Abstract
Background
This study investigated the therapeutic effects of the peroxisome proliferator-activated receptor-γ (PPARγ) agonist pioglitazone in ob/ob mice with obesity-related glomerulopathy (ORG).
Material/Methods
A total of 24 mice were divided into 3 groups: wild-type C57BL/6 mice (n=8), ob/ob mice (n=8), and ob/ob mice receiving pioglitazone treatment (n=8). Body mass, blood glucose, serum adiponectin (ADP), and urine microalbumin (mALB) levels were determined. Renal histology was examined using light and electron microscopy. Wilms tumor 1 (WT1), Zonula occludens-1 (ZO-1), AMP activated protein kinase (AMPK), and NADPH oxidase-4 (NOX-4) expression were evaluated by immunohistochemistry and Western blot.
Results
Serum ADP did not alter between weeks 0 and 12 in the control group, while the ob/ob mice showed a time-dependent decrease that was prevented by pioglitazone. Urinary mALB did not alter between week 0 and 12 in the control group, but was higher in week 0 and week 12 in the ob/ob group. Pioglitazone prevented the rise in urinary mALB in week 12. Histology revealed glomerulomegaly, mesangial proliferation, focal segmental glomerulosclerosis, and foot processes fusion in the ob/ob group, which were ameliorated by pioglitazone treatment. Compared to the control group, ob/ob mice had a higher kidney index and glomerular diameter, which were reduced by pioglitazone treatment. Immunohistochemical and Western blot experiments revealed lower expression levels of WT1, ZO-1, and AMPK and higher NOX-4 expression level in the ob/ob group, which was prevented by pioglitazone treatment.
Conclusions
Pioglitazone, a PPARγ agonist, can prevent ORG, probably by reducing oxidative stress.
MeSH Keywords: Nephrology, Obesity, Oxidative Stress, Podocytes, PPAR gamma, Thiazolidinediones
Background
Obesity has now become a global epidemic with a rising incidence over the past decades. The proportion of overweight and obese adults (BMI ≥25 kg/m2) worldwide has increased from 28.8% to 36.9% in men, and from 29.8% to 38.0% in women between 1978 and 2013 [1]. This epidemic has led to a higher prevalence of metabolic syndrome, which is characterized by insulin resistance, chronic inflammation, oxidative stress, and injury to different organs [2]. For example, obesity-related glomerulopathy (ORG) is now becoming an important clinical problem leading to significant morbidity and mortality [3]. Obesity-related glomerulopathy is morphologically defined as glomerulomegaly and focal segmental glomerulosclerosis (FSGS), clinically featuring proteinuria and renal disfunction.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated transcription factor regarded as a master regulator of fat deposition. Previous studies have shown that activation or over-expression of PPARγ can stimulate lipogenesis and adipogenesis [4]. Therefore, PPARγ is considered as a therapeutic target for obesity. Thiazolidinediones (TZDs) are PPARγ agonists that act to increase insulin sensitivity. They have been shown to increase gene expression related to fat metabolism and improve pancreatic islet cell function, thereby improving insulin sensitivity and reducing blood glucose and dyslipidemia. Through binding with PPARγ, they also exert anti-oxidant and anti-inflammatory effects.
In the recent years, the potential protective effects of PPARγ agonists on the kidneys have attracted increasing attention. However, whether these agents are effective in treating obesity-related glomerulopathy has not been elucidated. Using an ob/ob mouse model, we explored potential renoprotective effects of pioglitazone and possible involvement of the oxidative stress pathway. It is expected that PPARγ agonists may provide new ideas for the prevention and treatment of clinical obesity-related glomerulopathy.
Material and Methods
Experimental animals
This study was approved by the Administration Committee of Laboratory Animals. All experimental procedures for animal use compiled fully with the MOST Guideline on Administration of Laboratory Animals. Eight-week-old male ob/ob mice and homologous C57BL/6 (wild-type) mice were purchased from Beijing HFK Bioscience Co. Ob/ob mice with leptin deficiency are known to suffer from obesity. These mice were kept in single cages in the animal laboratory at the Chinese Academy of Medical Sciences with 12-h light-dark cycles at 22±2°C and 50±5% relative humidity. Free access to food and water was provided.
