Abstract
Purpose
To report an accelerated course of visual recovery in a case of Leber's hereditary optic neuropathy (LHON) following treatment with idebenone and hormone replacement therapy (HRT). We hereby demonstrate the clinical utility of estrogen's protective role in LHON in vivo.
Methods
We present a case of LHON in a menopausal woman carrying the 10197 mitochondrial DNA (mtDNA) mutation, who experienced loss of vision shortly after discontinuing her estrogen replacement regimen. Functional visual outcomes are reported following treatment with idebenone and HRT.
Results
The patient exhibited an accelerated course of visual recovery, experiencing improvement in vision as early as one month and complete reversal of vision loss by eight months post-therapy.
Conclusion
Idebenone treatment combined with HRT may have a synergistic effect in enhancing cellular bioenergetics and may explain the patient's accelerated visual improvement.
Keywords: Leber's hereditary optic neuropathy, Mitochondrial disease, Hormone replacement therapy, Accelerated visual improvement
Introduction
Leber's hereditary optic neuropathy (LHON), a maternally inherited disease characterized by bilateral central vision loss, remains without cure.[1], [2], [3], [4] Recent in vitro studies have highlighted estrogen's role in preserving vision by increasing mitochondrial biogenesis.[5], [6] However, estrogen's protective role in vivo has yet to be reported.
We report a case of LHON in a menopausal woman, who experienced accelerated visual recovery following treatment with idebenone and hormone replacement therapy (HRT).
Case report
A 48-year-old woman presented with a four-month history of painless, progressive loss of vision beginning with the left eye followed by subsequent involvement of the right eye two months later. The patient was previously treated with intravenous (IV) corticosteroids for presumed optic neuritis, however, her vision worsened. Best corrected visual acuities (BCVA) were 20/60 OD and 20/200 OS. Intraocular pressures were 13 OU. Color vision on Ishihara plate testing was 1/14 OD and 0/14 OS. Humphrey visual field (HVF) testing showed bilateral cecocentral scotomas (Fig. 1); dilated funduscopy revealed temporal pallor OU. Neurological examination of motor and sensory function, muscle bulk, tone, and gait were intact. Magnetic resonance imaging (MRI) showed a normal optic nerve appearance bilaterally without enhancement or periventricular white matter lesions. Lumbar puncture cerebrospinal fluid analysis, autoimmune panel, immunoglobulins, and complement levels were all negative. The patient's mother had bilateral vision loss at 58 years of age. Of note, the patient consumed more than a half-bottle of wine daily. She was previously on contraceptive hormonal therapy including etonogestrel 0.12 mg and ethinyl estradiol 0.015 mg daily. Her visual symptoms began three months after discontinuing the hormonal regimen. The patient's presentation was concerning for mitochondrial optic neuropathy. She began idebenone 300 mg TID with vitamin C, resumed her original hormonal regimen of etonogestrel 0.12 mg and ethinyl estradiol 0.015 mg daily and minimized alcohol consumption. Genetic testing of the patient and her mother for OPA1 and LHON mitochondrial DNA (mtDNA) mutations (11778G > A, 14484T > C and 3460G > A) was negative. Intriguingly, the patient's vision markedly improved post-therapy, having BCVAs of 20/40 OD and 20/60 OS by one month; 20/25 OU by two months; 20/20 OD and 20/25 OS by eight months. Color vision significantly improved on Ishihara plate testing, scoring 2/14 OD and 1/14 OS by one month; 6/14 OD and 2/14 OS by six months. 30-2 HVF also markedly improved OU by two months (Fig. 2) and continued to eight months (Fig. 3). Comprehensive mitochondrial genome sequencing genetically confirmed LHON, revealing a 10197 mtDNA mutation.
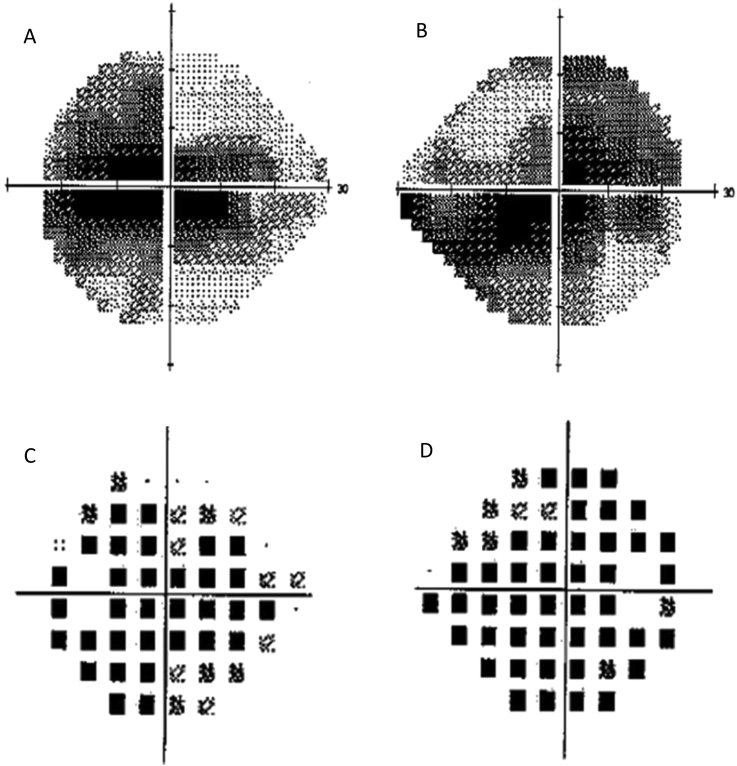
Fig. 1.
