Abstract
The present study was to investigate the effect of cellulose matrix and oligosaccharide on solid state and morphology characteristics of freeze-dried cationic dimethyldioctadecylammonium (DDA)-based liposomes encapsulating ovalbumin (OVA). The OVA-containing liposomes were protected using cellulose derivative matrix and oligosaccharide. Despite the fact that saccharides are known to preserve protein and lipid membranes during drying, however, collapse structure are often addressed. In other side, cellulose matrix potentially prevents collapsing as it has been widely used for matrix in drug delivery formulations to increase the mass for compact matrices of resultant products. Their solid state characteristics were determined in terms of their crystallinity using X-Ray diffraction (XRD), thermal properties and detection of phase separation using differential scanning calorimetry (DSC). Furthermore, their morphology was observed using scanning electron microscopy and transmission electron microscopy. The study revealed that formulation with either oligosaccharide and cellulose matrix demonstrated a miscible mixture with DDA and soy phosphatidylcholine (SPC) that might construct stable dried liposomal formulation. Phase separation was not observed in formula with combination of oligosaccharide and cellulose matrix where their DSC thermograms showed glass transition indicating amorphous structure and miscible mixture. XRD confirmed the absence of crystal-like properties, demonstrating prevented crystallization. The dry products were porous with spherical liposomes trapped in the matrices, signifying the ease in reconstitution. Furthermore, OVA were well-preserved as its recovery was more than 80%. The preservation of both liposomes and protein antigen were found to be dependent upon the incorporation of both oligosaccharide and cellulose matrix included in the formulation.
Keywords: Cellulose matrix, Liposomes, Oligosaccharide, Ovalbumin, Vaccine
INTRODUCTION
Most commercial vaccines are administered as liquids, despite the fact that most of them are stored as solids and require reconstitution prior to administration. In general, such vaccines are thermo-labile, high cost, and disadvantageous for people who are needle-phobic. To eliminate such disadvantages, there is still a need to provide vaccines that are more acceptable and convenient. Characteristics of an ideal vaccine should consider the safety, efficacy, heat stability and ease of administration (1,2). Many particulate delivery systems for vaccine have been developed including nanoparticles, microparticles, microemulsion, niosomes, and liposomes (3). Among those particulate delivery systems, liposomes have attracted much attention as they have several advantages compared with others (4).
Furthermore, dry vaccines offer advantages in terms of their physical and chemical stability at elevated temperatures (5,6). Problems such as aggregation, denaturation, hydrolysis, oxidation, and thermo-labile properties can be avoided and preferably be cheaper to make them more attractive for developing countries.
Saccharides with many hydroxyl groups (polyhydroxy compound) have been widely used as lyoprotectant in freeze drying process (7,8).
Proposed mechanism is that they interact directly with phosphate groups in liposomal lipid bilayers via hydrogen bonds, thus displace the water lost (9). Other mechanism is the formation of a glassy matrix that protects proteins and lipid bilayers from aggregating and preventing conformational changes (10). Among the well-known lyoprotectants used in freeze-drying are disaccharides such as trehalose, sucrose, maltose, and oligosaccharides such as maltodextrin (MAL). Some studies reported that MAL perform well or even better as lyoprotectants than sucrose and maltose due to its nature amorphous structure (11).
However, fibrous, membranous, and sheet-like structures materials demonstrate collapse phenomena that occur after drying (12,13). This phenomenon is one of the important physicochemical characteristics that obtain optimal cycle in freeze drying (14). Many issues have been raised as concern in event of collapse. The use of lyoprotectant often fails to prevent collapse as the resultant product could not maintain their solid structure following drying (15). On the other hand, addition of a cellulose derivative matrix can be an alternative strategy to strengthen the macrostructure of the dry products as well as protecting the proteincontaining liposomes. Cellulose derivative matrix such as hypromellose (HYP) may increase the mass for compact matrices of the resultant products and avoid collapse. Moreover, due to its large molecular size and complexity of the structure, such matrix may prevent particles from coalescent or agglomerate (16). This study aimed to investigate the solid state characteristics and phase behavior of dried-ovalbumin (OVA)-containing liposomes. Combination of lyoprotectant oligosaccharide and cellulose derivative matrix was used in the formulation of the dehydrated cationic dimethyldioctadecylammonium (DDA)-based liposomes. DDA is one of particular interests in the developed formula as it shows strong evidence in stimulating immune responses (17,18).
MATERIALS AND METHODS
Materials
Dimethyl-dioctadecyl ammonium bromide (Sigma Aldrich, Singapore) and soy phosphatidylcholine S-100 (Lipoid GmBh, Germany) were used as liposomal membrane constituent, while cholesterol (Sigma-Aldrich, Singapore) was used as the liposomal membrane stabilizer. MAL (DE:13-17) (Sigma-Aldrich, Singapore) was used as lyoprotectants, while HYP 90SH (Shin-Etsu, Japan) was selected as cellulose derivative matrix. OVA (Sigma-Aldrich, Singapore) was used as antigen model. Methanol (E. Merck, Singapore) was selected as a solvent to facilitate the mixing of liposomal ingredients. The solvent used was of analytical grade.
