Abstract
The mammalian target of rapamycin (mTOR) signaling plays a critical role in lipid synthesis and immune responses. The T regulatory cells (Treg) as suppressor of T cells, are a subset of T cells that modulate the immune system, maintain tolerance, and prevent autoimmune diseases.. The interleukin (IL) -10 derived from the Treg and T helper (Th) 2 is an anti-inflammatory cytokine in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). Due to the exclusive roles of rapamycin (RAPA) in mTOR inhibition, we evaluated the regulatory effect of the hemp seed oil/evening primrose oil (HSO/EPO) supplement in comparison with RAPA in EAE. EAE was induced by using myelin oligodendrocyte glycoprotein peptide and complete freund’s adjuvant (CFA) in C57BL/6 mice, total mRNA was extracted from local lymph nodes and real-time polymerase chain reaction was used to evaluate the expression level of the rapamycin-insensitive companion of mTOR complex 2 (RICTOR) and IL-10 genes. The expression of IL-10 and RICTOR genes were significantly increased in HSO/EPO group. In contrast with RAPA groups, histological findings have shown that the HSO/EPO treated group remarkably reduced cell infiltration and promoted remyelination. The EPO/HSO has beneficial effects on the repair of myelin, which was confirmed by immunological and histological findings.
Keywords: Autoimmune encephalomyelitis, Demyelination, Immune tolerance, Lymph node, Rapamycin
INTRODUCTION
The mammalian target of rapamycin (mTOR) signaling, a central integrator of nutrient-sensing pathways in all mammalian cells, forms two complexes of mTORC1 and mTORC2 both of which play distinct and complementary roles to generate cell proliferation with the available nutrients, mainly lipids. The mTORC2 is essential for T helper (Th) 2 differentiation, whereas mTORC1 is important for Th1 and Th17 differentiation (1,2). Experimental autoimmune encephalomyelitis (EAE), as an animal model of multiple sclerosis (MS), is induced by immune responses of Th1 cells, whereas the immune responses of Th2 cells produce interleukin (IL)-4 and IL-10 and can prevent and/or reverse EAE (3). Recent reports show the effectiveness of rapamycin (RAPA) as an inhibitor of mTOR signaling in the development of tolerance by the expansion of T regulatory (Treg) cells. Treg cells secrete inhibitory or regulatory cytokines including IL-10 and transforming growth factor (TGF)-β (2). RAPA, also known as sirolimus, is a natural antibiotic displaying antitumor and immunosuppressive activities. It has been generally used in the prevention of clinical allograft rejection and the treatment of some autoimmune diseases (4).
On the other hands, many studies suggest that decreasing dietary intake of omega-6 and omega-3-polyunsaturated fatty acid (ω6-PUFAs/ω3-PUFAs) may be involved in the course of MS (5). Also, ω3-PUFAs-mediated function reduces mTORC1 activity and can increase the maturity and function of Treg cells. The quality of fat, including reduced proportions of ω6/ω3 fatty acids or increased consumption of ω3-PUFAs, as well as administration of RAPA, could mitigate autoimmune diseases and up-regulate Treg cells through the control of mTOR (6). Therefore, because of the favorable content of ω6/ω3 fatty acids in the combination of hemp seed oil (HSO) and evening primrose oil (EPO), it can reduce symptoms of MS. The hemp seed oil/evening primrose oil HSO/EPO reduces proinflammatory cytokines in MS patients (7,8) and may be able to reduce neuro-inflammation and demyelination in the central nerves system (CNS) in an animal model of MS. Since the reduction in antioxidant deficiencies in the course of MS is a result of chronic inflammation accompanied with increased oxidative stress (9), the antioxidant properties of HSO/EPO can reduce the pro-inflammatory cytokines and targets this key mechanism of disease and works like approved treatments (7,8). Also, the beneficial activities of HSO/EPO could be linked to the activation of Th2 and play a role in this signaling pathway activity in the inflammatory autoimmune conditions such as MS (10,11). Therefore, the current study was conducted to investigate the regulatory effects of RAPA, HSO/EPO, and co-administration of the two agents on histological findings and the expression of rapamycin-insensitive companion of mTORC2 (RICTOR) and IL-10 genes in lymph nodes mononuclear cells in of EAE mice.
