailed t test. C, GST-RBD pull-down assay of forebrain lysates from 6-, 9-, or 13-month-old WT and APP/PS1 mice. The pull-down proteins and lysates were subjected to immunoblotting with anti-RhoA antibody. The bait proteins amount was examined by Coomassie blue staining from 5% of pull-down samples. D, Quantification of RhoA-GTP/total RhoA ratio by densitometric analysis. The ratio was normalized to age-matched WT. Data are graphed as mean ± SEM (n = 6 mice for each condition). **p < 0.01, ***p < 0.001 by Student's two-tailed t test. E, Data were normalized with the lowest value = 0 and the highest value = to 100. Data are graphed as mean ± SEM. The Aβo level and synapse density loss data were derived from Figure 5J and L, respectively.
Pyk2-Graf1c interaction drives dendritic spine loss through RhoA activation
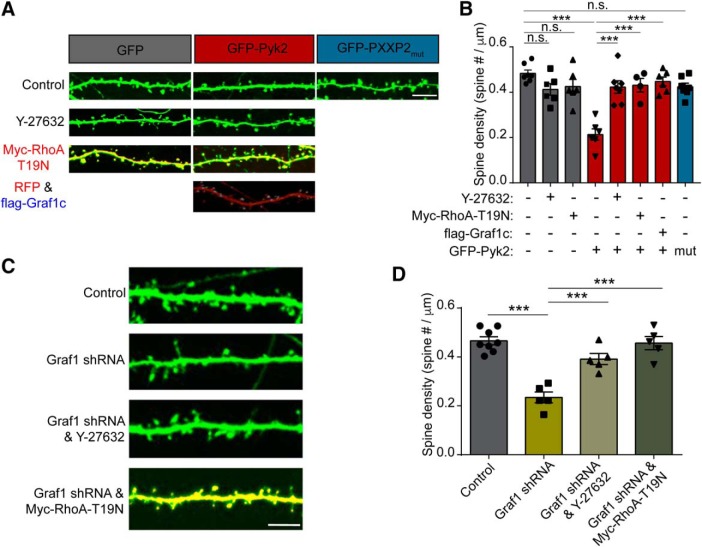
Next, we evaluated whether RhoA activity is necessary for Pyk2-dependent decrease in dendritic spine density. As described above, overexpression of GFP-Pyk2 induces loss of half of hippocampal neuron dendritic spines. Consistent with the Pyk2-Graf1c-RhoA interaction mediating this spine density reduction, the Pyk2 PRD mutant PXXP2mut, which fails to interact with Graf1c or regulate RhoA, does not display reduced spine density compared with GFP control (Fig. 8A,B). Treating neurons with the Rho/Rock pathway inhibitor Y-27632 (Bito et al., 2000), or coexpression of dominant-negative RhoA-T19N (Gebbink et al., 1997), each fully rescue GFP-Pyk2-induced spine loss. Overexpression of Graf1c counteracts the ability of Pyk2 to suppress dendritic spine density. Furthermore, knockdown of Graf1c expression induces a significant decrease of spine density, and both Y-27632 treatment and RhoA-T19N coexpression rescue Graf1c-knockdown-induced spine loss (Fig. 8C,D). Therefore, we conclude that, in response to Aβo, Pyk2 interacts with Graf1c within dendritic spines to allow increased RhoA-GTP levels and subsequent reduced synapse density.
Figure 8.
Pyk2 and Graf1c regulate RhoA activity and dendritic spine loss. A, Representative GFP fluorescence images of the cultured hippocampal neurons. Neurons were transfected with GFP, GFP and Myc-RhoA-T19N, GFP-Pyk2, GFP-Pyk2 and Myc-RhoA-T19N, GFP-Pyk2 and flag-Graf1c, or GFP-PXXP2mut then incubated in the presence or absence of 10 μm Y-27632 at 17 DIV and then fixed at 21 DIV. The Myc-RhoA-T19N and flag-Graf1c expressions were examined by immunostaining with anti-Myc and anti-flag antibodies. Scale bar, 10 μm. B, Quantification of dendritic spine density. Data are graphed as mean ± SEM (GFP: n = 7; GFP and Y-27632: n = 6; GFP and Myc-RhoA-T19N: n = 6; GFP-Pyk2: n = 9; GFP-Pyk2 and Y-27632: n = 7; GFP-Pyk2 and Myc-RhoA-T19N: n = 5; GFP-Pyk2 and flag-Graf1c: n = 6; GFP-PXXP2mut: n = 7 coverslips from 3 different cultures). ***p < 0.001 by ANOVA, Tukey's multiple-comparisons test. C, Representative fluorescence images of cultured hippocampal neurons. Neurons were transfected with U6 vector (Control), Graf1 shRNA, Graf1 shRNA, and 10 μm Y-27632, or Graf1 shRNA and Myc-RhoA-T19N at 14 DIV and then fixed at 21 DIV. Scale bar, 10 μm. D, Quantification of dendritic spine density in the transfected neurons. Data are graphed as mean ± SEM (Control: n = 8; Graf1 shRNA: n = 5; Graf1 shRNA and Y-27632: n = 5; Graf1 shRNA and Myc-RhoA-T19N: n = 5 coverslips from 3 different cultures). ***p < 0.001 by one-way ANOVA, Tukey's multiple-comparisons test.