Experimental design
We divided 16 ob/ob mice fed with high-fat diet containing crude fat ≥6% (Beijing HFK Bioscience Co., Ltd.) into 2 groups: the obesity group (ob/ob) and the obesity with pioglitazone group (ob/ob+PGZ). Eight C57BL/6 mice fed with normal diet served as the control group (WT). The mice in the ob/ob+PGZ group were administered 12.5 mg·kg−1·d−1 PGZ by gavage daily for 12 weeks. The same dosage of distilled water was given to the mice in the WT group and ob/ob group at the same time.
Data collection
Body mass and blood glucose were measured weekly. Serum adiponectin (ADP) and urine microalbumin (mALB) in all the mice were tested by enzyme-linked immunosorbent assay (ELISA, Abcam Co., Shanghai, China) at week 0 and 12 of the experiment. Blood was obtained from the inner canthus of the mice and 24-h urine was obtained individually.
The mice were sacrificed at week 12. Both kidneys were removed and the mass of the kidney was measured to calculate the kidney index (kidney mass/body mass ratio). Half of the right kidney was placed into formaldehyde and embedded in paraffin for light microscopy. The kidney histomorphometry was examined in the presence of hematoxylin-eosin (HE) staining. A total of 10 glomeruli, including capillary and urine pole, were examined, and their diameters were measured and averaged. The other half of the right kidney was placed into glutaraldehyde and embedded in paraffin for electron microscopy, in which podocytes were observed. The left kidney was placed into liquid nitrogen for protein assay by Western blot.
Immunohistochemistry
Wilms tumor 1 (WT1), Zonula occludens-1(ZO-1), AMP-activated protein kinase (AMPK), and NADPH oxidase-4 (NOX-4) were positioned by immunohistochemistry (IHC, Abcam, Shanghai, China). Podocyte number marked by WT1 and ZO-1, AMPK, and NOX-4 expression level were evaluated. WT1, ZO-1, AMPK, and NOX-4 were scored semi-quantitatively by pathology image analysis software in 10 randomly selected glomeruli [5]. Positive staining was indicated by brown color. Podocyte number marked by WT1 was expressed by percentage of positive cells. ZO-1, AMPK, and NOX-4 were expressed by the product of the positive staining area and intensity. The positive staining area grade was: <1%=0, 1~10%=1, 11~50%=2, 51~80%=3, and 81~100%=4. The positive staining intensity grade was: negative=0, weak positive=1, positive=2, and strongly positive=3. The investigators performing these measurements were blinded to grouping.
Western blot
The kidney tissues were lysed in RIPA buffer, lysates were resolved, and we conducted electrophoresis in SDS-polyacrylamide gels. Proteins on SDS-Page were subsequently transferred onto nitrocellulose membranes. Membranes were incubated in blocking buffer at 22°C for 1 h, and with primary antibody (anti-WT1, ZO-1, AMPK, NOX-4, Abcam, Shanghai, China) diluted in blocking buffer at 4°C overnight, followed by another 1-h secondary HRP antibody (anti-rabbit, Abcam, Shanghai, China) incubation at 22°C after washing with TBS-Tween. Enhanced chemiluminescence detection was performed using the ECL Kit (Abcam, Shanghai, China).
Statistical analyses
Comparisons between 3 groups were analyzed by single-factor analysis of variance. Data of 2 groups were compared with the independent-samples t test. All statistical analyses were performed using SPSS 22. P<0.05 was considered to indicate statistical significance.