MATERIALS AND METHODS
Animal
Adult female C57BL/6 mice were purchased from the Pasteur Institute of Iran, the Production and Research Complex (Tehran, I.R. Iran). Mice were immunized at 6-8 weeks of age. All procedures were conducted in accordance with the animal care and the protocol of Urmia University of Medical Science, Urmi, I.R. Iran (Ethics committee approval number: IR.umsu.rec.1396.73).
Experimental autoimmune encephalomyelitis induction
Myelin oligodendrocyte glycoprotein 35-55 (300 μg/mouse, Sigma, USA) and 400 mg Mycobacterium tuberculosis (500 μg/mouse, Sigma-Aldrich, USA) in an emulsion with complete Freund’s adjuvant (CFA, Sigma-Aldrich, USA) dissolved in phosphate buffer saline (PBS, Sigma-Aldrich, USA) were injected subcutaneously into two different flanking sites to each mouse. The mice also received 500 ng pertussis toxin (500 ng/mouse, Sigma-Aldrich, USA) intraperitoneally (i.p.) and intravenously at the same time as the peptide and again 2 days later (12).
Clinical evaluation of experimental autoimmune encephalomyelitis
Mice were observed daily and the severity of the disease was scored using a standard scale by the investigatorsblinded to mouse identity: (0 = no disease; 0.5 = partially limp tail; 1 = paralyzed tail; 2 = back limb paresis; 2.5 = one back limb paralyzed; 3 = both back limbs paralyzed; 3.5 = back limbs paralyzed and weakness in forelimbs; 4 = forelimbs paralyzed and 5 = moribund state (13).
Experimental animal groups
EAE mice were randomly assigned to three groups (EAE/administered) in comparison with two control groups (EAE and naive) and each group contained 6 mice. Group A, EAE mice treated with HSO/EPO (50 λ/mouse) (7) and RAPA (1 mg/kg/50 λ) (2); group B, EAE mice treated with RAPA (1 mg/kg/50 λ) (2); group C, EAE mice treated with HSO/EPO (50 λ/mouse) (7); group D, EAE control mice treated with 1% ethyl alcohol and diluted with distilled water (2); group E, naive control mice treated with 1% ethyl alcohol diluted with distilled water (2). When the clinical signs of EAE started to appear (beginning with active disease), the mice were treated until sacrificed. RAPA was injected daily (i.p.) into groups A and B immediately after the onset of disease symptoms (about 14 days after immunization) and HSO/EPO was administered orally to groups A and C. Based on the reference, the prescribing dose of HSO/EPO was calculated based on the previous study (mL or g/kg of body weight) (7), and given that it was a diet supplement, no specific timing for taking it was considered, but the treatments were performed at the same time in all days.
Rapamycin and hemp seed/evening primrose oil treatment
Virgin oil of hemp seed and evening primrose were extracted from the commercial seeds with a cold pressing standardized method, a mechanical extraction process, at Giah Essence Agro-Industry & Phytopharm Company, Gorgan, Golestan Province, I.R. Iran. The analysis of the extracted oils was determined by gas chromatography (Table 1). RAPA in powder form (Santa Cruz Biotechnology, United States) was dissolved in 1 mL ethyl alcohol (Merck, Germany) and diluted with distilled water. The RAPA solution was kept at 4 °C in the dark according to the manufacturer’s instruction. The control solution contains only 1% ethyl alcohol (Merck, Germany) and diluted with distilled water.
Table 1.
Fatty acid profiles (%) of hemp seed and evening primrose oils.
| Oils | Essential fatty acid | |||
|---|---|---|---|---|
| ALA | GLA | SDA | PUFA | |
| Hemp seed oil | 22 | 7 | 2.5 | 83.5 |
| Evening primrose oil | 0 | 9 | 0 | 84 |
ALA, alpha-linolenic acid; PUFA, polyunsaturated fatty acids (ω6/ω3-PUFAs); SDA, stearidonic acid; GLA, gammalinolenic acid.
