Abstract
Treatment of acute lymphoblastic leukemia (ALL) has been promising in last decades, but side effects still persist and searching for the least toxic agents continue. Pterostilbene (PTE) is a natural compound with several anti-cancer and anti-oxidant properties. Fas, as a member of death inducing family of tumor necrosis factor (TNF) receptors with an intracellular death domain, can initiate the extrinsic apoptosis signaling pathway. Here after the half maximal inhibitory concentration (IC50) determination in cell lines, we searched for PTE effects on Fas, both in mRNA and surface levels in two ALL cell lines, Jurkat and Molt-4. After harvesting cells in optimum situations, MTS assay was used to determine IC50 concentrations. Real-time polymerase chain reaction (RT-PCR) and flow cytometry were performed for Fas mRNA and surface expression variations after exposure to PTE. The findings showed that PTE decreases cell viability with different extent in two ALL cell lines. In addition to inducing apoptosis, it can increase Fas in both gene and cell surface expression in the same concentrations. Pterostilbene as a natural anti-cancer agent can increase Fas expression both in mRNA and surface levels that results in apoptosis signal transduction improvement which sensitizes cells to apoptosis by immune effector cells. As a result, abnormal cells removal would be more efficiently with the minimum side effects on normal cells.
Keywords: Acute lymphoblastic leukemia, Fas, Flowcytometry, Pterostilbene, RT-PCR
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the malignancy of hematopoietic ancestor stem cells that occurs in both children and adults but its prevalence is around 2 to 5 years old (1).The main character of ALL is influx of immature lymphocytes from bone marrow to blood stream and other peripheral organs (2). It comprises 25% of cancers happening before the age of 15 years and 19% among those younger than 20 years (3). Usual treatment methods are chemotherapy and radiotherapy that are not always completely efficient and have many side effects (4). Bone marrow transplantation is another treatment approach, but has only really remarkable outcome in up to 40% in average (5). Today, cancer scientists are looking for natural anti-oxidants which are less harmful. Among several candidates, stilbenes which are naturally found in blueberries and grapes have attracted lots of research interest. The best known stilbenes are resveratrol (RES) and pterostilbene (PTE) (6). Resveratrol (trans-3,5,4 V–trihydroxystilbene), is a phytoalexin in grapes especially red grapes that shows a variety of pharmacological properties like cancer and inflammation prevention, cell cycle arrest and apoptosis induction.
It has three hydroxyl groups which decreases its bioavailability in oral prescription and faces concerns in clinical uses (7). Pterostilbene (trans-3, 5- dimethoxy- 4- hydroxy stilbene) is a natural dimethylated analog of RES that is primarily found in blueberries and proposes anticancer, antiinflammation, anti-oxidant, apoptosis induction, and anti-proliferation features (8). Structural differences of PTE, which make it different from RES are two methoxy and one hydroxyl groups that leads to its better bioavailability (9). Additional to absorption improvement, some studies have demonstrated that PTE may have greater antioxidant and anticancer effects than RES.
All of these resulted in introduction of PTE as a potential anticancer drug in clinical uses. Some researchers have shown that PTE can induce anti-proliferative and apoptosis induction effects on solid tumors such as lung cancer (10) as well as liquid malignancies such as chronic myelogenous leukemia and lymphoblastic leukemia (11). The exact mechanism especially on leukemic cells has not been explained yet.
Apoptosis is a form of programmed cell elimination, which is classified as type I morphological cell death in multicellular organisms and has the main role in embryonic development, proliferation control, and auto reactive lymphocyte removing (12). As an advantage, compared to the other cell death types such as necrosis, apoptotic cell removal occurs with no immune response to apoptotic cell antigens and neighboring tissue damaging (13). According to the signal initiators, there are two main apoptosis pathways: intrinsic and extrinsic. In extrinsic or cytoplasmic way, following apoptotic ligands binding to death receptors from tumor necrosis factor receptor (TNFR) family, such as CD95 (APO-1/Fas) and TRAIL receptors, signals will be activated (14). Today we know that while Fas ligand (FasL) expression is limited to cytotoxic T cells, natural killer cells, and immune privilege tissues, Fas can be found on almost all cells (15). After binding to FasL or mAbs, Fas homo-trimerization occurs and conformation changes make it ready for signaling transduction which proceed to death complex formation and eventually make DNA fragmented and causes cell death (16).