Results
Morphometric and biochemical measurements
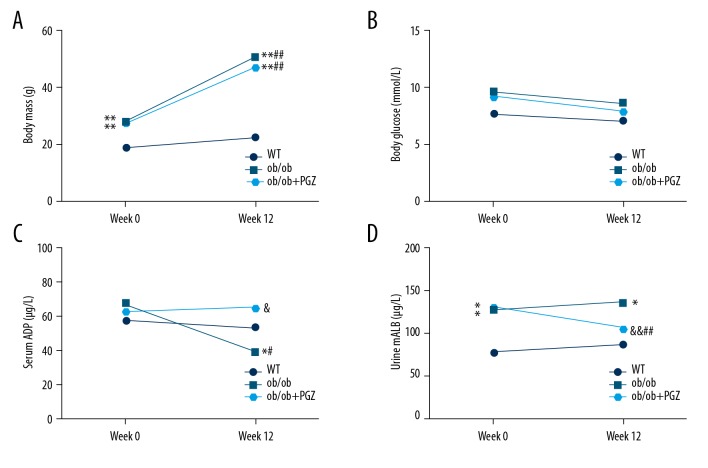
Body mass increased from 28.13±1.55 to 50.50±3.21 g between week 0 and 12 in the ob/ob group (P<0.01), and there was a similar trend in the ob/ob+PGZ group (27.50±1.20 to 47.13±5.67 g; P<0.01). Pioglitazone had no significant effects on the body mass of ob/ob mice (P>0.05) (Figure 1A). Moreover, there was no significant difference in glucose of the 3 groups at week 0 and 12 (P>0.05) (Figure 1B). Serum ADP did not alter between week 0 and 12 in the control group (57.90±6.88 vs. 53.68±9.87 μg/L; P>0.05), while the ob/ob mice showed a time-dependent decrease from 68.03±19.41 to 39.57±15.11 μg/L (P<0.05), which was prevented by pioglitazone at week 12 (65.53±4.97 μg/L; P<0.05) (Figure 1C). Urine mALB levels did not significantly differ between week 0 and 12 in the control group (76.27±10.12 vs. 85.58±17.16 μg/L; P>0.05), but was higher in week 0 (127.25±18.04 μg/L; P<0.05) and week 12 (136.43±17.94; P<0.05) in the ob/ob group. Pioglitazone treatment prevented the increase of urine mALB at week 12 (105.79±17.43 μg/L; P<0.01) (Figure 1D).
Figure 1.
Morphometric and biochemical measurements, including body mass (A), blood glucose (B), serum ADP (C), and urine mALB (D), in control (WT), ob/ob mice (ob/ob), and ob/ob mice receiving pioglitazone (ob/ob+PGZ). Data are displayed as mean. * P<0.05, ** P<0.01 compared to WT group at the same time; & P<0.05, && P<0.01 compared to ob/ob group at the same time; # P<0.05, ## P<0.01 compared to week 0 of the experiment at the same group.
Renal histology
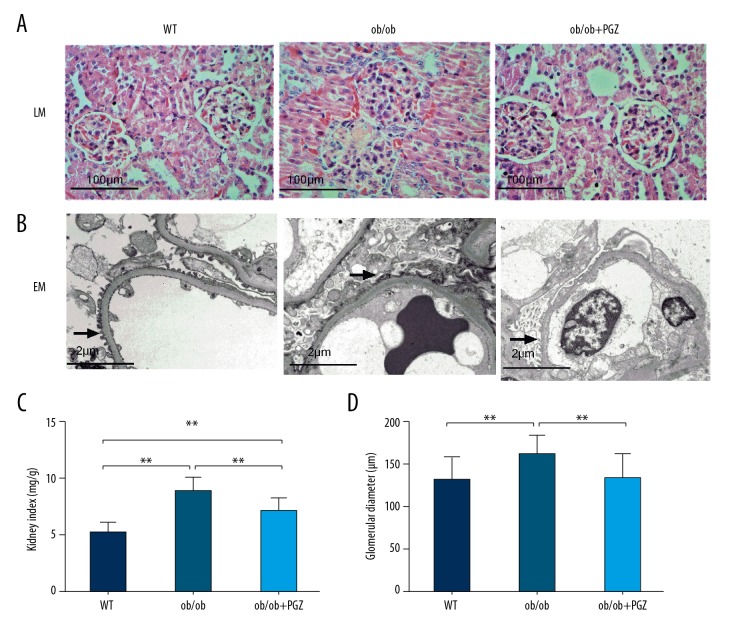
In light microscopy, histology revealed glomerulomegaly, FSGS and mesangial proliferation in the ob/ob group, which were ameliorated by pioglitazone treatment (Figure 2A). In electron microscopy, the foot processes fused in the ob/ob group, while the foot process fusion was improved in the ob/ob+PGZ group (Figure 2B). Compared to the control group, ob/ob mice had a higher kidney index and glomerular diameter (both P<0.01), which were reduced by pioglitazone treatment (P<0.01) (Figure 2C, 2D).