Histological assessment
At the end of the experiment, which followed until the 28th day of EAE induction, animals were killed after i.p. administration of ketamine/xylazine (80/10 mg/kg), and samples were taken from the brain tissues to evaluate the infiltration of inflammatory cells and demyelination lesions, as disease indices. Histologically, this form of EAE is characterized by major cerebellar or brainstem involvement. The brain of the mice in each group was enucleated and fixed in a mixture of 10% formaldehyde and deionized water for 24 h, then dehydrated in graded ethanol solutions and fixed in paraffin wax. The fixed tissue was cut into 4-6-μm-thick sections. The tissue was cut into 4-6-μm-thick sections. The sections for histological examination were subjected to routine staining of hematoxylin and eosin (H&E) for inflammatory lesions and to confirm the demyelination of axons, we performed luxol fast blue (LFB) staining, which stains lipoproteins in myelin rendering them a blue appearance when examined under light microscopy (×400) (14). The resulting slides of each areas of the brain were stained with H&E and LFB were graded on a 4-point scale: 0 = not any pathology; 1 = not any tissue damage but minor inflammation; 2 = modest inflammation, primary tissue damage and demyelination; 3 = moderate tissue destruction (demyelination, neuronal loss, tissue damage, cell death, neuronal vacuolation, and neuronophagia); 4 = necrosis (loss of all tissue elements entirely with associated cellular remains). The zone with extreme tissue damage was used to estimate each brain area (14).
Real time-polymerase chain reaction
In order to investigate the expression of mTORC2 and IL-10 mRNA, RT-PCR was performed. Total RNA was extracted from cells lymph node using Kit (Gene All, South Korea) after separation from the mice and the isolated RNA was reverse-transcribed by random hexamer primers and reverse transcriptase (Gene All, South Korea). The extracted RNA purity was assessed by evaluating the ratio of optical density at 260 to 280 nm. Also, RNA integrity was assessed by agarose gel electrophoresis. The cDNA was amplified using the SYBR-green PCR master mix kit (Ampliqon, Denmark) according to the manufacturer’s directions. RT-PCR with genespecific primers for RICTOR, IL-10, and β-actin were performed under following condition: initial denaturation, 95 °C for 51 s; annealing, 60.5 °C for 60 s and β-actin was used as an internal control. The primers sequences are presented in Table 2.
Table 2.
The primers sequences to evaluate expression of RICTOR, IL-10, and β- actin genes in lymph node cells.
| Target gene | Primer sequence | Product size (bp) | |
|---|---|---|---|
| β-actin | Forward | 5´CGTTGACATCCGTAAAGACC 3´ | 285 |
| Reverse | 5´CAGTAACAGTCCGCCTAGAA 3´ | ||
| RICTOR | Forward | 5´GGAGCACACGGATGACAAT 3´ | 153 |
| Reverse | 5´TCTAAGGGGTTGTGGATCGT 3´ | ||
| IL-10 | Forward | 5’CCCTTTGCTATGGTGTCCTT 3´ | 185 |
| Reverse | 5’GCCACAGTTTTCAGGGATGA 3’ |
RICTOR, rapamycin-insensitive companion of mammalian target of rapamycin complex 2; IL-10, interleukin-10, IFN- γ, interferon-gamma.
A melting curve analysis was used to confirm the specificity of the amplification reactions. For calculation relative quantitative, the 2-ΔΔCT formula was used (15,16).
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine statistical analysis and differences between groups. The RT-PCR results of all experiments were repeated in duplicate. Data are presented as the mean ± standard error of mean (SEM). A P-value of less than 0.05 was considered statistically significant.
RESULTS
The influence of rapamycin and hemp seed/evening primrose oil on experimental autoimmune encephalomyelitis course
All EAE mice exhibited chronic disease. Treatment with RAPA + HSO/EPO, RAPA, and HSO/EPO was initiated from the 15th-day post-immunization, and progression of the disease was monitored daily. The treatment of mice with HSO/EPO significantly reduced the severity of EAE and delayed the progression of the disease compared to the RAPA + HSO/EPO and RAPA groups (Fig. 1).
Fig. 1.
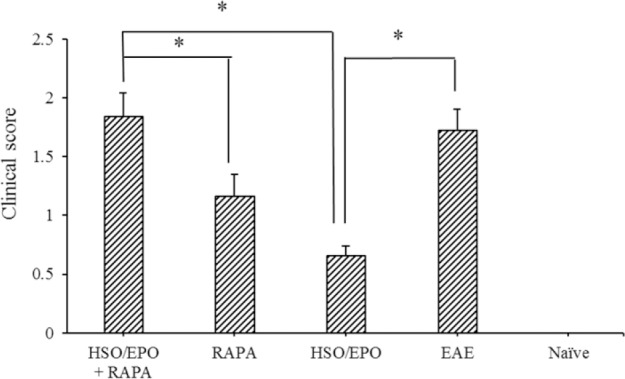
Clinical scores for the severity of experimental autoimmune encephalomyelitis in mice. Data are presented as mean ± SEM. * Shows significant difference between groups, P ≤ 0.05.