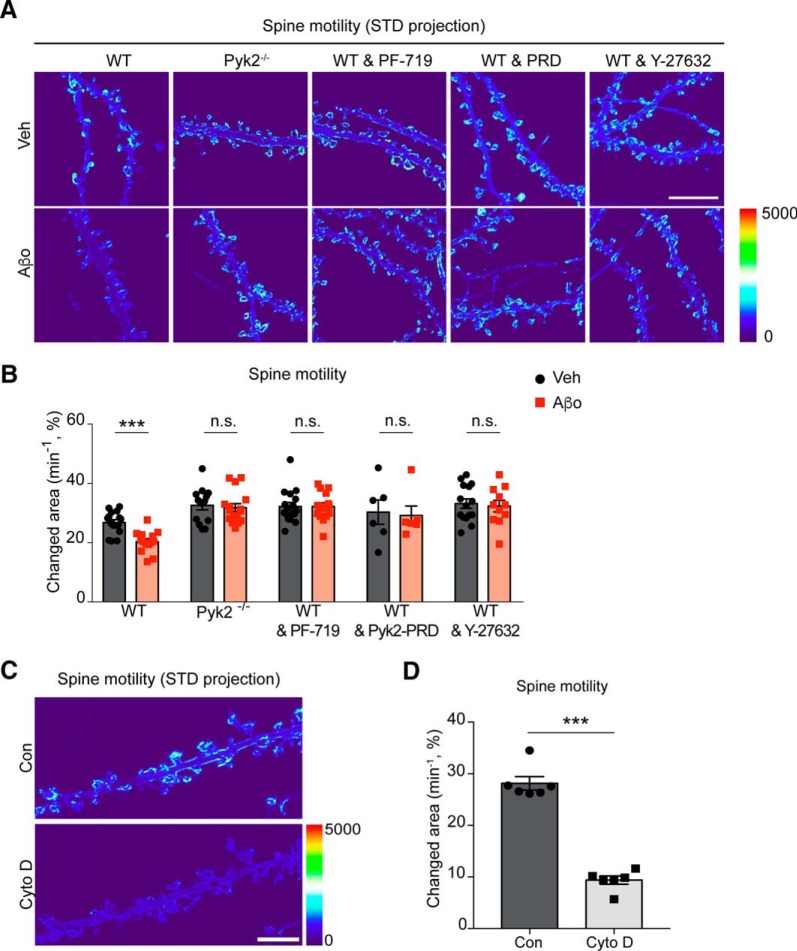
Pyk2 signaling mediates Aβo-dependent dendritic spine deficits in primary neurons
These effects of a postsynaptic Pyk2 pathway suggests that AD-mediated gain of Pyk2 function might underlie synapse loss by altering actin-based spine dynamics (Fischer et al., 1998; Tashiro et al., 2000). We monitored actin-dependent spine motility in primary hippocampal neurons treated with vehicle or Aβo for 24 h (Fig. 9A,B). We quantified motility as the time-dependent SD of change in dendritic spine profiles during 5 min with 0.1 Hz image captures. There is a significant decrease in spine motility from WT neurons treated with Aβo compared with WT vehicle treated neurons. To ensure that the spine motility we measured is actin-dependent, we treated neurons with cytochalasin D, an inhibitor of actin polymerization (Cooper, 1987), and observed near complete cessation of spine motility (Fig. 9C,D). Consistent with a gain-of-function hypothesis for Pyk2, genetically deleting Pyk2, inhibiting Pyk2 with PF-719, blocking the Pyk2-Graf1c interaction with Pyk2-PRD, or inhibiting Rho/Rock signaling with Y-27632 are each able to fully rescue the Aβo-induced decrease in spine motility (Fig. 9A,B). Thus, the Pyk2/Graf1c/RhoA pathway mediates an Aβo driven deficit in spine motility.
Figure 9.