According to the above mechanism, using methods that enhance Fas expression at both gene and surface levels in leukemic cells will improve apoptosis induction and efficient clearance by immune cells from blood. In this study we examined the PTE effects on Fas mRNA level and its surface appearance on Jurkat and Molt-4 lymphoblastic cells.
MATERIALS AND METHODS
Cell culture
Jurkat (C121) and Molt-4 lymphoblastic cell lines were purchased from Pasteur Institute of Iran, I.R. Iran, and were cultured in RPMI 1640 medium (GibcoR, USA) supplemented with 10% fetal bovine serum (FBS),100 U/mL penicillin, and 100 mg/mL streptomycin. Cells were maintained in cell culture flasks under standard cell culture conditions (humidified atmosphere of 5% CO2, in 37 °C incubator) and were passaged every 2-3 days (17). The cells were stained with trypan blue and counted on a hemocytometer to evaluate the number of live and dead neoplastic cells.
In vitro cytotoxicity assay
Cells were seeded at 104 cells/well in 96-well microtiter plates and treated with various concentrations (0, 20, 40, 60, 80, 100, 120, 140, 160, and 180 μM) of PTE for 24, 48, and 72 h to evaluate IC50s in the presence of PTE. Pterostilbene was purchased from Sigma-Aldrich, Germany. Stock solution was prepared in dimethyl sulfoxide (DMSO), stored at -20 °C and diluted with culture medium prior to the experiment. Each test was carried out 3 times. Afterward, the cytotoxic effects of the PTE on cell viability were assessed using MTS assay (17). Solubilized formazan absorbance was read at 490 nm using an ELISA reader (Stat Fax 3200, Awareness Technology, USA).
Real-time polymerase chain reaction
About 2 × 105 cells from each line were treated with different concentrations of PTE in 6-well plates for 24, 48, and 72 h.
Total RNA was extracted using RNX reagent according to the manufacturer’s instructions. The total RNA concentrations were measured by 260/280 nm absorbance ratio using NanoDrop spectrophotometer (Thermo, USA). Complementary DNA (cDNA) was prepared from RNA using a synthesis kit (Takara Bio Inc .Japan) using 1μg total RNA according to the manufacturer’s instructions.
The procedure of cDNA reverse transcription was carried out using Prime Script reagent kit (Takara Bio Inc .Japan). Cycles were performed as heat denaturation 3 min at 60 °C, annealing with oligo (dT) primers for 45 min at 42 °C and reactions were stopped by heat inactivation for 5 min at 90 °C. Later, aliquots of 10 μL of cDNA were amplified by real-time polymerase chain reaction (RT-PCR using SYBR green PCR master mix (Qiagen, Germany) in the presence of specific primers for Fas (18).
The primers were designed using Oligo 6.0 (Molecular Biology Insights, Cascade, CO, USA) and confirmed by the blast (NCBI), Table 1. They were purchased from Eurogentec (Seraing, Belgium). Fas gene expression was detected using Rotor-Gene 3000 (Corbett, Australia) for each mentioned concentration.
Table 1.
GAPDH and Fas primer sequences.
| Gene | Sequences | Product | TM |
|---|---|---|---|
| GAPDH | Forward: 5′ACACCCACTCCTCCACCTTTG3/ | 129 | 60 |
| Reverse: 5′CCACCACCCTGTTGCTGTAG3/ | |||
| Fas | Forward: 5′-TGAAGGACATGGCTTAGAAGTG-3′ | 118 | 60 |
| Reverse: 5′- GGTGCAAGGGTCACAGTGTT-3′ |
The temperature profile for the reaction was an initial denaturation stage of 95 °C at 10 min, then a three-step program was developed for 40 cycles including 95 °C for 15 sec, 61 °C for 25 sec, and 72 °C for 30 sec respectively. A housekeeping gene, GAPDH, was quantified as an endogenous control gene for the normalization of Fas expression.