The highest scores were found in EAE and RAPA + HSO/EPO groups, and the lowest scores were found primarily in the HSO/EPO group. The analysis of the different groups showed significant reductions in RAPA group compared to RAPA + HSO/EPO group, whereas there was no significant difference between RAPA + HSO/EPO and EAE control groups at 28 days after disease induction.
Histological examination of brain
The histological evaluations showed extensive and severe infiltration of inflammatory cells in sections of the brain of RAPA + HSO/EPO, RAPA, and EAE groups, which indicate the occurrence of immunological functions. In contrast, only a few infiltrations of inflammatory cells in sections of the HSO/EPO group were clearly visible and showed some degree of inflammatory inhibition. As for the HSO/EPO mice, no significant abnormalities were identified, which was characterized by normal brain tissue (Fig. 2).
Fig. 2.
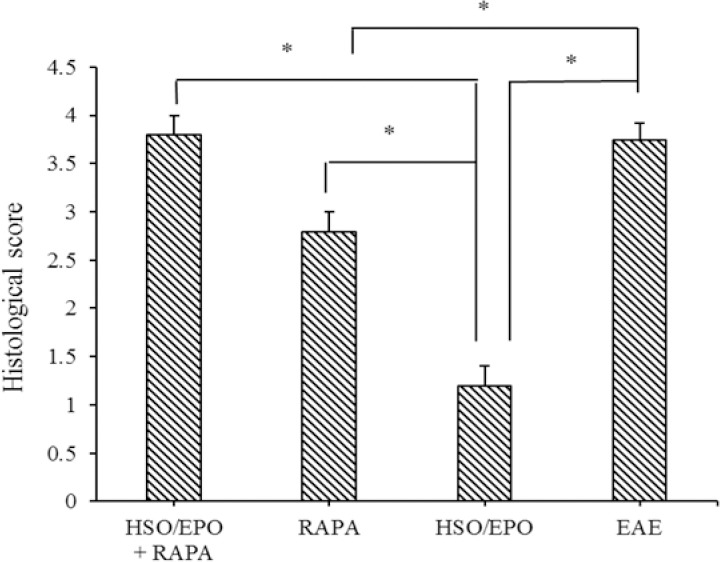
Histological score indicating the degree of inflammation, neuronal and axonal changes and demyelination. Data are presented as mean ± SEM. * Shows significant difference between groups, P ≤ 0.05.
Similarly, pathologic investigations of RAPA + HSO/EPO, RAPA, and EAE groups revealed extensive demyelination and tissue damage in the white matter areas associated with immune cell infiltrations compared to the HSO/EPO group. In contrast to RAPA group, RAPA anti-inflammatory effects were not seen in the RAPA + HSO/EPO group. Also, the neuronal vacuolation and spongiotic degeneration zones reported in the RAPA group, of course with an excessive level in the RAPA + HSO/EPO and EAE groups that was not seen in HSO/EPO group (Fig. 3). Hematoxylin stains nuclear dark-purple, while eosin stains cytoplasm pink to red. LFB stains lipoproteins in myelin and gives them a blue appearance under the microscope.
Fig. 3.
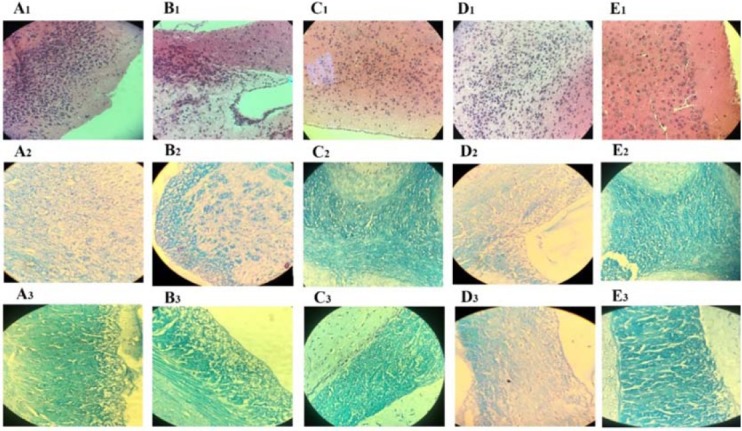
Histological findings of brain sections of mice. Group A, RAPA + HSO/EPO-treated mice show (A1) extensive infiltration of inflammatory cell, neurophagia, (A2) spongy tissue, and (A3) extensive demyelination; group B, RAPA-treated mice show (B1) focal infiltration of inflammatory cells, (B2) spongiotic zones, and (B3) demyelination; group C, HSO/EPO-treated mice show (C1) a few inflammatory cells (C2,3) without spongy lesions and demyelination; group D, control EAE mice show (D1) a great number of inflammatory cells, (D2) extensive degeneration, spongy tissue, and (D3) demyelination; group E, the section of the brain of naive mice exhibiting (E1-3) no clinical signs. The first row was stained with H&E and the second and third rows were stained with LFB. EAE, experimental autoimmune encephalomyelitis; HSO/EPO, hemp seed oil/evening primrose oil; RAPA, rapamycin; H&E, hematoxylin and eosin; LFB, luxol fast blue.