Figure 2.
Renal histology by light microscopy (LM, ×200, A) and electron microscopy (EM, ×10 000, B). Arrows point to podocyte foot processes. Kidney index (C) and glomerular diameter (D) in control (WT), ob/ob mice (ob/ob), and ob/ob mice receiving pioglitazone (ob/ob+PGZ). Data are displayed as mean and SD. ** P<0.01.
Immunohistochemistry
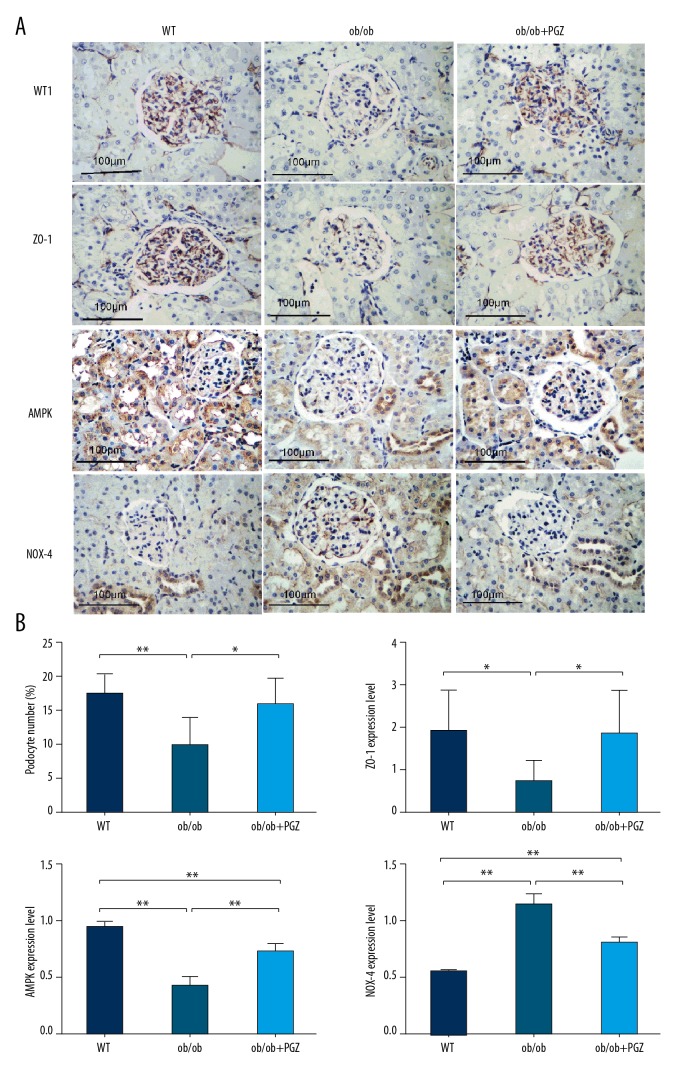
WT1 was expressed in the podocyte nucleus, ZO-1 was expressed in the podocyte cytoplasm, and AMPK and NOX-4 were expressed in the glomerulus, renal tubule, and interstitium. Compared to the control group, lower expression levels of WT1 (P<0.01), ZO-1 (P<0.05), and AMPK (P<0.01) and higher NOX-4 expression level (P<0.01) were detected in the ob/ob group. These abnormalities were prevented by pioglitazone treatment (Figure 3A, 3B).
Figure 3.
Immunohistochemical experiments showing the WT1, ZO-1, AMPK, and NOX-4 expression (IHC, ×200, A), with quantification (B). Data are displayed as mean and SD. * P<0.05; ** P<0.01.