Flowcytometric analysis
For investigating the effects of PTE on surface Fas expression, 2 × 105 cells from each cell lines were seeded in each well of 6-well plate and after incubation with different concentrations of PTE (0, 20, 40, 60, 80 μM for Jurkat and 0, 120, 140, 160, 180 μM for Molt-4 cells) for 48 h, they were prepared for flowcytometry analysis. For each cell line, the tests were done 3 times. After washing twice with phosphate buffered saline (PBS), 106 cells of each concentration was resuspended in 1 mL ice-cold PBS.
Fluorescein isothiocyanate (FITC)-conjugated F(ab’)2 fragments of Fas antihuman mouse IgG MoAb (ABclonal USA) were used to determine the expression of surface Fas in Jurkat and Molt-4 cells with and without PTE treatment (18).
Statistical analysis
Quantitative data were reported as the mean ± SD for the individual experiments. Data were analyzed on SPSS and graphs were traced with the program GraphPad Prism. Statistical analysis was done using the Student’s t-test and P values below 0.05 were considered statistically significant. RT-PCR data were analyzed by Livak method and IC50 values were calculated with probit analysis.
RESULTS
Effect of pterostilbene on cell viability
As shown in Fig. 1, cell proliferation has been affected by PTE treatment in a dosedependent manner after each treatment period.
Fig. 1.
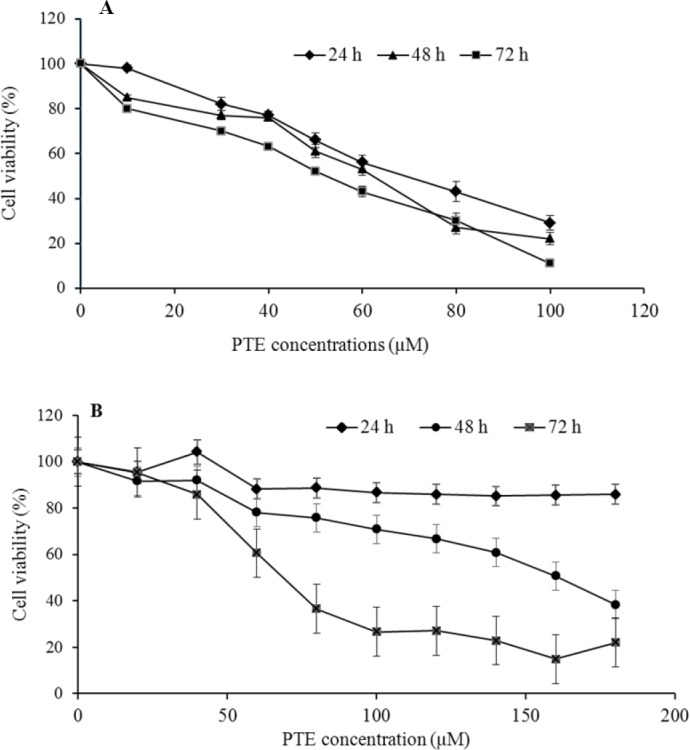
(A), Jurkat and (B), Molt-4 cells were treated with different concentrations of PTE for indicated periods. The experiments have been repeated at least 3 times for all used concentrations. For each indicated time point, cell viability decreased in a dose-dependent manner, but has instability at 24 h for Molt-4 cells.