Aβo-inhibition of F-actin-dependent dendritic motility is prevented by deletion of Pyk2 and RhoA inhibition in primary neurons. A, Representative images of dendritic spine motility by SD projection of time-lapse image. Myristoyl-GFP-expressing hippocampal neurons (21 DIV) from WT or Pyk2−/− mice were imaged for 5 min with 10 s intervals after Veh or Aβo (1 μm monomer, 10 nm oligomer estimate) pre-incubation with or without 1 μm PF-719, RFP-Pyk2-PRD (PRD), or 10 μm Y-27632 for 24 h. The SD value for each pixel is shown by thermal color scale. Scale bar, 10 μm. B, Quantification of spine motility, expressed as percentage of changed area of spines with subtraction of changed area of dendritic shaft as background [changed area = (ΔAspine/Aspine × 100) − (ΔAshaft/Ashaft × 100)]. Data are graphed as mean ± SEM (Veh in WT: n = 16; Aβo in WT: n = 14; Veh in Pyk2−/−: n = 14; Aβo in Pyk2−/−: n = 16; Veh in WT and PF-719: n = 17; Aβo in WT and PF-719: n = 16; Veh in WT and Pyk2-PRD: n = 6; Aβo in WT and Pyk2-PRD: n = 6; Veh in WT and Y-27632: n = 15; Aβo in WT and Y-27632: n = 11 neurons from 3 different cultures). ***p < 0.001 by Student's two-tailed t test. C, Representative images of dendritic spine motility by SD projection of time stacks. Hippocampal neurons (21 DIV) expressing myristoyl-GFP were imaged for 5 min with 10 s intervals as a control and then incubated with 1 μm cytochalasin D for 20 min, imaged again with same acquisition protocol as control image at the same neuron. To display the spine motility in time-lapse images, the time stack images over 5 min of each conditions were projected to SD using ImageJ and color-coated with thermal color scale. Scale bar, 10 μm. D, Quantification of spine motility, expressed as percentage of changed area of spines with subtraction of dendritic shaft area change [changed area = (ΔAspine/Aspine × 100) − (ΔAshaft/Ashaft × 100)]. Mean ± SEM (n = 5 coverslips).
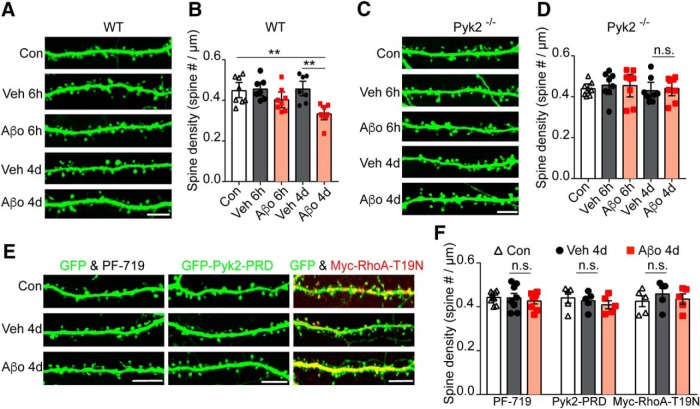
We considered whether the acute effects of Aβo-induced, Pyk2-mediated decrease on spine motility might be translated into reduced spine number over longer time periods. We measured dendritic spine density in populations of vehicle and Aβo-treated primary hippocampal neurons. Previously, we demonstrated that tracking single spines over 6 h detects a loss of ∼6–8% of spines in the presence of Aβo, but not vehicle (Um et al., 2012, 2013). At 6 h this loss is not significant by endpoint population analysis of dendritic spine density (Fig. 10A,B). Nonetheless, after 4 d, there is significant net reduction of spine density by 25% in the Aβo-treated WT cultures (Fig. 10A,B). In contrast, Pyk2−/− neurons treated with Aβo display no dendritic spine loss at 6 h or 4 d compared with vehicle-treated controls (Fig. 10C,D). Consistent with the Pyk2 gain-of-function hypothesis, small molecule inhibition of Pyk2 with PF-719, blocking the Pyk2-Graf1c interaction with Pyk2-PRD, or inhibiting RhoA activity with dominant-negative RhoA-T19N is each able to fully rescue Aβo-dependent spine loss (Fig. 10E,F). Furthermore, PF-719, Pyk2-PRD, or RhoA-T19N alone had no effect on spine density. These data suggest that Pyk2 mediates Aβo-dependent dendritic spine deficits in primary neurons.
Figure 10.
Aβo-induced dendritic spine loss are prevented by deletion of Pyk2 or RhoA inhibition. A, C, Representative images of 21 DIV GFP transfected hippocampal neurons (21 DIV) from WT mice (A) or Pyk2−/− mice (C) after non-treated (Con), Veh, or Aβo treatment for 6 h or 4 d. B, D, Quantification of dendritic spine density for conditions shown in A and C. Data are graphed as mean ± SEM (n = 7–8 coverslips from 3 different cultures). **p < 0.01 by ANOVA, Tukey's multiple-comparisons test. E, F, GFP, GFP-Pyk2-PRD, or GFP and Myc-RhoA-T19N transfected neurons were incubated with Veh or Aβo for 4 d and then fixed and stained with anti-Myc antibodies at 21 DIV. Mean ± SEM (n = 5–8 coverslips from 3 different cultures). One-way ANOVA, Tukey's multiple-comparisons test.