Preparation of liposomes
Liposomes formula was prepared using thin film hydration methods. Soy phosphatidylcholine (SPC), DDA, and cholesterol were dissolved in methanol in the molar ratio of SPC:DDA:cholesterol (9:3:1). The solvent was then evaporated under vacuum at 45 °C using rotary evaporator (Büchi, Germany) for 60 min. Thin film was then hydrated with 5 mL pre-warmed solution that contain MAL at various concentrations and OVA at concentration of 0.25 mg/mL. Hydration process was conducted at 50 °C for 10 min. The liposomes formation was signed by the appearance of white-milky suspension. Liposomes suspension was sonicated in water bath sonicator for 5 min. HYP powder was weighed accordingly and dispersed in purified water to form corresponding HYP gel concentrations. The liposomes suspension was incorporated into HYP gel and stirred homogeneously at room temperature to make up weight ratio (%) of MAL and HYP as follows: (F1), 5:2.5; (F2), 10:2.5; (F3), 5:7.5; and (F4), 10:7.5. They were then dispensed into vials before freeze-drying (Virtiz Lyophilizer, US). The freezing temperature was conducted at -80 °C for 24 h and continued by drying at -20 °C for 48 h.
Morphology characterization of freeze-dried liposomes
Scanning electron microscopy
The morphology of the liposomes contained in the solid matrix was analyzed using scanning electron microscopy (SEM). The portions of the dried product were scattered and glued onto 25 mm diameter plates, which were attached to SEM specimen mounts. The specimens were sputter-coated with a layer of Au-palladium approximately 5 nm in thickness and specimens were examined with an electron microscope (Phenom, USA).
Transmission electron microscopy
The morphology of the liposomes in liquid samples was observed using transmission electron microscopy (TEM). Samples were prepared on a copper grid (S162 Formvar/Carbon 200 mesh Cu; Agar Scientific UK) at room temperature by conventional negative staining method using 0.2% phosphotungstic acid. Samples were viewed under transmission electron microscope (TEM, Philips CM 100, The Netherlands).
Solid state characterization of freeze-dried liposomes
X-Ray diffraction analysis
Powder X-Ray diffraction instrument (Phillips X’Pert PRO PANalytical, Netherlands) was used to observe crystallinity of the dried products. The sample was placed on the sample holder and flatted to prevent particle orientation during preparation. The condition of analysis was as follows: Cu metal target, Kα filter, the voltage of 40 kV, 40 mA performed at room temperature. The analysis was carried out in 2 theta range of 5-40°.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) instrument (Mettler Toledo FP 85, Switzerland) was used to detect the solid state of the dried product. Samples were placed in aluminum crucibles and scanned from 30 °C to 250 °C at the heating rate of 10 °C/min.
Quantification of ovalbumin recovery
To allow determination of OVA antigen recovery in the freeze-dried liposomes formulation, the samples were treated with Bradford’s reagent to obtain colored solution. The intensity of the color absorbance should be in linear correlation with the protein concentration. OVA recovery was measured by determining the amount of OVA and comparing to the theoretical total loaded OVA. Samples (500 μL) were filtered using 0.2-μm Whatman filter paper (USA) before treated with 500 μL Bradford’s reagent and incubated for at least 10 min. The absorbance was measured at 595 nm using a UV spectrophotometer (Shimadzu UV-1800, Japan). Measurements were made in triplicate.
RESULTS
Morphology of freeze-dried liposomes
The effect of incorporation of cellulose derivative matrix (HYP) and oligosaccharide (MAL) on visual appearance of freeze-dried cakes is shown in Fig. 1. The cake, which was incorporated neither with cellulose derivative matrix nor with oligosaccharide, was totally shrunken and collapsed. The cake that was only incorporated with oligosaccharide was considerably shrunken and collapsed. Only the cake that incorporated cellulose derivative matrix maintained the structure without significant loss. It can be seen from Fig. 1B that the combined cellulose derivative matrix and saccharide cakes survived the freezedrying and maintained the structures.
Fig. 1.

Visual images of ovalbumin-containing formulations. (A1) the cake without maltodextrin (MAL) or hypromellose (HYP), (A2) the cake with MAL only, and (A3) the cake with HYP only. B1-B3 is the cakes containing combination of MAL and HYP regardless of their concentrations.
Scanning electron microscopy
It can be seen from Fig. 2 that cakes combining cellulose derivative matrix and oligosaccharide appeared to be highly porous. They consisted of large trails of water vapor flow. The spherical liposomes were trapped and preserved in the solids. The structures indicated that the solid products were amorphous. However, later analysis of X-Ray diffraction (XRD) results showed the formulation without oligosaccharide (MAL 0%) and cellulose matrix (HYP 0%) exhibited crystallinity as it has been predicted.