The IC50 values for Molt-4 cell line were found to be 46.92 ± 2.15, 126.9 ± 3.21, and 63.32 ± 2.45 μM after 24, 48, and 72 h exposure to PTE, respectively. We confirmed our previous data on Jurkat cells and PTE displayed an IC50 of 67.78 ± 3.88, 60.97 ± 3.36, and 52.11 ± 2.50 μM after 24, 48 and 72 h incubation, respectively (17). The results indicate that PTE can potently inhibit proliferation of Molt-4 and Jurkat leukemic cell lines.
Effect of pterostilbene on Fas mRNA expression levels
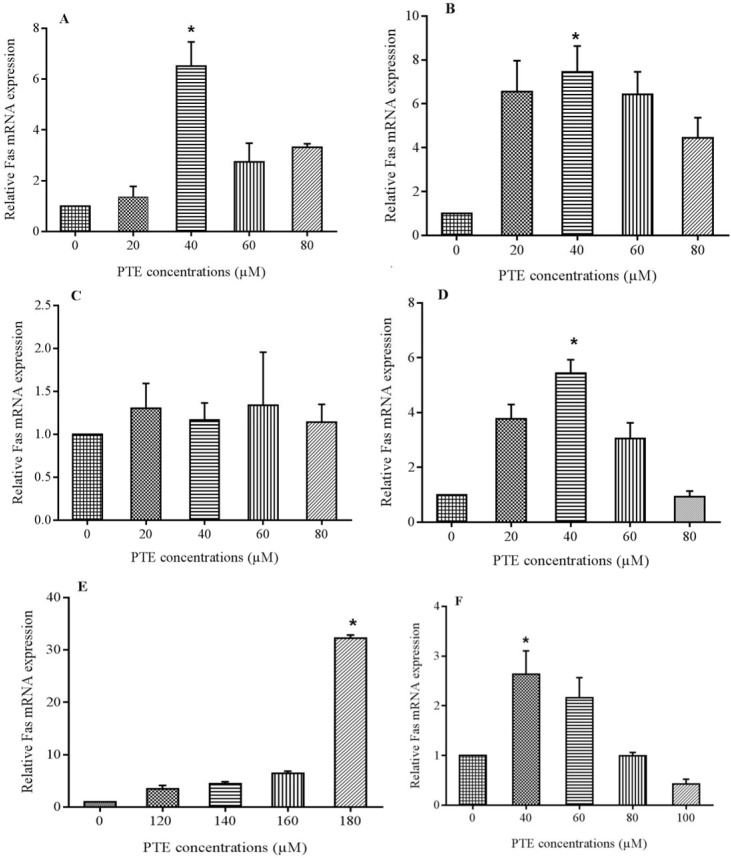
Fas expresses on almost all cell types but its frequency is decreased on the surface of leukemic cells to escape from immune responses. Real-time polymerase chain reaction was used to determine PTE effects on Fas mRNA levels (Fig. 2). The expression of GAPDH and Fas were determined in the same reaction system. Fas was expressed in base line level in cells but treatment with PTE increased its frequency in to a significant level at 40 μM after 24 and 48 h and 80 μM after 72 h treatment in Jurkat cells and 40 μM after 24 and 72 h and 180 μM after 48 h treatment in Molt-4 cells. In Jurkat cells the expression was more than 6 times at 20 and 60 μM and about 8 times at 80 μM concentration of PTE after 48 h treatment. However in Molt-4 cells the expression was more than 20 times of control at 180 μM concentration after 48 h treatment, but the most increase at 24 and 72 h incubation was about 2 and 5 times of control that occurred at 40 μM concentration.
Fig. 2.
Fas mRNA expression after 3 different treatment periods with pterostilbene (PTE). (A-C), demonstrate expression in 24, 48, and 72 h respectively for Jurkat cells. The maximum gene expression increase was observed at 40 μM concentration after 48 h treatment. In Molt-4 cells (E), the maximum gene expression increase was at 180 μM after 48 h treatment but (D and F) in 40 μM concentration after 24 and 72 h incubation time. * shows significant difference with control (P < 0.05).