Hemp seed/evening primrose oil treatment mediates activation of mammalian target of rapamycin complex 2
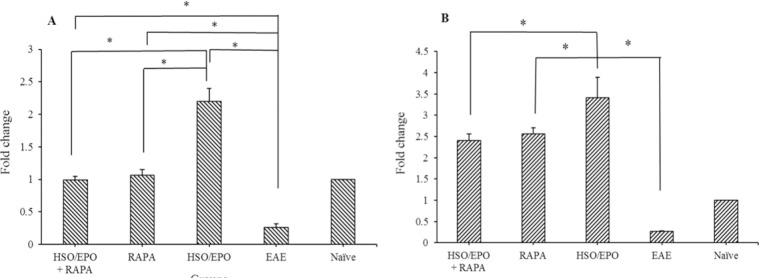
Results of RT-PCR indicated expression of RICTOR and IL-10 mRNA were significantly lower in EAE group compared to other groups, while the expression of RICTOR and IL-10 mRNA were significantly increased in the HSO/EPO group compared to other groups.
Significant increase in the expression of IL-10 in RAPA groups without any difference in the expression of RICTOR gene compared to the naive group indicates the absolute role of RAPA in the development of Treg cells and induction of tolerance (Fig. 4).
Fig. 4.
Fold changes of the mRNA expression of (A) mTORC2 and (B) IL-10 genes. Data presented as mean ± SEM. * Shows significant difference between groups, P ≤ 0.05. mTORC2, mammalian target of rapamycin complex 2; IL-10, intraperitoneal; HSO/EPO, hemp seed oil/evening primrose oil.
DISCUSSION
MS, a chronic demyelinating disease of the CNS, is associated with an activated inflammatory response. Role of antioxidants and long chain ω-6 and ω-3 PUFAs in immunosuppression, cellular function, and remyelination are important subjects of etiologic research in MS (17). An increased risk for MS was found to be associated with high animal fat intake without fish fat (5). Previous studies have shown that daily administration of HSO/EPO has beneficially affected PUFAs of total cell lipids extracted from blood cell phospholipids. Changes in red blood cell membranes were evaluated for multiple fatty acids. There was a significant increase in the PUFAs of red blood cells. The MS patients had significantly higher levels of PUFAs, eicosapentaenoic acid, arachidonic acid, linolenic acid and lower myristic acid and omega-9 fatty acids (MUFAs) (10). HSO has over 80% PUFAs and is a rich source of the two EFAs, linoleic acid and alpha-linolenic acid. The ω6/ω3-PUFA ratio in HSO is normally between 2:1 and 3:1, which is measured optimally for human health (18). From the nourishing point of view, up to 7% gamma linolenic acid (GLA) and 2.5% stearidonic acid is very notable (Table 1) (19). The corporation of both GLA and stearidonic acid in HSO, naturally at a favorable ω6/ω3 ratio of 2:1, allows the enzymatic phase of delta-6-desaturase to be efficiently bypassed (20) so that delta-6-desaturase concentrations significantly decreased after 6 months of HSO/EPO interventions in MS patients (11). The delta-6-desaturase, which is the slowest and rate-limiting step in the metabolic pathway to GLA, catalyzes this reaction. GLA is rapidly elongated to dihomo GLA (DGLA) by the enzyme elongase. DGLA is acetylated and integrated into the cell membrane phospholipids. DGLA competes with arachidonic acid for cyclooxygenase enzymes then the metabolites of DGLA and arachidonic acid are produced by the prostaglandins (PG) E3 and PGE2, respectively. Actions of PGE3 and PGE2 are opposed to each other. The ratio of 2 to 1 ω6 to ω3 fatty acids in HSO/EPO increases the production of anti-inflammatory PGE3 and PGE1 instead of inflammatory PGE2 (20). After an inflammatory stimulus in MS, pro-inflammatory eicosanoids derived from arachidonic acid are PGE2, whereas GLA and DGLA produce anti-inflammatory eicosanoids including PGE1 and PGE3 that inhibit the secretion of interferon-gamma (IFN-γ) (21). Also, an analysis revealed that HSO contains over 3.6-6.7 g of antioxidant/kg oil including terpenes, phytosterols, tocopherols, and polyphenols (19). Variety in antioxidant properties of HSO can lead to scavenging of free radicals and regulate signaling pathways for modulating inflammatory reactions (22). EPO is a rich source of GLA (Table 1), the precursor of antiinflammatory PGE1, which are elements of cell membranes. Because of the effective