Fig. 2.
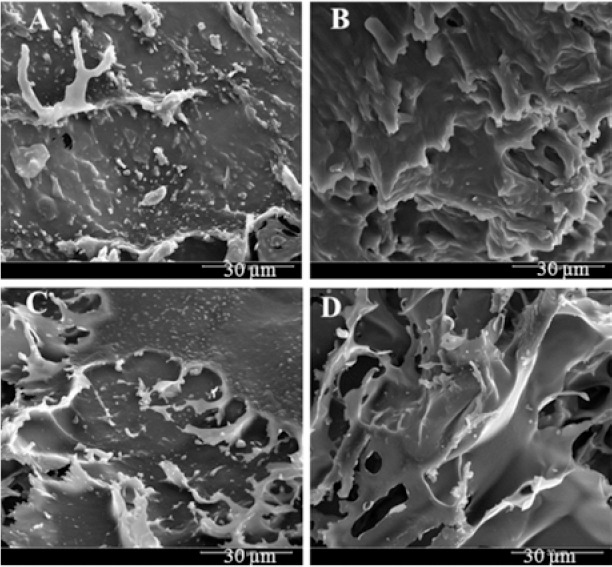
Scanning electron micrographs of liposomal ovalbumin-containing cakes using combination of maltodextrin (MAL) and hypromellose (HYP). (A) 5% MAL and 2.5% HYP, (B) 10% MAL and 2.5% HYP, (C) 5% MAL and 7.5% HYP, and (D) 10% MAL and 7.5% HYP.
Transmission electron microscopy
Further characterization of the vesicles, the freeze-dried liposomes were negatively stained by phosphotungstic acid and observed by TEM. The OVA-containing liposomes appeared as spherical structure, confirming the vesicular and lamellarity characteristics (Fig. 3). Its spherical structure and lamellarity can be maintained after rehydration of the freeze-dried cakes. The membranes appear uniform and continuous throughout the liposome. The spheroidal particles may be coexistence of uni-or multilamellar vesicles.
Fig. 3.
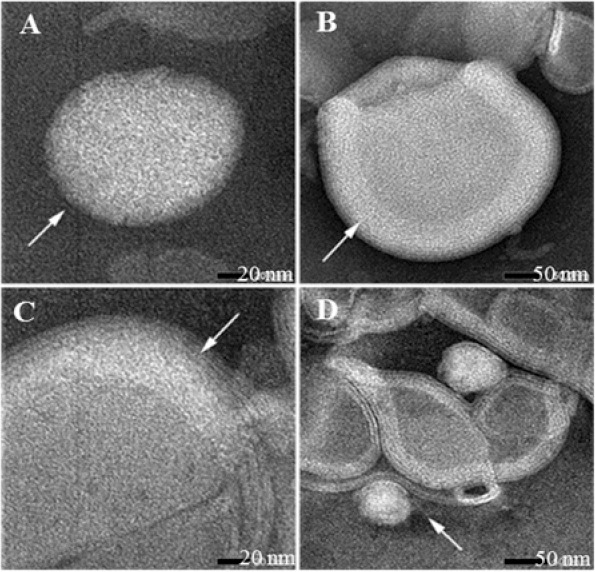
Transmission electron microscopy microphotographs of ovalbumin-containing liposomes. (A) and (B) show the liposome spherical shape and their lamellarity before freeze-drying. (C) and (D) show the shape and lamellarity following reconstitution of the freeze-dried cakes.
Solid state of freeze-dried liposomes
X-Ray diffraction
Figure 4 shows the X-ray diffraction patterns of all single materials used in the formulation. The XRD diffractogram of all materials are in amorphous solids except the DDA and cholesterol. Sharp peaks at a diffraction angle of 2θ 6.7, 7.2, 14.5, 19.5, 20.5, and 22.7° are observed, indicating that these materials are present as crystalline structure. Their crystalline-indicating peaks were detectable in the sample without saccharide (MAL 0%) and cellulose derivative matrix (HYP 0%) as presented in Fig. 5. This was in contrast with those formula using MAL and HYP, regardless their concentrations. This confirmed their potential utilization in the developed formulations for amorphization of freeze-dried liposomes formulations (Fig. 5).
Fig. 4.
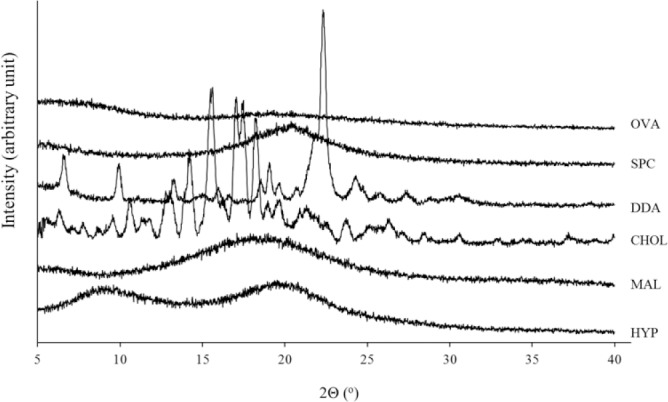
X-Ray diffraction diffractograms of single components used in the developed formulations. OVA, ovalbumin; SPC, soy phosphatidylcholine; DDA, dimethyldioctadecylammonium; CHOL, cholesterol; MAL, maltodextrin; HYP, hypromellose.