(A, B) Humphrey visual field (HVF) 24-2 maps of the visual field examination on initial presentation, revealing significant cecocentral scotomas in the left and right eye, respectively. (C, D) Depict total deviation of the visual field examination on initial presentation in the left and right eye, respectively. Mean deviation: –15.66 dB OS, –18.25 dB OD.
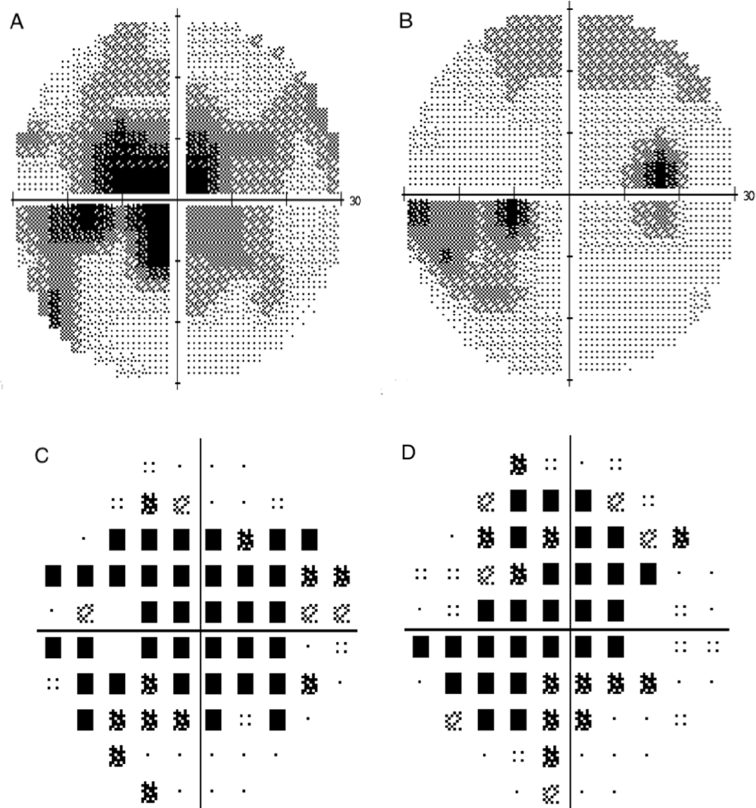
Fig. 2.
(A, B) Humphrey visual field (HVF) 30-2 maps of the visual field examination at two months, revealing mild decrease in cecocentral scotomas in the left and right eye, respectively. (C, D) depict total deviation of the visual field examination on initial presentation in the left and right eye, respectively. Mean deviation: −8.11 dB OD, −13.14 dB OS.
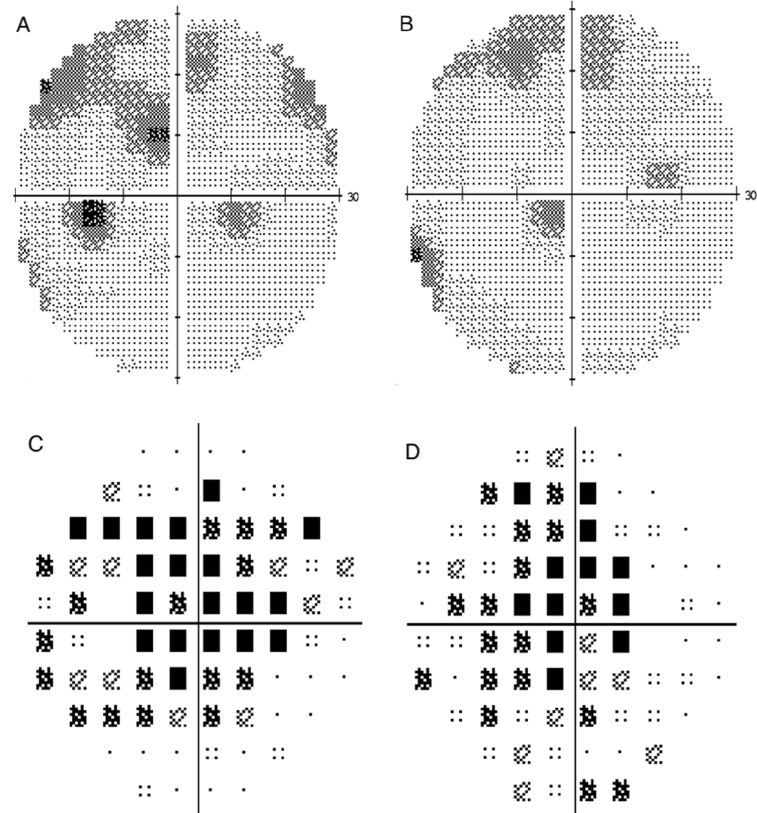
Fig. 3.