Western blot
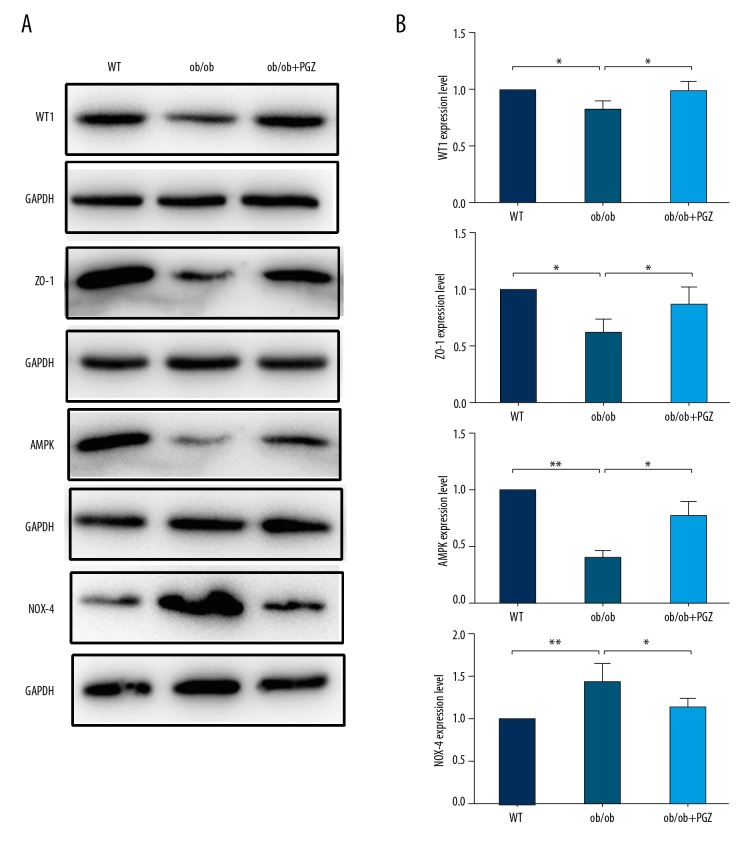
Western blot analysis demonstrated lower expression levels of WT1 (P<0.05), ZO-1 (P<0.05), and AMPK (P<0.01) and higher NOX-4 expression level (P<0.01) in the ob/ob group compared to the control group. These abnormalities were prevented by pioglitazone treatment (Figure 4A, 4B).
Figure 4.
Western blot experiments showing the WT1, ZO-1, AMPK, and NOX-4 expression (A), with quantification (B). Data are displayed as mean and SD. * P<0.05; ** P<0.01.
Discussion
Mouse models are useful systems for modelling human disease conditions owing to their amenability to both genetic alterations and pharmacological manipulation [6,7]. For obesity, a commonly used model is ob/ob mice [8], which demonstrate at least 20% higher body mass index compared to control mice, thereby satisfying the definition of obesity [9]. Our study showed clinical and pathological changes in the kidneys of ob/ob mice, as reflected by increased urine microalbumin levels, higher kidney index, and the presence of glomerulomegaly accompanied by focal segmental glomerulosclerosis.
We found lower expression levels of WT1 and ZO-1 in the kidneys of ob/ob mice. WT1 is widely used as a podocyte marker within the glomerulus [10]. Podocyte number is a key factor in the development of obesity-related glomerulopathy, termed podocytic disease. ZO-1, a binding protein related to glomerular filtration membrane, is located in the cytoplasmic side of foot processes, into which the slit diaphragm inserts. ZO-1 can interact with the actin cytoskeleton, anchoring proteins in the slit diaphragm to the cytoskeleton, thereby maintaining the structural integrity of the foot processes [11]. In other animal models of obesity-related glomerulopathy, glomerulus and podocyte volume both increased in relation to body weight gain. However, the volume increase rate of podocytes is lower than that of glomerulus, creating a mismatch between them [12]. As podocyte hypertrophy is limited, glomerulus volume expands, leading to mechanical strain acting on podocytes. Podocytes fall off, resulting in denudation of the glomerular basement membrane (GBM) and subsequent adhesions to the Bowman capsule and parietal cell coverage, forming a nidus for glomerulosclerosis [13]. In obese patients, microscopy can reveal podocyte swelling, increased width of podocytes, and fusion of foot processes [14]. Previous studies have also reported associations between body mass index and extent of podocyte hypertrophy, podocyte number, and degree of proteinuria in patients with obesity-related glomerulopathy [15–17].