Fig. 5.
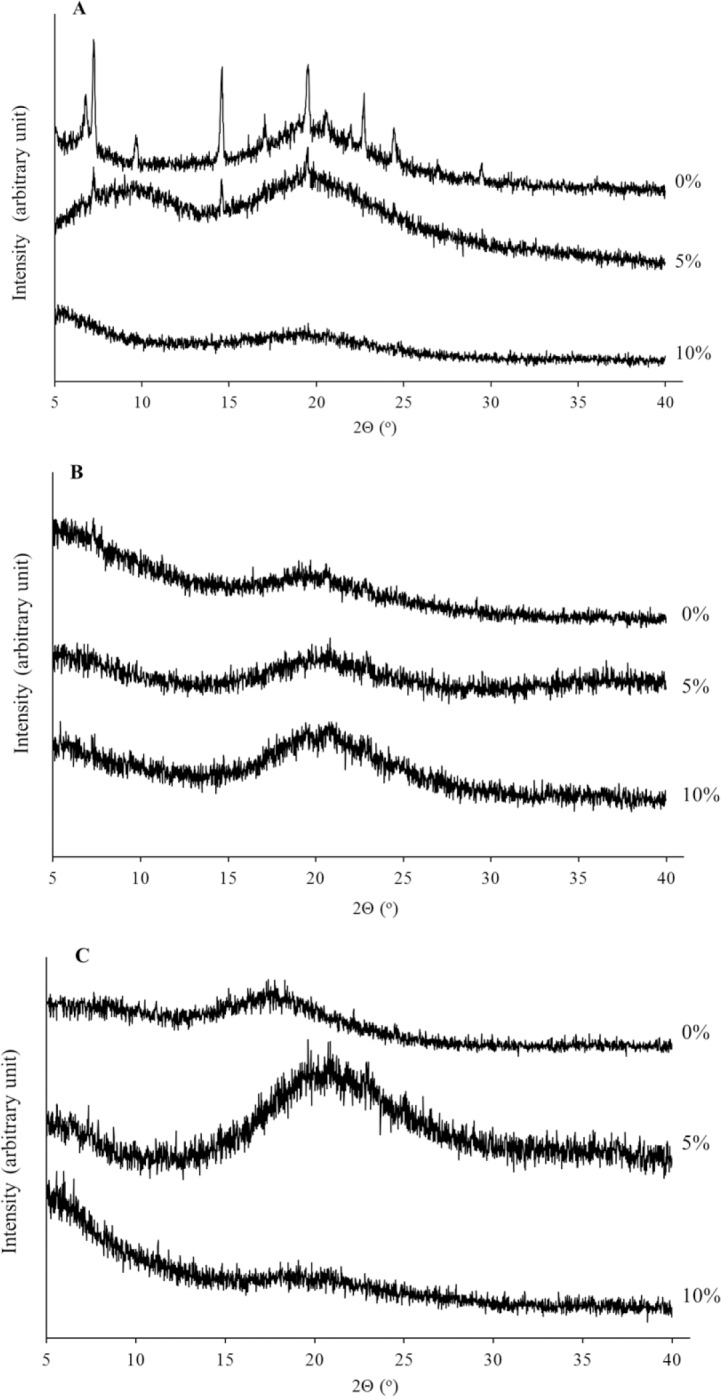
X-Ray diffraction diffractogram of all formulations combining saccharides maltodextrin at concentrations of 0%, 5%, and 10% with hypromellose (A) 0%, (B) 2.5%, (C) and 7.5%.
Differential scanning calorimetry
The OVA as antigen model provides a wide endothermic peak at 68.22 °C, similar with other components including SPC, MAL, and HYP at 99.54, 237.86, and 88.37 °C respectively. This indicates that the initial forms of the materials are amorphous. In contrast, the components of DDA cationic lipid showed sharp endothermic peak at 90.18 and 158.87 °C, as well as cholesterol at 149.31 °C, indicating that both materials are crystalline (Fig. 6).
Fig. 6.
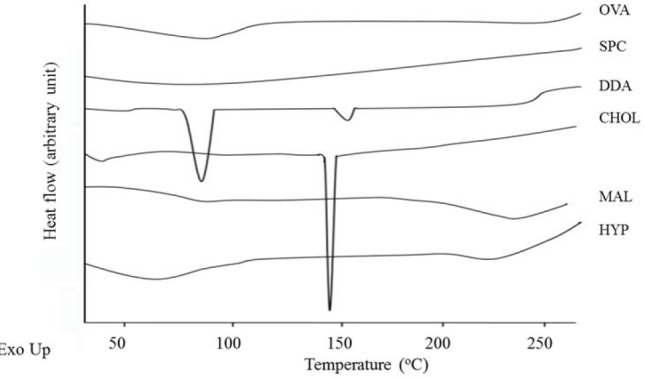
Differential scanning calorimetry thermograms of single components used in the developed formulations.