Discussion
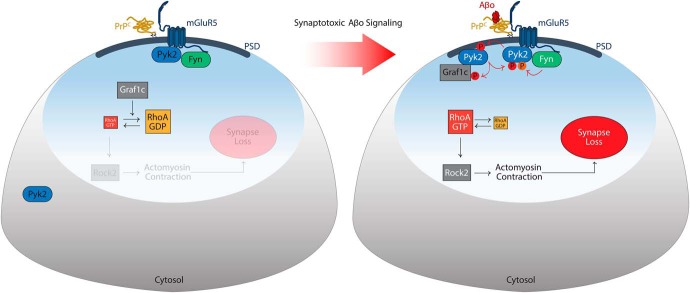
The present study demonstrates that activation of the AD risk gene product Pyk2 functions locally in neurons to mediate synapse loss. The RhoA GAP protein, Graf1c, is a physical interactor and substrate of Pyk2, and RhoA activation with altered actin dynamics mediates Pyk2-induced net retraction of dendritic spines. Aβ oligomeric species stimulate Pyk2 to translocate to the PSD, to engage this Graf1c/RhoA pathway and to reduce the density of synaptic structures. In AD, the Pyk2 protein is activated by an Aβo/PrPC/mGluR5/Fyn complex to reduce dendritic spine density (Fig. 11). Thus, inhibition of Pyk2 kinase provides a potential target for disease-modifying AD intervention.
Figure 11.
Model for Pyk2 action in AD risk. The proposed mechanism for Pyk2 in Aβo-induced AD signaling at the PSD of dendritic spines is illustrated. In the healthy basal state at left, Pyk2 and Fyn activation is limited (black lettering) and Graf1c actively (white lettering) suppresses RhoA signaling to the actin cytoskeleton to maintain synaptic morphology. In AD (right), Aβo binds to PrPC to engage mGluR5 and activate Fyn kinase (white lettering). Fyn phosphorylates Pyk2 kinase (white lettering, orange P) this leads to Pyk2 activation (white lettering), autophosphorylation (red P) and Pyk2 recruitment to the PSD. Pyk2 physically interacts with Graf1c at the PSD, phosphorylates Graf1c (red P) and inactivates Graf1c GAP activity (black lettering). Loss of Graf1c function allows increased active RhoA GTPase and subsequent ROCK2-dependent actin contractility, to retract dendritic spines and cause synapse loss. Human genetic variants at the Pyk2 locus, which have been shown to increase Pyk2 mRNA expression, enhance this pathway and thereby increase AD risk.
Our analysis of brain proteins interacting with Pyk2 identified Graf1c, consistent with a previous report (Ohba et al., 1998). Here, we show that the two proteins colocalize at postsynaptic sites. Furthermore, their interaction is functionally bidirectional in the sense that Graf1c increases synaptic localization of Pyk2, whereas Pyk2 titrates Graf1c activity as a RhoA GAP. Interaction of the two proteins requires the Pyk2 PRD, and Pyk2 is able to directly phosphorylate Graf1c. Additionally, Pyk2 kinase activity is essential for Pyk2 suppression of the GAP activity of Graf1c for RhoA. Graf1c recruits Pyk2 to postsynaptic sites but the basis of Graf1c localization to those sites will require further study. Fyn kinase functions synergistically with Pyk2 and also localizes at postsynaptic sites (Um et al., 2012, 2013; Kaufman et al., 2015; Haas et al., 2016; Li and Götz, 2018). These data place Pyk2 at the center of postsynaptic signaling complex that controls dendritic spine morphology.
Graf1c links Pyk2 function to RhoA GTPase activity. The GTP-bound active RhoA is known to inhibit axonal and dendritic growth, to cause dendritic spine retraction and to titrate synaptic plasticity (Jin and Strittmatter, 1997; Tashiro et al., 2000; Rex et al., 2009; Murakoshi et al., 2011). The presence of kinase active Pyk2 but not inactive Pyk2 limits the activity of Graf1c to terminate RhoA activity by stimulating the GTPase. Consistent with a central role for Pyk2 in synaptic AD related signaling, Aβo and AD transgenes also increase RhoA activation. The ability of Pyk2 overexpression and Aβo stimulation to activate RhoA are consistent with Pyk2/Graf1c complex acting via this mechanism. Neuronal stimulation has been shown to increase active RhoA association with class I mGluRs (Schubert et al., 2006), which are upstream of Fyn/Pyk2 in the Aβo/PrPC signaling cascade (Um et al., 2013; Haas et al., 2016). Others have observed RhoA activation in AD or AD model systems (Petratos et al., 2008; Huesa et al., 2010; Chacon et al., 2011; Herskowitz et al., 2013; Pozueta et al., 2013; Yang et al., 2013; Bolognin et al., 2014; Chang et al., 2015; Henderson et al., 2016), consistent with this mechanism.