(A, B) Humphrey visual field (HVF) 30-2 maps of the visual field examination at eight months, revealing further decrease of the scotomas in the left and right eye, respectively. (C, D) depict less total deviation of the visual field examination on second visit in the left and right eye, respectively. Mean deviation: −4.56 dB OD, −6.61 dB OS.
Discussion
We present a case of LHON in a menopausal woman whose vision loss began shortly after discontinuing estrogen replacement. While causality cannot be definitively proven, the patient's presentation in association with estrogen deficiency and the absence of alternative triggers is highly suggestive of estrogen's protective role in LHON. Also, LHON 10197, characterized by an ND3 subunit defect in mitochondrial complex I, has been solely identified in patients with Leigh syndrome, Leigh-like syndrome, and LHON and dystonia.[7], [8] Our case provides the first reporting of this mutation with isolated LHON, as the patient had no accompanying neurological deficits.
Idebenone can absorb reactive oxygen species and serve as an electron carrier in the mitochondrial respiratory chain. Visual improvement typically does not occur until two or more years post-therapy, and rarely occurs by one year.[9], [10] Our patient's vision improved shortly after one month with complete visual recovery by eight months. Therefore additional mechanisms must be considered. Recent studies have highlighted the important role of estrogen in preserving vision in LHON. This may explain not only the reduced prevalence of disease in women relative to men overall, but also the increased incidence of disease associated with a decline in estrogen as frequently seen in menopausal patients.[5], [6] In vitro studies have elucidated estrogen's mechanism of action. Through activation of the estrogen β receptor, which localizes to the mitochondrial network of retinal ganglion cells, estrogen upregulates antioxidant enzyme production of superoxide dismutase 2, increases mitochondrial biogenesis, and enhances cellular energy competence.[5], [6] Idebenone treatment combined with HRT may have a synergistic effect in enhancing cellular bioenergetics and may explain the patient's accelerated visual improvement. Nevertheless, additional studies are needed to confirm estrogen's protective role in vivo.
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interest regarding the publication of this paper.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Riordan-Eva P., Sanders M.D., Govan G.G. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(Pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Carelli V., Ross-Cisneros F., Sadun A.A. Mitochondrial disfunction as cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Sadun A.A., Carelli V., Salomao S.R. A very large Brazilian pedigree with 11778 Leber's hereditary optic neuropathy. Trans Am Ophthalmol Soc. 2002;100:169–178. Discussion 178–9. [PMC free article] [PubMed] [Google Scholar]
- 4.De Marinis M. Optic neuropathy after treatment with anti-tuberculous drugs in a subject with Leber's hereditary optic neuropathy mutation. J Neurol. 2001;248(9):818–819. doi: 10.1007/s004150170103. [DOI] [PubMed] [Google Scholar]
- 5.Giordano C., Montopoli M., Perli E. Oestrogens ameliorate mitochondrial dysfunction in Leber's hereditary optic neuropathy. Brain. 2011;134(Pt 1):220–234. doi: 10.1093/brain/awq276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisano A., Preziuso C., Iommarini L. Targeting estrogen receptor β as preventive therapeuthic strategy for Leber's hereditary optic neuropathy. Hum Mol Genet. 2015;24(24):6921–6931. doi: 10.1093/hmg/ddv396. [DOI] [PubMed] [Google Scholar]
- 7.Huang T.L., Wang J.K., Pang C.Y., Tsai R.K. Leber's hereditary optic neuropathy associated with the m.10197G>A mutation. J Clin Exp Ophthalmol. 2017;8(673):4–8. [Google Scholar]
- 8.Chae J.H., Lee J.S., Kim K.J. A novel ND3 mitochondrial DNA mutation in three Korean children with basal ganglia lesions and complex I deficiency. Pediatr Res. 2007;61(5 Pt 1):622–624. doi: 10.1203/pdr.0b013e3180459f2d. [DOI] [PubMed] [Google Scholar]
- 9.Sadun A.A., La Morgia C., Carelli V. Leber's hereditary optic neuropathy. Curr Treat Options Neurol. 2011;13(1):109–117. doi: 10.1007/s11940-010-0100-y. [DOI] [PubMed] [Google Scholar]
- 10.Carelli V., La Morgia C., Valentino M.L. Idebenone treatment in Leber's hereditary optic neuropathy. Brain. 2011;134(Pt 9):e188. doi: 10.1093/brain/awr180. [DOI] [PubMed] [Google Scholar]