Phase separation in liposomal membrane may have major disadvantage for their application as delivery systems, including drug leakage and collapse. Therefore, the effect of MAL and HYP on the phase behavior (i.e. miscibility and phase separation) of dehydrated phospholipids mixtures was investigated. The results showed that phase separation occurred in formulation which are only contain MAL (Fig. 7A). This is observed from the presence of two endothermic peaks at temperature in the range of 80-120 °C and 200-250 °C.
Fig. 7.
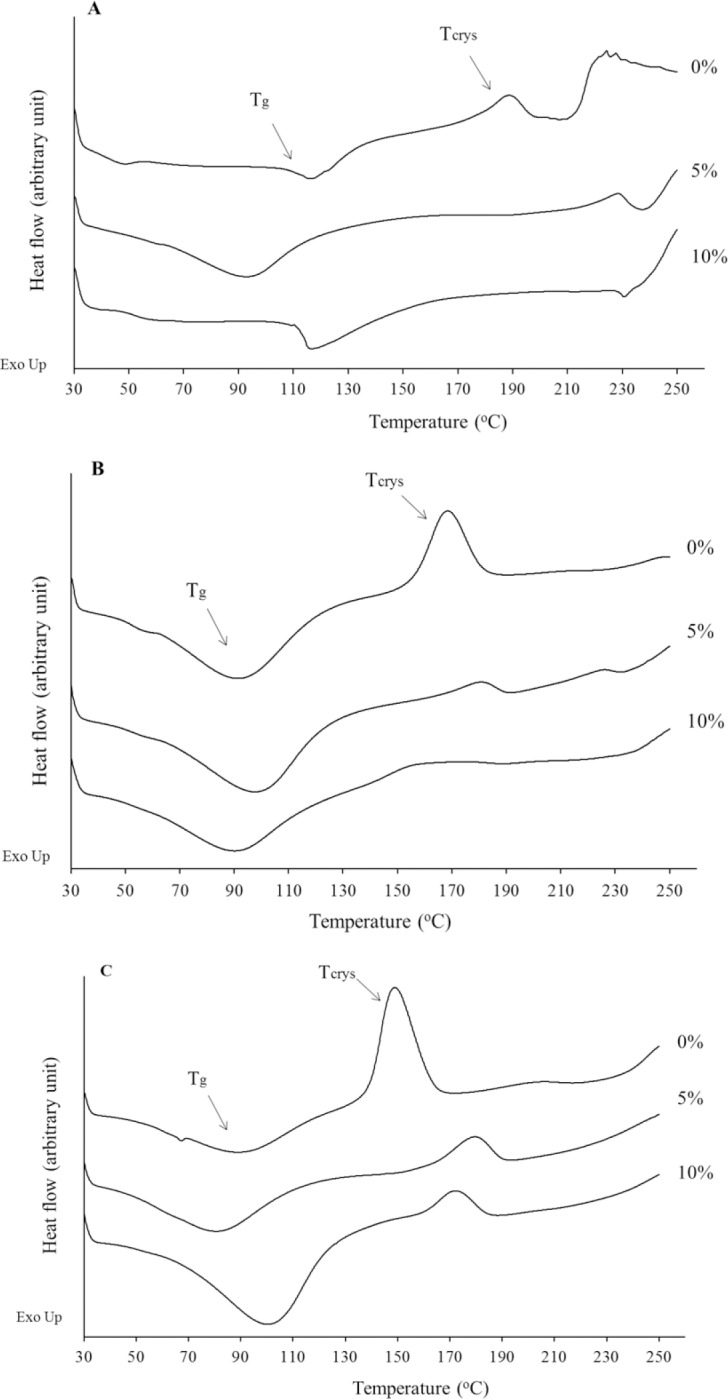
Differential scanning calorimetry thermograms of all formulations containing combination of saccharides and maltodextrin at concentrations of 0, 5, and 10% with hypromellose (A) 0, (B) 2.5, and (C) 7.5%. Tg, glass transition temperature; Tcrys, crystallization temperature.
The formulas using combination of MAL and HYP provide a relatively homogeneous mixture as specific peaks that correspond to single materials were no longer appear in the dry product. The DSC thermogram showed glass transition temperature (Tg) for all formulations incorporating HYP and MAL (Fig. 7B and 7C), indicating that the dry cakes were amorphous solid. The values were at range of 80-100 °C which were relatively high and far above room temperature at which storage and reconstitution are normally performed. Furthermore, exothermic peaks i.e. crystallization (Tcrys) were also observed in temperature range of 140-190 °C (Fig. 7B and 7C). The exothermic peak implies the rearrangement of molecules in the mixture as they were heated above their Tg. The Tcrys values allow gaps around 60-90 °C with the corresponding Tg. This indicated that the developed formulations were stable at room temperature and small chance of crystallization below the Tcrys.