Another important finding in our study was that serum adiponectin levels were lower in ob/ob mice compared to control mice at week 12, in agreement with previous findings [18]. Normally, adiponectin maintains the normal function of filtration barrier and may additionally exert anti-oxidant and anti-inflammatory actions. It can also function as an adipokine to regulate kidney cells in response to mechanical stress induced by ultrafiltration, and is involved in cell hypertrophy, extracellular matrix alteration, and fibrosis [19]. In a mouse adiponectin-knockout model [18], the fusion of foot processes, glomerular basement membrane nudity, decreased ZO-1 expression in podocytes, and albuminuria were observed. These abnormalities were rescued by treatment with recombinant adiponectin. Podocyte lesions in obesity-related glomerulopathy may be associated with decrease of serum adiponectin, and increasing adiponectin can ameliorate podocyte functions and reduce proteinuria.
The results of the present study confirmed that pioglitazone can prevent decreases in adiponectin, improve the expression levels of WT1 and ZO-1 in the kidneys, maintain podocyte number, reverse structural abnormalities of podocytes, and reduce the degree of proteinuria in obesity. We found higher AMPK and lower NOX-4 levels, thereby suggesting a reduction in oxidative stress as a possible mechanism for its renal-protective effects. Thus, previous studies have reported adiponectin levels are regulated by the PPARγ pathway [20–22], with higher adiponectin level associated with increased PPARγ co-activator-1α expression [23,24]. Adiponectin acts through its receptor, AdipoR1, to activate AMPK, which in turn negatively regulates NOX-4, which is responsible for generating much of the reactive oxygen species in the kidneys [25]. In our study, pioglitazone exerted protective effects on podocytes through PPARγ activation, leading to restoration of adiponectin, upregulating AMPK, downregulating NOX-4, and inhibiting oxidative stress. The study of Sharma et al. [18] found lower AMPK and higher NOX-4 levels in adiponectin-knockout mice, which was prevented by recombinant adiponectin. The addition of adiponectin or AMPK activator in the culture medium of podocytes in vitro can increase the AMPK expression or activity and subsequently decrease NOX-4 expression, thereby promoting podocyte repair. Another study showed that PPARγ is expressed in the glomerular podocytes, suggesting that pioglitazone acts directly on podocytes [26]. Antioxidants such as heme oxygenase and NOX-4 inhibitors possess similar renal-protective effects in obesity-related glomerulopathy [27]. Our conclusion is strengthened by the fact that the mechanism of pioglitazone action is to decrease oxidative stress. These pre-clinical results are in keeping with clinical findings that pioglitazone increased adiponectin levels and decreased proteinuria in patients with diabetic nephropathy [28].
Finally, it should be noted that the blood glucose in ob/ob mice was less than 16.7 mmol/L, excluding diabetes. Moreover, we found no significant effects of pioglitazone on blood glucose in ob/ob mice, suggesting that the protective effects on the kidneys were independent of glycemic control. In addition, obesity-related glomerulopathy is known to have an inflammatory component, and PPARγ is also associated with inflammatory pathways. The anti-inflammatory effects of PPARγ agonist in obesity-related glomerulopathy warrants further study. Other interesting research revealed that pioglitazone ameliorates endothelial dysfunction in obese rats with nephropathy [29]. These results demonstrate that pioglitazone prevents obesity-related glomerulopathy via multiple targets.
Conclusions
The peroxisome proliferator-activated receptor-γ (PPARγ) agonist, pioglitazone, can prevent obesity-related glomerulopathy (ORG), probably by reducing oxidative stress.
Footnotes
Source of support: Science Fund of Tianjin Medical University (grant number 2010ky50)
Conflict of interest
None.