Pyk2 is a tyrosine kinase capable of auto-phosphorylation and Graf1c phosphorylation. Although Graf1c interaction occurs via the isolated PRD domain of Pyk2, the regulation of Graf1c and RhoA-GTP requires Pyk2 kinase activity. It is likely that both Pyk2 auto-phosphorylation and activation by Fyn contribute to Pyk2 regulation of Graf1c. Although our data show that Graf1c and RhoA play an essential role, additional kinase substrates of Pyk2 may contribute to the ability of Pyk2 and Aβo to induce loss of dendritic spines.
We have previously observed Pyk2 activation in the transgenic APPswe/PS1ΔE9 Alzheimer model, in parallel with Aβ accumulation, synapse loss, and memory impairment (Kaufman et al., 2015; Haas et al., 2016, 2017). In such studies, genetic or pharmacological targeting of the PrPC/mGluR5/Fyn pathway normalized Pyk2 activation, suggesting that Pyk2 is downstream of mGluR5 and Fyn activation by Aβ oligomers. Indeed, Pyk2 physically associates with mGluR5 complexes in brain tissue (Haas et al., 2016). Recently, we demonstrated that Pyk2 is required for memory impairment and synapse density loss in APPswe/PS1ΔE9 mice (Salazar et al., 2018). Here, we delineate a downstream mechanism for the biochemical and dendritic morphology changes requiring Pyk2 function in neurons. Without Pyk2, cultured neurons are protected from Aβo-induced reduction in dendritic spine motility and dendritic spine loss. This suggests that increased Pyk2 function enhances AD risk and is consistent with the observation that the AD risk SNP exhibits elevated Pyk2 expression in blood (Chan et al., 2015). Very recently, decreased pY402-Pyk2 was reported in 5XFAD transgenic mice, and viral Pyk2 overexpression reduced behavioral deficits in that strain (Giralt et al., 2018), opposite to the previous APP/PS1 studies and the prediction from the cellular studies here. The basis for these disparate findings is not clear, but the onset of 5XFAD phenotypes at juvenile or early adult stages, as opposed to late adult onset for APPswe/PS1ΔE9 mice may be relevant. Obviously, onset and progression of phenotypes during aging is more consistent with the human AD condition. Separately from AD models, Pyk2-null synaptic phenotypes reported for young mice (Giralt et al., 2017) resolve with age (Salazar et al., 2018). In this setting, the APP/PS1 may test Pyk2 effects after any development phenotypes have resolved. In addition, it should be noted that both of these transgenic lines overexpress familial AD mutant proteins, such that future testing of Pyk2 genotype effects in APP mutant knock-in mice (Saito et al., 2014) may be informative.
The role of Pyk2 in AD is likely to include an influence of Tau pathology, as well as Aβ-triggered neuronal dysfunction, based on phenotypic analysis in Drosophila (Dourlen et al., 2017) and biochemical interaction studies (Li and Götz, 2018). Furthermore, Pyk2 and Tau both interact with Fyn, and Pyk2 activates the Tau kinase Gsk3β (Dikic et al., 1996; Hartigan et al., 2001; Sayas et al., 2006; Ittner et al., 2010; Usardi et al., 2011). Thus, Pyk2 may be juxtaposed to link and modulate the two major pathologies of AD through both microtubule and actin cytoskeletal elements. The biochemical analysis here demonstrates that Pyk2 activity couples to a postsynaptic signaling pathway for synapse loss via RhoA and F-actin that is central to brain dysfunction in AD.
Footnotes
This work was supported by Grants from the Falk Medical Research Trust to S.M.S., and from the NIH to S.V.S. and S.M.S. We thank Stefano Sodi for expert technical assistance.
The authors declare no competing financial interests.