Quantification of ovalbumin recovery
All the developed formulations showed good recovery after the freeze-drying process as indicated by their % of recovery that reached > 80% (Fig. 8). These results suggest that OVA were well protected by the incorporation of MAL and HYP in the formulations.
Fig. 8.
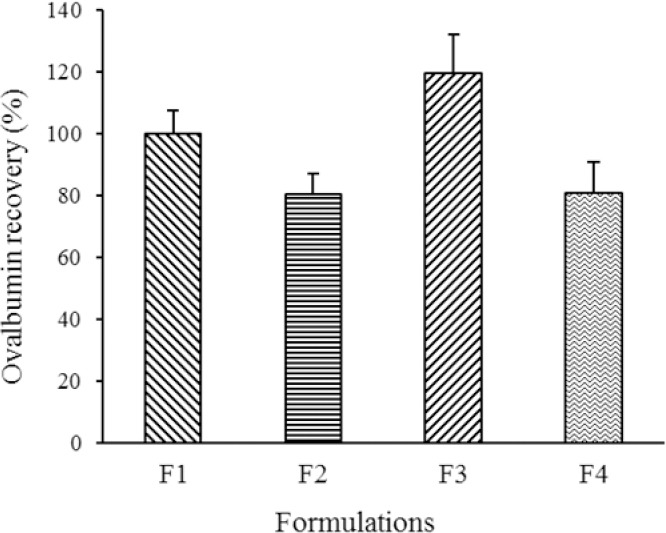
Ovalbumin recovery after reconstitution of dry products. Data are presented as mean ± SD, n = 3.
DISCUSSIONS
One of the essential factors in drying process of an OVA-containing liposome formula is to prevent collapse upon water removal. The addition of oligosaccharide and cellulose derivative matrix was not only to preserve the OVA-containing liposomes, but also to provide a pharmaceutically elegant freeze-dried solid. For instance, damaged protein and lipid membranes during drying, is often the results of collapsed structure (19,20). Thus combining the two excipients i.e. oligosaccharide and cellulose derivative matrix potentially prevent collapse as they can increase the mass for compact matrices of the resultant products. The physical form of an excipient depends on the intended function of that excipient. All excipients must remain amorphous to provide a matrix in which the labile OVA-containing liposome is molecularly dispersed.
The formulations we studied contained heat-labile OVA, phospholipids, oligosaccharide, and cellulose derivative polymer (HYP). It was desirable to prevent damaging crystallization because phase separation may lead to membrane disruption and protein leakage. MAL used in this study is naturally amorphous and can provide vitrification effects so that the liposomal integrity can be maintained (10,21). HYP was selected because of its ability to prevent particle aggregation and prevent crystallization (22,23).
It is evident that addition of MAL and HYP is essential since the macroscopic structure of the lyophilized product proved elegant appearance. The addition of MAL alone was not sufficient to maintain the macro structure of the solid products. In contrast, all formulas using combination of MAL and HYP regardless of their concentrations resulted in elegant and non-collapsing solids. The ability of HYP to increase the mass and viscosity of the system, may have been the cause of preventing deformation and collapse due to their cellulose derivative structure (24,25). It is expected that HYP-containing formulations; regardless of their concentrations, have higher collapse temperature than those without HYP. This is advantageous in terms of process, since lyophilization takes long time and requires high energy (26). When the process can be carried out at relatively higher temperatures, the required drying time is also shorter (27).
The SEM images showed that the dried-OVAcontaining liposomes were amorphous and porous, formed by the loss of ice crystals during drying. This might ease rehydration of the products (28). Moreover, the cakes were not collapsed as the macroscopic structure was maintained, showing the beneficial effect of combining MAL with HYP. The rigid structure as a function of MAL and HYP provide good matrix to maintain the structure after sublimation of ice (29,30). Thus, collapse could be avoided at temperature exceeded the Tg of the amorphous solid. XRD data of formulations combining MAL and HYP shows that large diffraction peaks of cholesterol and DDA were no longer observed, confirming that they were no longer crystalline. Protection mechanism through vitrification is evident, where vesicles were entrapped and prevented from aggregation and fusion (31). In contrast, XRD data of formulations that do not contain MAL or HYP revealed the existence of imperfect crystals referring to CHOL and DDA. The crystals might be randomly distributed throughout the solid as DSC analysis of this sample did not show any phase separations.
The TEM images showed that the spherical structure and lamellarity of OVA-containing liposomes were maintained after rehydration of the freeze-dried cakes. This indicated that combination of MAL and HYP work synergistically to preserve the liposomes structure. The membranes appear uniform and continuous throughout the liposome. Rehydration might have converted many of the liposomes into asymmetrical, roughly spheroidal particles from 50 to 500 nm in diameter as large, multilamellar liposomes were also observed in the sample. The undamaged liposomes may contain fewer structural defects after freeze-drying, making them more resistant to disruption. This result was also relatively comparable with other published work on liposomes where the structural intactness was observed by TEM (32).