antiinflammatory activity of GLA, EPO is regularly recommended for the treatment of inflammatory and autoimmune disorders. The earliest results in the use of EPO and colchicine combined therapy in MS patients suggest that it may be of considerable value (23). RAPA is produced by Streptomyces hygroscopic strain, which inhibits differentiation of effector T cells by inhibiting mTORC1 and mitigating Th1 and Th17 differentiation but not Th2 cells. mTORC1 tends to promote Th1 differentiation, while mTORC2 may bias the response to Th2 (2). Inhibition of PI3K/mTOR by RAPA blocked both mTORC1 and mTORC2 kinase signaling. The PI3K/Akt/mTORC2 pathway is an essential cellular signaling involved in a wide range of fundamental physiological functions, including cell growth, proliferation, and metabolism (24,25). Furthermore, the importance of PI3K/Akt/mTORC2 activation pathway for oligodendrocyte survival and myelination was indicated in EAE model (26). Long-term and chronic treatment of RAPA exhibits inhibitory effects on mTORC2 activity (27), while simultaneously increasing the percentage of Treg cells (6). The functional aspects of Treg cells depend on the secretion of IL-10 and TGF-β (2). In contrast, EAE induction resulted in a decrease in the amount of Treg cells in the blood, peripheral lymph node, and spleen associated with the increased production of IFN-γ and a reduction in IL-10 production (3). Previously, IL-10 was termed as a factor produced by Th2 cells, which inhibits the creation of cytokines by Th1 cells that may play a main role in the modulation of EAE (28). But nowadayes, it has been reported that IL-10 may be expressed by almost all T cell types (27,29). Our results showed HSO/EPO can lead to elevation of the expression of IL-10 that may play a main role in the modulation of EAE. The RT-PCR analysis showed the elevation of expression of RICTOR and IL-10 genes in HSO/EPO group as a result of the modulation of the mTORC1 signaling pathway. Specifically, the enhanced gene expression of RICTOR as a critical component of PI3K/Akt/mTORC2 shows that its activation is mediated by the involved protein phosphorylation (25). As it is known, the mTORC2 signaling is a significant factor beneficial for neuronal function following neuronal damage by activating Th2 cells (30). Results clearly demonstrated a downregulation of the RICTOR pathway and IL-10 in EAE group (Fig. 4). It is possibly due to increasing the expression of mTORC1 (or RAPTOR) gene and related inflammatory cytokines compared to other groups. On the contrary, a significant increase in the expression RICTOR and IL-10 genes were observed in HSO/EPO group compared to other groups. Results showed that EAE induction caused a decrease in the expression of Th2 cells immune responses, while HSO/EPO treatment increased RICTOR and IL-10 expression levels in comparison with all groups. According to our previous research (7,8,10) HSO/EPO promoted immune response to Th2 cells by production of IL-4 and improved inflammation and regeneration in the erythrocytes membranes. Concomitant with immunological findings, histological findings showed minimal inflammatory cell infiltration in the HSO/EPO treated animals without clinical signs and demyelination. It clearly indicates stimulation of the PI3K/Akt/mTORC2 pathway to promote remyelination in these animals.
Meanwhile, statistical analysis showed that there was no significant difference in the expression of mTORC2 in RAPA + HSO/EPO and RAPA groups in comparison with the naive group. It is likely that increasing of IL-10 could be influenced by Treg immune cells. RAPA has widely suppressed the expression of pro-inflammatory factors, including IFN-γ and IL-17, and increased regulatory factors such as Treg cells and IL-10, in line with our results.