Effect of pterostilbene on surface Fas expression
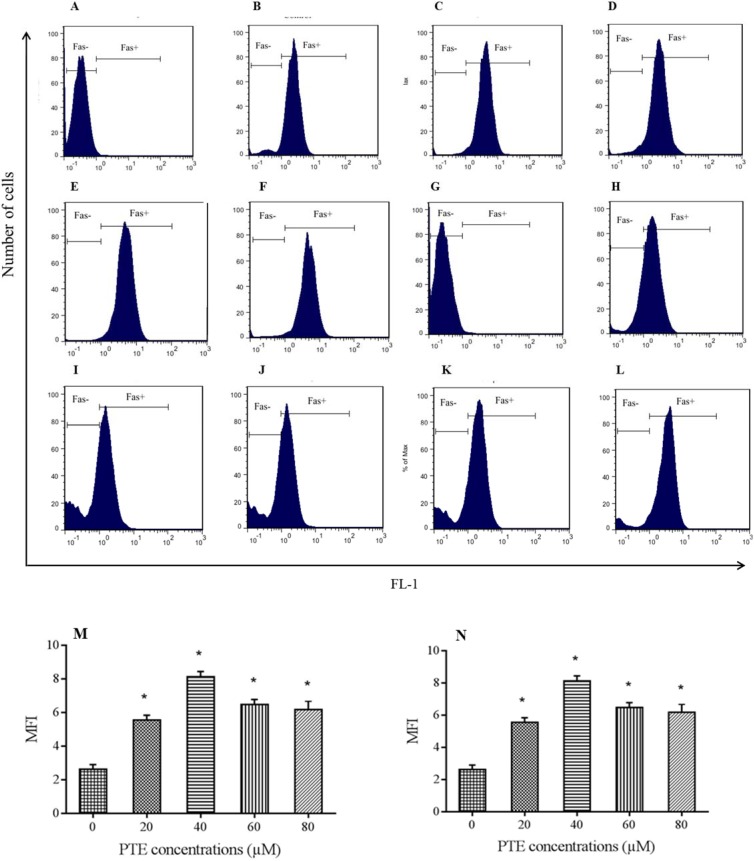
Surface analysis of Fas expression was performed after 48 h incubation with PTE using flow cytometry. As Fas mRNA had the highest expression at 48 h (Fig. 2), this time point was considered for further study of the surface expression. Mean fluorescent intensity of stained cells was compared to that of the control. As shown in Fig. 3A-3F, in Jurkat cells the highest expression was observed at 40 μM which declined again with increasing the PET concentration, however, in Molt-4 cells all concentrations of PTE increased surface Fas expression compared to control with the highest increase at 180 μM concentration (Fig. 3G-3L).
Fig. 3.
Flowcytometric analysis of surface Fas expression. Cells treated for 48 h with pterostilbene (PTE) or left untreated and then stained with anti-CD95 (Fas) monoclonal antibody according to manufacturer instruction. Parts A-F are examples of flow cytometric analyasis of treated jurkat cells with 0, 20 ,40, 60 and 80 μM concentration of PTE respectively and graph M compares mean fluorescent intensity (MFI) of Fas expression in the presence of above concentrations. All used concentrations increased surface Fas expression with the maximum increase at 40 μM concentration (more than 2 times of control). Parts G-L are examples of flow cytometric analyasis of treated Molt-4 cells with 0, 120 ,140, 160 and 180 μM concentration of PTE respectively and graph N compares MFI of Fas expression in the presence of above concentrations. Treatment with PTE increased the levels of Fas expression in Molt-4 with the maximum increase in 180 μM concentration. * shows significant difference compared to control group (P < 0.05).