Interestingly, DSC data showed the presence of two endothermic peaks at temperature in the range of 80-120 °C and 200-250 °C as the results of the absence of HYP. We hypothesized that the sample mixture has two populations i.e. MAL poor region (peak at lower temperature) and rich region (peak at higher temperature). The presence of MAL in the poor region and rich region could phase separate during the drying process. The appearance of broad peak indicated that the MAL might be present around the liposomes and not directly interacting with the lipid, which further might be phase separated from the lipid mixture.
Tg of all formulas containing MAL and HYP were in the range of 81 °C to 100 °C and experienced crystallization in temperature range of 140-200 °C. This indicates that the formed matrices were metastable where increased molecular mobility at temperatures above the Tg; enabling them to re-crystallize and may damage biomolecules such as OVA (33,34). However, the developed formula has good potential in terms of physical stability as indicated by the high Tg values of formulas with MAL and HYP. The entrapped protein will not be damaged as the result of prevented crystallization (35). The present study demonstrated that incorporation of cellulose derivative matrix added two benefits. First, collapse is prevented due to increased mass and complexity of the matrix that maintain the formation of solid cakes. Second, as the cake is amorphous, the rupture of liposome membrane is prevented due to absence of phase separation in the mixture.
CONCLUSIONS
The present study exhibited solid structural collapse of the dried OVA-containing liposomes as a result of dropped resistance due to functional failure of oligosaccharide alone during the drying process. Addition of cellulose derivative matrix produces amorphous, porous structure and elegant matrix that prevents the collapse. Thus, the well preserved liposomes as well as OVA are the results of synergistic effect of oligosaccharide and cellulose derivative matrix.
ACKNOWLEDGEMENT
The study was financially supported by Research Grant of Directorate General of Higher Education, Ministry of Research, Technology and Higher Education of the Republic of Indonesia.
REFERENCES
- 1.Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015;10(5):791–808. doi: 10.2217/fmb.15.19. [DOI] [PubMed] [Google Scholar]
- 2.Amorij JP, Kersten GF, Saluja V, Tonnis WF, Hinrichs WL, Slütter B, et al. Towards tailored vaccine delivery: needs, challenges and perspectives. J Control Release. 2012;161(2):363–376. doi: 10.1016/j.jconrel.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Shahiwala A, Vyas TK, Amiji MM. Nanocarriers for systemic and mucosal vaccine delivery. Recent Pat Drug Deliv Formul. 2007;1(1):1–9. doi: 10.2174/187221107779814140. [DOI] [PubMed] [Google Scholar]
- 4.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cellmediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langford A, Bhatnagar B, Walters R, Tchessalov S, Ohtake S. Drying technologies for biopharmaceutical applications: Recent developments and future direction. Dry Technol. 2017;36(6):677–684. [Google Scholar]
- 6.Lovalenti PM, Anderl J, Yee L, Nguyen V, Ghavami B, Ohtake S, et al. Stabilization of live attenuated influenza vaccines by freeze drying, spray drying, and foam drying. Pharm Res. 2016;33(5):1144–1160. doi: 10.1007/s11095-016-1860-1. [DOI] [PubMed] [Google Scholar]
- 7.Guan P, Lu Y, Qi J, Niu M, Lian R, Wu W. Solidification of liposomes by freeze-drying: the importance of incorporating gelatin as interior support on enhanced physical stability. Int J Pharm. 2015;478(2):655–664. doi: 10.1016/j.ijpharm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Pikal MJ. Mechanisms of Protein Stabilization During Freeze-Drying Storage: the Relative Importance of Thermodynamic Stabilization and Glassy State Relaxation Dynamics. In: Rey L, May JC, editors. Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products. 3rd ed. Vol. 213. CRC Press; 2016. pp. 210–244. [Google Scholar]
- 9.Shiraga K, Suzuki T, Kondo N, De Baerdemaeker J, Ogawa Y. Quantitative characterization of hydration state and destructuring effect of monosaccharides and disaccharides on water hydrogen bond network. Carbohyd. Res. 2015;406:46–54. doi: 10.1016/j.carres.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Díaz SB, Ale NM, Altabef AB, Tymczyszyn E, Gomez-Zavaglia A. Interaction of galactooligosaccharides and lactulose with dipalmitoylphosphatidilcholine lipid membranes as determined by infrared spectroscopy. RSC Adv. 2017;7(39):24298–24304. [Google Scholar]
- 11.Soltanizadeh N, Mirmoghtadaie L, Nejati F, Najafabadi LI, Heshmati MK, Jafari M. Solid-state protein-carbohydrate interactions and their application in the food industry. Compr Rev Food Sci Food Saf. 2014;13(5):860–870. [Google Scholar]