Despite the increased expression of IL-10 mRNA in RAPA groups, significant improvement was not observed.
We found a deterioration status despite the ability of the immune system to produce IL-10 in the RAPA + HSO/EPO and RAPA groups. It seems that the inhibitory effect of RAPA on mTOR inhibits the remyelination process, especially in the RAPA + HSO/EPO group. Instead of participating in the cell membrane structure, HSO/EPO fatty acids increase fat levels in the bloodstream, causing metabolic disorders, and exacerbating inflammation (31). The inhibition of mTORC1 by RAPA blocks the expression of sterol regulatory element binding protein 1 and target genes of acetyl-CoA carboxylase, delta-6-desaturase, and stearoyl CoA desaturase that shows the role of mTORC1 in fatty acids biosynthesis (32,33,34). Due to impairment of cellular metabolism of fatty acids following administration of RAPA, histopathological findings showed that the infiltration of inflammatory cells, cell immigration, and demyelination in the RAPA treated groups is prominent. Myelin damage and spongiotic degeneration may cause functional disorders in the CNS of RAPA groups as shown in Fig. 3. The inhibitory effects of RAPA were found to limit myelination during treatment, representing myelin loss in RAPA treated groups. Therefore, our findings in accordance with reports from other researchers (35) showed that RAPA could inhibit the actions of remyelination after demyelination in the neurons of EAE mice. Taken together, these findings suggest that HSO/EPO can increase Th2 cellular response and can be used through oral route to improve EAE in mice. This suggests that the effects of dietary PUFA with an ideal balance of ω6/ω3 differentially affect inflammatory functions and the production of cytokines from mononuclear cells through intervention. This indicates that natural oils used in our study may have a therapeutic effect on MS.
CONCLUSION
In conclusion, it was indicated that HSO/EPO was more potent down-regulator of EAE compared to RAPA. The HSO/EPO could exert its effects through the immunomodulation and remyelination activities, which could be a major strategy for MS treatment.
ACKNOWLEDGMENTS
This project was financially supported (Grant NO. 1395-01-00-2835) by the Cellular and Molecular Research Center, Cellular and Molecular Medicine Institute, Urmia University of Medical Sciences, Urmia, I.R. Iran.
REFERENCES
- 1.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23(6):744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Lisi L, Navarra P, Cirocchi R, Sharp A, Stigliano E, Feinstein DL, et al. Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2012;243(1-2):43–51. doi: 10.1016/j.jneuroim.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161(7):3299–3306. [PubMed] [Google Scholar]
- 4.Kelly PA, Gruber SA, Behbod F, Kahan BD. Sirolimus, a new, potent immunosuppressive agent. Pharmacotherapy. 1997;17(6):1148–1156. [PubMed] [Google Scholar]
- 5.Ghadirian P, Jain M, Ducic S, Shatenstein B, Morisset R. Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol. 1998;27(5):845–852. doi: 10.1093/ije/27.5.845. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wang W, Xu J, Le Q. Effect of rapamycin and interleukin-2 on regulatory CD4+CD25+Foxp3+ T cells in mice after allogenic corneal transplantation. Transplant Proc. 2013;45(2):528–537. doi: 10.1016/j.transproceed.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 7.Rezapour-Firouzi S, Arefhosseini SR, Mehdi F, Mehrangiz EM, Baradaran B, Sadeghihokmabad E, et al. Immunomodulatory and therapeutic effects of Hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complement Ther Med. 2013;21(5):473–480. doi: 10.1016/j.ctim.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Rezapour-Firouzi S, Arefhosseini SR, Farhoudi M, Ebrahimi-Mamaghani M, Rashidi MR, Torbati MA, et al. Association of expanded disability status scale and cytokines after intervention with cosupplemented hemp seed, evening primrose oils and hot-natured diet in multiple sclerosis patients♦. Bioimpacts. 2013;3(1):43–47. doi: 10.5681/bi.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besler HT, Comoglu S, Okcu Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci. 2002;5(3):215–220. doi: 10.1080/10284150290029205. [DOI] [PubMed] [Google Scholar]
- 10.Rezapour-Firouzi S. Herbal Oil Supplement With Hot-Nature Diet for Multiple Sclerosis. In: Watson RR, Killgore WDS, editors. Nutrition and Lifestyle in Neurological Autoimmune Diseases. USA: Academic Press; 2017. pp. 229–245. [Google Scholar]