DISCUSSION
A usual treatment method for ALL like other cancers is chemo and radiotherapy. Although treatment procedures of ALL have promoted over the last four decades and caused survival of almost 80%, the main issue is reducing treatment-related side effects, which is observed in more than two thirds of survivors (19). Side effects that can affect patients life quality even after complete recovery of disease as the main limitation of usual methods from one side and cancer rate growth worldwide from another side, have encouraged searching for new safe and more efficient treatments. Resistance to apoptosis is the main mechanism by which cancer cells maintain in body fluids and tissues. Today, herbal products with apoptosis induction ability have become a great point of view in anti-cancer agent development. Pterostilbene can be viewed as a well-known therapeutic polyphenol and despite the fact that its principal mechanism is not still completely clear, it can interfere in multiple signaling pathways that are necessary for cancer development. In this study, we first focused on PTE IC50s after three time periods that induces cell death in lymphoblast cell lines. Values of 72- h IC50 was lower than that of 48-h ones, as it has been reported for other plants-derived extracts on leukemia cells (20). Remsberg et al. introduced PTE as a significant antiinflammatory, antioxidant, antitumor, and analgesic factor. They demonstrated that 65 μM concentration of PTE for 24 h could inhibit the cell growth of MCF-7 breast cancer up to 50% (21). This finding is similar to our data for jurkat but not for Molt-4 cells. Suppression of cell proliferation and G0/G1 cell cycle arrest has been reported in myeloid cell lines upon treatment with PTE. The induction of cell apoptosis has been found through mithochondrial dependent pathway (22).
Tolomeo et al. reported that this dimethylated stilbene is an apoptotic-inducing operative in various leukemia cells, which are resistant to apoptosis induced by several anticancer agents and PTE toxicity on normal blood progenitor cells was less than on leukemia and lymphoma cells (23). Pterostilbene exhibited the highest bioavailability of about 80% among stilbenoids. Two methoxy groups in the PTE structure make it more lipophilic and thus more bioavailable than RES (8). Pterostilbene is also known to be more metabolically stable because of its free hydroxyl group (24). As PTE has higher lipophilicity, it causes more powerful inhibitory effect on some cancer cells than RES (25). We found that PTE IC50 on Molt-4 cells was 126.9 μM after 48 h. Considering 48 h IC50, our results also indicate that Molt-4 cells are more resistant to PTE compared to Jurkat cells. This type of resistance for Molt-4 cells has also been demonstrated in a study that resulted in unefficient effect of algal extract on Molt-4 cells compared to Jurkat cells (26). As we know cancer treatment and its complete uproot relies on malignant cell removal from body which means their death without any harm for normal cells, so understanding different kinds of cell death in detail, will yield a molecular basis for new treatment strategies targeting these mechanisms in all forms of cancer including resistant types (27). Pan et al. showed that in human gastric adenocarcinoma cell lines, PTE activates caspase cascade via both mitochondrial and Fas/FasL pathways. Growth inhibitory effects and apoptosis induction on gastric cancer cells arrest them in the G0/G1 phase (28). Treatment of HL-60 cells by PTE at IC90 resulted in G0/G1 cell cycle arrest (29). Roslie et al. have reported phosphatidyl serine externalization and loss of mitochondrial integrity in K562 leukemia cell line. They also found early caspase 9 activation in these cells (30). These data confirmed the variation of PTE effects depending on the cancer types and sites and improved it as a useful drug alone or along with other cancer therapy methods. Furthermore, administration of PTE at a dose of 125 mg twice daily for 6-8 weeks was found to be safe and did not induce any significant adverse reactions (31). As reduction in Fas expression has been known as an immune escape mechanism by malignant cells, restoration and improvement of Fas expression has been studied in several researches. We have previously reported induction of apoptosis and enhancement of caspase-3 after PTE treatment in lymphoblastic leukemia cell line (under review). Here we also investigated PTE effects on Fas expression both at gene and surface levels that resulted in introducing the appropriate concentrations of PTE for Fas expression on both cell lines. As the gene expression may not necessarily result in protein expression, the surface Fas was also studied. Such effects have already been reported by other plants-derived component in lymphoblastic leukemia cells (32). Pterostilbene also induces apoptosis through the caspase-independent pathway in both sensitive and chemo-resistant lymphoid leukemia cell lines (33). Gulbins showed that cytotoxic drugs enhance Fas gene transcription as well as surface expression improvement. Also it has been shown that anticancer agents increase Fas receptor by enhancing Fas gene expression in tumor cells (34). Our data demonstrated that the Fas gene and surface protein expression have the same pattern in both cell lines.