References
- Alzheimer's Association (2012) 2012 Alzheimer's disease facts and figures. Alzheimers Dement 8:131–168. 10.1016/j.jalz.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J (2001) Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem 276:20130–20135. 10.1074/jbc.M102307200 [DOI] [PubMed] [Google Scholar]
- Bartos JA, Ulrich JD, Li H, Beazely MA, Chen Y, Macdonald JF, Hell JW (2010) Postsynaptic clustering and activation of Pyk2 by PSD-95. J Neurosci 30:449–463. 10.1523/JNEUROSCI.4992-08.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S (2000) A critical role for a rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 26:431–441. 10.1016/S0896-6273(00)81175-7 [DOI] [PubMed] [Google Scholar]
- Bolognin S, Lorenzetto E, Diana G, Buffelli M (2014) The potential role of rho GTPases in Alzheimer's disease pathogenesis. Mol Neurobiol 50:406–422. 10.1007/s12035-014-8637-5 [DOI] [PubMed] [Google Scholar]
- Chacon PJ, Garcia-Mejias R, Rodriguez-Tebar A (2011) Inhibition of RhoA GTPase and the subsequent activation of PTP1B protects cultured hippocampal neurons against amyloid beta toxicity. Mol Neurodegener 6:14. 10.1186/1750-1326-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, White CC, Winn PA, Cimpean M, Replogle JM, Glick LR, Cuerdon NE, Ryan KJ, Johnson KA, Schneider JA, Bennett DA, Chibnik LB, Sperling RA, Bradshaw EM, De Jager PL (2015) CD33 modulates TREM2: convergence of Alzheimer loci. Nat Neurosci 18:1556–1558. 10.1038/nn.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RY, Etheridge N, Nouwens AS, Dodd PR (2015) SWATH analysis of the synaptic proteome in Alzheimer's disease. Neurochem Int 87:1–12. 10.1016/j.neuint.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Chang S, De Camilli P (2001) Glutamate regulates actin-based motility in axonal filopodia. Nat Neurosci 4:787–793. 10.1038/90489 [DOI] [PubMed] [Google Scholar]
- Collins M, Bartelt RR, Houtman JC (2010a) T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Mol Immunol 47:1665–1674. 10.1016/j.molimm.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JC (2010b) The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. J Leukocyte Biol 87:691–701. 10.1189/jlb.0409227 [DOI] [PubMed] [Google Scholar]
- Cooper JA. (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105:1473–1478. 10.1083/jcb.105.4.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J (1996) A role for Pyk2 and src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383:547–550. 10.1038/383547a0 [DOI] [PubMed] [Google Scholar]
- Doherty GJ, Åhlund MK, Howes MT, Morén B, Parton RG, McMahon HT, Lundmark R (2011) The endocytic protein GRAF1 is directed to cell-matrix adhesion sites and regulates cell spreading. Mol Biol Cell 22:4380–4389. 10.1091/mbc.e10-12-0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourlen P, Fernandez-Gomez FJ, Dupont C, Grenier-Boley B, Bellenguez C, Obriot H, Caillierez R, Sottejeau Y, Chapuis J, Bretteville A, Abdelfettah F, Delay C, Malmanche N, Soininen H, Hiltunen M, Galas MC, Amouyel P, Sergeant N, Buée L, Lambert JC, et al. (2017) Functional screening of Alzheimer risk loci identifies PTK2B as an in vivo modulator and early marker of tau pathology. Mol Psychiatry 22:874–883. 10.1038/mp.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A (1998) Rapid actin-based plasticity in dendritic spines. Neuron 20:847–854. 10.1016/S0896-6273(00)80467-5 [DOI] [PubMed] [Google Scholar]
- Gebbink MF, Kranenburg O, Poland M, van Horck FP, Houssa B, Moolenaar WH (1997) Identification of a novel, putative rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol 137:1603–1613. 10.1083/jcb.137.7.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, Strittmatter SM (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci 30:6367–6374. 10.1523/JNEUROSCI.0395-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Brito V, Chevy Q, Simonnet C, Otsu Y, Cifuentes-Díaz C, de Pins B, Coura R, Alberch J, Ginés S, Poncer JC, Girault JA (2017) Pyk2 modulates hippocampal excitatory synapses and contributes to cognitive deficits in a Huntington's disease model. Nat Commun 8:15592. 10.1038/ncomms15592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, de Pins B, Cifuentes-Díaz C, López-Molina L, Farah AT, Tible M, Deramecourt V, Arold ST, Ginés S, Hugon J, Girault JA (2018) PTK2B/Pyk2 overexpression improves a mouse model of Alzheimer's disease. Exp Neurol 307:62–73. 10.1016/j.expneurol.2018.05.020 [DOI] [PubMed] [Google Scholar]
- Haas LT, Strittmatter SM (2016) Oligomers of amyloid β prevent physiological activation of the cellular prion protein-metabotropic glutamate receptor 5 complex by glutamate in Alzheimer disease. J Biol Chem 291:17112–17121. 10.1074/jbc.M116.720664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC, Strittmatter SM (2016) Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain 139:526–546. 10.1093/brain/awv356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas LT, Salazar SV, Smith LM, Zhao HR, Cox TO, Herber CS, Degnan AP, Balakrishnan A, Macor JE, Albright CF, Strittmatter SM (2017) Silent allosteric modulation of mGluR5 maintains glutamate signaling while rescuing Alzheimer's mouse phenotypes. Cell Rep 20:76–88. 10.1016/j.celrep.2017.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan JA, Xiong WC, Johnson GV (2001) Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem Biophys Res Commun 284:485–489. 10.1006/bbrc.2001.4986 [DOI] [PubMed] [Google Scholar]