References
- 1.GBD 2013 Risk Factors Collaborators. Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H. Obesity and metabolic inflammation. Drug Discov Today Dis Mech. 2013;10:21–25. doi: 10.1016/j.ddmec.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morandi A, Maffeis C. Urogenital complications of obesity. Best Pract Res Clin Endocrinol Metab. 2013;27:209–18. doi: 10.1016/j.beem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Kim E, Kim EJ, Seo SW, et al. Meta-review of protein network regulating obesity between validated obesity candidate genes in the white adipose tissue of high-fat diet-induced obese C57BL/6J mice. Crit Rev Food Sci. 2014;54:910–23. doi: 10.1080/10408398.2011.619283. [DOI] [PubMed] [Google Scholar]
- 5.Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–45. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Wong P, Laxton V, Srivastava S, et al. The role of gap junctions in inflammatory and neoplastic disorders (review) Int J Mol Med. 2017;39:498–506. doi: 10.3892/ijmm.2017.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YT, Lin HY, Chan YW, et al. Mouse models of atherosclerosis: A historical perspective and recent advances. Lipids Health Dis. 2017;16:12. doi: 10.1186/s12944-016-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–45. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien HJ, Peng SJ, Hua TE, et al. 3-D imaging of islets in obesity: Formation of the islet – duct complex and neurovascular remodeling in young hyperphagic mice. Int J Obes. 2015;40:685–97. doi: 10.1038/ijo.2015.224. [DOI] [PubMed] [Google Scholar]
- 10.Szeto HH, Liu S, Soong Y, et al. Protection of mitochondria prevents high-fat diet – induced glomerulopathy and proximal tubular injury. Kidney Int. 2016;90:997–1011. doi: 10.1016/j.kint.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 11.González-Mariscal L, Betanzos A, Ávila-Flores A. MAGUK proteins: Structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–24. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda A, Chowdhury MA, Venkatareddy MP, et al. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23:1351–63. doi: 10.1681/ASN.2012030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453–71. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 14.Chen H-M, Li S-J, Chen H-P, et al. Obesity-related glomerulopathy in China: A case series of 90 patients. Am J Kidney Dis. 2008;52:58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 15.Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73:947–55. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 16.Chen H-M, Liu Z-H, Zeng C-H, et al. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48:772–79. doi: 10.1053/j.ajkd.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Camici M, Galetta F, Abraham N, et al. Obesity-related glomerulopathy and podocyte injury: A mini review. Front Biosc (Elite Ed) 2012;4:1058–70. doi: 10.2741/e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–56. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briffa JF, McAinch AJ, Poronnik P, et al. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F1629–36. doi: 10.1152/ajprenal.00263.2013. [DOI] [PubMed] [Google Scholar]
- 20.Niranjan G, Mohanavalli V, Srinivasan AR, et al. Serum lipid peroxides and magnesium levels following three months of treatment with pioglitazone in patients with type-2 diabetes mellitus. Diabetes Metab Syndr. 2013;7:35–37. doi: 10.1016/j.dsx.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Gensch C, Clever YP, Werner C, et al. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 23.Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav H, Quijano C, Kamaraju AK, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedeek M, Callera G, Montezano A, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1348–58. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 26.Xing Y, Ye S, Hu Y, et al. Podocyte as a potential target of inflammation: Role of pioglitazone hydrochloride in patients with type 2 diabetes. Endocr Pract. 2012;18:493–98. doi: 10.4158/EP11378.OR. [DOI] [PubMed] [Google Scholar]
- 27.Camici M, Galetta F, Abraham N, et al. Obesity-related glomerulopathy and podocyte injury: A mini review. Front Biosci. 2012;4:1058–70. doi: 10.2741/e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki Y, Cersosimo E, Triplitt C, et al. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int. 2007;72:1367–73. doi: 10.1038/sj.ki.5002516. [DOI] [PubMed] [Google Scholar]
- 29.Namikoshi T, Satoh M, Tomita N, et al. Pioglitazone ameliorates endothelial dysfunction in obese rats with nephropathy. Biochem Biophys Res Commun. 2007;361:835–40. doi: 10.1016/j.bbrc.2007.07.136. [DOI] [PubMed] [Google Scholar]