- 12.Horn J, Friess W. Detection of collapse and crystallization of saccharide, protein and mannitol formulations by optical fibers in lyophilization. Front Chem. 2018;6:4–12. doi: 10.3389/fchem.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brougham CM, Levingstone TJ, Shen N, Cooney GM, Jockenhoevel S, Flanagan TC, et al. Freezedrying as a novel biofabrication method for achieving a controlled microarchitecture within large, complex natural biomaterial scaffolds. Adv Healthc Mater. 2017;6(21) doi: 10.1002/adhm.201700598. DOI: 10.1002/adhm.201700598. [DOI] [PubMed] [Google Scholar]
- 14.Passot S, Tréléa IC, Marin M, Fonseca F. The Relevance of Thermal Properties for Improving Formulation and Cycle Development: Application to Freeze-Drying of Proteins. In: Rey L, May JC, editors. Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products. 3rd ed. Vol. 213. CRC Press; 2016. p. 136. [Google Scholar]
- 15.Lale SV, Goyal M, Bansal AK. Development of lyophilization cycle and effect of excipients on the stability of catalase during lyophilization. Int J Pharm Investig. 2011;1(4):214–221. doi: 10.4103/2230-973X.93007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiew TY, Cheow WS, Hadinoto K. Preserving the supersaturation generation capability of amorphous drug-polysaccharide nanoparticle complex after freeze drying. Int J Pharm. 2015;484(1-2):115–123. doi: 10.1016/j.ijpharm.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 17.van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf H, Ali AA, Orr N, Tunney MM, McCarthy HO, Kett VL. Novel freeze-dried DDA and TPGS liposomes are suitable for nasal delivery of vaccine. Int J Pharm. 2017;533(1):179–186. doi: 10.1016/j.ijpharm.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Flood A, Estrada M, McAdams D, Ji Y, Chen D. Development of a freeze-dried, heat-stable influenza subunit vaccine formulation. PloS One. 2016;11(11):e0164692. doi: 10.1371/journal.pone.0164692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18(1):24–36. doi: 10.1002/pro.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki K, Hayashi M. Chem Pharm Bull. 1. Vol. 45. Tokyo: 1997. The effects of glucose oligomers (maltodextrins) on freeze-drying liposomes; pp. 165–170. [DOI] [PubMed] [Google Scholar]
- 22.Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8(2) doi: 10.3390/pharmaceutics8020016. pii: E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edueng K, Mahlin D, Larsson P, Bergström CAS. Mechanism-based selection of stabilization strategy for amorphous formulations: Insights into crystallization pathways. J Control Release. 2017;256:193–202. doi: 10.1016/j.jconrel.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harnkarnsujarit N, Charoenrein S, Roos YH. Microstructure formation of maltodextrin and sugar matrices in freeze-dried systems. Carbohydr Polym. 2012;88(2):734–742. [Google Scholar]
- 25.AlHusban F, Perrie Y, Mohammed AR. Formulation of multiparticulate systems as lyophilised orally disintegrating tablets. Eur J Pharm Biopharm. 2011;79(3):627–634. doi: 10.1016/j.ejpb.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Tang XC, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21(2):191–200. doi: 10.1023/b:pham.0000016234.73023.75. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142(3):299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J Pharm Sci. 2010;99(3):1254–1264. doi: 10.1002/jps.21903. [DOI] [PubMed] [Google Scholar]
- 29.Wessman P, Mahlin D, Akhtar S, Rubino S, Leifer K, Kessler V, et al. Impact of matrix properties on the survival of freeze-dried bacteria. J Sci Food Agr. 2011;91(14):2518–2528. doi: 10.1002/jsfa.4343. [DOI] [PubMed] [Google Scholar]
- 30.Pieters S, De Beer T, Kasper JC, Boulpaep D, Waszkiewicz O, Goodarzi M, et al. Near-infrared spectroscopy for in-line monitoring of protein unfolding and its interactions with lyoprotectants during freeze-drying. Anal Chem. 2012;84(2):947–955. doi: 10.1021/ac2022184. [DOI] [PubMed] [Google Scholar]
- 31.Abdelwahed W, Degobert G, Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63(2):87–94. doi: 10.1016/j.ejpb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Ruozi B, Belletti D, Tombesi A, Tosi G, Bondioli L, Forni F, et al. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int J Nanomedicine. 2011;6:557–563. doi: 10.2147/IJN.S14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhugra C, Pikal MJ. Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state. J Pharm Sci. 2008;97(4):1329–1349. doi: 10.1002/jps.21138. [DOI] [PubMed] [Google Scholar]
- 34.Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42. doi: 10.1016/s0169-409x(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 35.Duddu SP, Zhang G, Dal Monte PR. The relationship between protein aggregation and molecular mobility below the glass transition temperature of lyophilized formulations containing a monoclonal antibody. Pharm Res. 1997;14(5):596–600. doi: 10.1023/a:1012196826905. [DOI] [PubMed] [Google Scholar]