- Heiss JK, Barrett J, Yu Z, Haas LT, Kostylev MA, Strittmatter SM (2017) Early activation of experience-independent dendritic spine turnover in a mouse model of Alzheimer's disease. Cereb Cortex 27:3660–3674. 10.1093/cercor/bhw188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BW, Gentry EG, Rush T, Troncoso JC, Thambisetty M, Montine TJ, Herskowitz JH (2016) Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer's disease and ROCK1 depletion reduces amyloid-beta levels in brain. J Neurochem 138:525–531. 10.1111/jnc.13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz JH, Feng Y, Mattheyses AL, Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC, Thambisetty M, Seyfried NT, Levey AI, Lah JJ (2013) Pharmacologic inhibition of ROCK2 suppresses amyloid-β production in an Alzheimer's disease mouse model. J Neurosci 33:19086–19098. 10.1523/JNEUROSCI.2508-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Taylor JM, Parsons JT (1996) An SH3 domain-containing GTPase-activating protein for rho and Cdc42 associates with focal adhesion kinase. Mol Cell Biol 16:3169–3178. 10.1128/MCB.16.6.3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712–716. 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kim MJ, Wang CF, Sheng M (2010) Proline-rich tyrosine kinase 2 regulates hippocampal long-term depression. J Neurosci 30:11983–11993. 10.1523/JNEUROSCI.1029-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF (2001) CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29:485–496. 10.1016/S0896-6273(01)00220-3 [DOI] [PubMed] [Google Scholar]
- Huesa G, Baltrons MA, Gómez-Ramos P, Morán A, García A, Hidalgo J, Francés S, Santpere G, Ferrer I, Galea E (2010) Altered distribution of RhoA in Alzheimer's disease and AβPP overexpressing mice. J Alzheimers Dis 19:37–56. 10.3233/JAD-2010-1203 [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J (2010) Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell 142:387–397. 10.1016/j.cell.2010.06.036 [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR (2003) Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol 62:1220–1227. 10.1093/jnen/62.12.1220 [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR (2004) Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13:159–170. 10.1093/hmg/ddh019 [DOI] [PubMed] [Google Scholar]
- Janus C, Flores AY, Xu G, Borchelt DR (2015) Behavioral abnormalities in APPSwe/PS1dE9 mouse model of AD-like pathology: comparative analysis across multiple behavioral domains. Neurobiol Aging 36:2519–2532. 10.1016/j.neurobiolaging.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM (1997) Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci 17:6256–6263. 10.1523/JNEUROSCI.17-16-06256.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, Robinson SA, Gunther EC, van Dyck CH, Nygaard HB, Strittmatter SM (2015) Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol 77:953–971. 10.1002/ana.24394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, Vortmeyer A, Strittmatter SM (2015) Prion-protein-interacting amyloid-β oligomers of high molecular weight are tightly correlated with memory impairment in multiple Alzheimer mouse models. J Biol Chem 290:17415–17438. 10.1074/jbc.M115.643577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostylev MA, Tuttle MD, Lee S, Klein LE, Takahashi H, Cox TO, Gunther EC, Zilm KW, Strittmatter SM (2018) Liquid and hydrogel phases of PrPC linked to conformation shifts and triggered by Alzheimer's amyloid-β oligomers. Mol Cell 72:426–443.e412. 10.1016/j.molcel.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, et al. (2013) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 45:1452–1458. 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457:1128–1132. 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Götz J (2018) Pyk2 is a novel tau tyrosine kinase that is regulated by the tyrosine kinase Fyn. J Alzheimers Dis 64:205–221. 10.3233/JAD-180054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucken-Ardjomande Häsler S, Vallis Y, Jolin HE, McKenzie AN, McMahon HT (2014) GRAF1a is a brain-specific protein that promotes lipid droplet clustering and growth, and is enriched at lipid droplet junctions. J Cell Sci 127:4602–4619. 10.1242/jcs.147694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, McMahon HT (2008) The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol 18:1802–1808. 10.1016/j.cub.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon A, Burgaya F, Baudot P, Dunlap DD, Girault JA, Valtorta F (1999) FAK+ and PYK2/CAKbeta, two related tyrosine kinases highly expressed in the central nervous system: similarities and differences in the expression pattern. Eur J Neurosci 11:3777–3788. 10.1046/j.1460-9568.1999.00798.x [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56–68. 10.1038/nrm1549 [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R (2011) Local, persistent activation of rho GTPases during plasticity of single dendritic spines. Nature 472:100–104. 10.1038/nature09823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR (2015) ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J Cell Biol 210:225–242. 10.1083/jcb.201504046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Ishino M, Aoto H, Sasaki T (1998) Interaction of two proline-rich sequences of cell adhesion kinase beta with SH3 domains of p130Cas-related proteins and a GTPase-activating protein, Graf. Biochem J 330:1249–1254. 10.1042/bj3301249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J (2003) Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci U S A 100:10740–10745. 10.1073/pnas.1834348100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME (2004) White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage 23:213–223. 10.1016/j.neuroimage.2004.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Avraham HK, Avraham S (2004) RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a src-independent manner. J Biol Chem 279:33315–33322. 10.1074/jbc.M313527200 [DOI] [PubMed] [Google Scholar]