- 11.Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Farhoudi M, Baradaran B, Torbati MA, et al. Erythrocyte membrane fatty acids in multiple sclerosis patients and hot-nature dietary intervention with co-supplemented hemp-seed and evening-primrose oils. Afr J Tradit Complement Altern Med. 2013;10(6):519–527. doi: 10.4314/ajtcam.v10i6.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, Fallah A, Tahoori MT, Amari A, et al. Evaluation of AD-MSC (adipose-derived mesenchymal stem cells) as a vehicle for IFN-beta delivery in experimental autoimmune encephalomyelitis. Clin Immunol. 2016;169:98–106. doi: 10.1016/j.clim.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Benson JM, Campbell KA, Guan Z, Gienapp IE, Stuckman SS, Forsthuber T, et al. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J Clin Invest. 2000;106(8):1031–1038. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangalam AK, Luo N, Luckey D, Papke L, Hubbard A, Wussow A, et al. Absence of IFN-gamma increases brain pathology in experimental autoimmune encephalomyelitissusceptible DRB1*0301. DQ8 HLA transgenic mice through secretion of proinflammatory cytokine IL-17 and induction of pathogenic monocytes/microglia into the central nervous system. J Immunol. 2014;193(10):4859–4870. doi: 10.4049/jimmunol.1302008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado AM, de Souza WM, de Padua M, da Machado ARSR, Figueiredo LT. Development of a one-step SYBR Green I real-time RT-PCR assay for the detection and quantitation of Araraquara and Rio Mamore hantavirus. Viruses. 2013;5(9):2272–2281. doi: 10.3390/v5092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.van Meeteren ME, Teunissen CE, Dijkstra CD, van Tol EA. Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur J Clin Nutr. 2005;59(12):1347–1361. doi: 10.1038/sj.ejcn.1602255. [DOI] [PubMed] [Google Scholar]
- 18.Simopoulos AP, Leaf A, Salem NJr. Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63(3):119–121. doi: 10.1054/plef.2000.0176. [DOI] [PubMed] [Google Scholar]
- 19.Matthaus B, Brühl L. Virgin hemp seed oil: An interesting niche product. Eur J Lipid Sci Technol. 2008;110(7):655–661. [Google Scholar]
- 20.Okuyama H, Kobayashi T, Watanabe S. Dietary fatty acids the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog Lipid Res. 1996;35(4):409–457. doi: 10.1016/s0163-7827(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 21.Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115–121. doi: 10.1097/00075197-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Oomah BD, Busson M, Godfrey DV, Drover JCG. Characteristic of hemp (Cannabis sativa L.) seed oil. Food Chemistry. 2002;76(1):33–43. [Google Scholar]
- 23.Horrobin DF. Multiple sclerosis: the rational basis for treatment with colchicine and evening primrose oil. Med Hypotheses. 1979;5(3):365–378. doi: 10.1016/0306-9877(79)90018-5. [DOI] [PubMed] [Google Scholar]
- 24.Altomare DA, Khaled AR. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr Med Chem. 2012;19(22):3748–3762. doi: 10.2174/092986712801661130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herasa-ndoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J Neurosci. 2014;34(13):4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156(1):5–7. [PubMed] [Google Scholar]
- 28.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444–3451. [PubMed] [Google Scholar]
- 29.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(1):123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlakis M, Goldfarb-Rumyantzev AS. Diabetes after transplantation and sirolimus: what’s the connection? J Am Soc Nephrol. 2008;19(7):1255–1256. doi: 10.1681/ASN.2008050474. [DOI] [PubMed] [Google Scholar]
- 32.Mauvoisin D, Rocque G, Arfa O, Radenne A, Boissier P, Mounier C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J Cell Commun Signal. 2007;1:113–125. doi: 10.1007/s12079-007-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22(15):5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soliman GA. The mammalian target of rapamycin signaling network and gene regulation. Curr Opin Lipidol. 2005;16(3):317–323. doi: 10.1097/01.mol.0000169352.35642.06. [DOI] [PubMed] [Google Scholar]
- 35.Sachs HH, Bercury KK, Popescu DC, Narayanan SP, Macklin WB. A new model of cuprizonemediated demyelination/remyelination. ASN Neuro. 2014;6(5) doi: 10.1177/1759091414551955. pii:1759091414551955. [DOI] [PMC free article] [PubMed] [Google Scholar]