- Petratos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH (2008) The β-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a rho-GTP mechanism. Brain 131:90–108. 10.1093/brain/awm260 [DOI] [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Ribe EM, Troy CM, Arancio O, Shelanski M (2013) Caspase-2 is required for dendritic spine and behavioural alterations in J20 APP transgenic mice. Nat Commun 4:1939. 10.1038/ncomms2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro SA, Nicoll AJ, Collinge J (2018) Prion protein as a toxic acceptor of amyloid-β oligomers. Biol Psychiatry 83:358–368. 10.1016/j.biopsych.2017.11.020 [DOI] [PubMed] [Google Scholar]
- Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A (1997) Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med 185:1253–1259. 10.1084/jem.185.7.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G (2009) Different rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol 186:85–97. 10.1083/jcb.200901084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC (2014) Single app knock-in mouse models of Alzheimer's disease. Nat Neurosci 17:661–663. 10.1038/nn.3697 [DOI] [PubMed] [Google Scholar]
- Salazar SV, Strittmatter SM (2017) Cellular prion protein as a receptor for amyloid-β oligomers in Alzheimer's disease. Biochem Biophys Res Commun 483:1143–1147. 10.1016/j.bbrc.2016.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar SV, Cox TO, Lee S, Brody AH, Chyung AS, Haas LT, Strittmatter SM (2018) Alzheimer's disease risk factor Pyk2 mediates amyloid-β-induced synaptic dysfunction and loss. J Neurosci. Advance online publication. Retrieved December 5, 2018. doi: 10.1523/JNEUROSCI.1873-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH (2006) GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol Biol Cell 17:1834–1844. 10.1091/mbc.e05-07-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V, Da Silva JS, Dotti CG (2006) Localized recruitment and activation of RhoA underlies dendritic spine morphology in a glutamate receptor-dependent manner. J Cell Biol 172:453–467. 10.1083/jcb.200506136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger SA, Yao X, Gross C, Bassell GJ (2011) Automated 4D analysis of dendritic spine morphology: applications to stimulus-induced spine remodeling and pharmacological rescue in a disease model. Mol Brain 4:38. 10.1186/1756-6606-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R (2000) Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10:927–938. 10.1093/cercor/10.10.927 [DOI] [PubMed] [Google Scholar]
- Taylor JM, Hildebrand JD, Mack CP, Cox ME, Parsons JT (1998) Characterization of Graf, the GTPase-activating protein for rho associated with focal adhesion kinase: phosphorylation and possible regulation by mitogen-activated protein kinase. J Biol Chem 273:8063–8070. 10.1074/jbc.273.14.8063 [DOI] [PubMed] [Google Scholar]
- Taylor JM, Macklem MM, Parsons JT (1999) Cytoskeletal changes induced by GRAF, the GTPase regulator associated with focal adhesion kinase, are mediated by Rho. J Cell Sci 112:231–242. [DOI] [PubMed] [Google Scholar]
- Tse KW, Lin KB, Dang-Lawson M, Guzman-Perez A, Aspnes GE, Buckbinder L, Gold MR (2012) Small molecule inhibitors of the Pyk2 and FAK kinases modulate chemoattractant-induced migration, adhesion and Akt activation in follicular and marginal zone B cells. Cell Immunol 275:47–54. 10.1016/j.cellimm.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM (2012) Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci 15:1227–1235. 10.1038/nn.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, Gunther EC, Nygaard HB, Strittmatter SM (2013) Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron 79:887–902. 10.1016/j.neuron.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usardi A, Pooler AM, Seereeram A, Reynolds CH, Derkinderen P, Anderton B, Hanger DP, Noble W, Williamson R (2011) Tyrosine phosphorylation of tau regulates its interactions with Fyn SH2 domains, but not SH3 domains, altering the cellular localization of tau. FEBS J 278:2927–2937. 10.1111/j.1742-4658.2011.08218.x [DOI] [PubMed] [Google Scholar]
- Yang H, Wittnam JL, Zubarev RA, Bayer TA (2013) Shotgun brain proteomics reveals early molecular signature in presymptomatic mouse model of Alzheimer's disease. J Alzheimers Dis 37:297–308. 10.3233/JAD-130476 [DOI] [PubMed] [Google Scholar]
- Zhang C, Lambert MP, Bunch C, Barber K, Wade WS, Krafft GA, Klein WL (1994) Focal adhesion kinase expressed by nerve cell lines shows increased tyrosine phosphorylation in response to Alzheimer's Aβ peptide. J Biol Chem 269:25247–25250. [PubMed] [Google Scholar]