CONCLUSION
In conclusion, we focused on PTE effects on Fas as one of the fundamental apoptosis signaling initiators, expressed on the surface of various mammalian cell types and essential for extrinsic pathway activation. For the first time, we also found that PTE can induce Fas surface and mRNA expression in a same concentration in both cell lines, which can be considered an advantage in PTE administration and is necessary before PTE can be considered for cancer treatment in vivo. Our findings also suggest that PTE-induced apoptosis in lymphoblastic cells may be dependent on extrinsic pathway. More detailed experiments at the molecular level and downstream signaling pathways can help to more comprehensive understanding of the involved mechanisms.
ACKNOWLEDGEMENT
This research was technically supported by Medical Plant Research Center of Shahrekord University of medical sciences. We wish to thank Deputy of Research and Technology, Shahrekord University of Medical Science for financial supports (Grant No. 2027).
REFERENCES
- 1.Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351(6):533–542. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Gaynon PS, Angiolillo AL, Carroll WL. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children’s Oncology Group Report. Leukemia. 2010;24(2):285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorns C, Bastian B, Pinkel D, Roydasgupta R, Fridlyand J, Merz H, et al. Chromosomal aberrations in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma unspecified: A matrix-based CGH approach. Genes Chromosomes Cancer. 2007;46(1):37–44. doi: 10.1002/gcc.20386. [DOI] [PubMed] [Google Scholar]
- 5.Neale GA, Coustan-Smith E, Pan Q, Chen X, Gruhn B, Stow P, et al. Tandem application of flow cytometry and polymerase chain reaction for comprehensive detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 1999;13(8):1221–1226. doi: 10.1038/sj.leu.2401459. [DOI] [PubMed] [Google Scholar]
- 6.McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173(2):e53–e61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 7.McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:575482. doi: 10.1155/2013/575482. DOI:10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of RES and its dimethylether analog, pterostilbene, in rats. Cancer chemother pharmacol. 2011;68(3):593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remsberg CM, Yáñez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother Res. 2008;22(2):169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Ding L, Wang X, Zhang J, Han W, Feng L, et al. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am J Transl Res. 2012;4(1):44–51. [PMC free article] [PubMed] [Google Scholar]
- 11.Seidlecka K, Jozwik A, Kaszubowska L, Kowalczyk A, Boguslawski W. Pterostilbene induces cell cycle arrest and apoptosis in Molt-4 human leukemia cells. Folia Histochem Cytobiol. 2012;50(4):574–580. doi: 10.5603/20257. [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14(7):1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp cell res. 2000;256(1):58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 15.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 16.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288(5475):2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 17.Rahimnejad T, Beshkar P, Shirzad H, Rafieian-kopaie M, Safdari V, Asgarian N, et al. Effect of pterostilbene on cellular proliferation inhibition and induction of apoptosis in lymphoblastic leukemia cell line. J Babol Univ Med Sci. 2014;16(12):32–38. [Google Scholar]
- 18.Sourani Z, Shirzad H, Shirzad M, Pourgheysari B. Interaction between gallic acid and asparaginase to potentiate anti-proliferative effect on lymphoblastic leukemia cell line. Biomed Pharmacother. 2017;96:1045–1054. doi: 10.1016/j.biopha.2017.11.122. [DOI] [PubMed] [Google Scholar]
- 19.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. New England Journal of Medicine. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haji Abbasi Tabrizi F, Irian S, Amanzadeh A, Heidarnejad F, Gudarzi H, Salimi M. Antiproliferative activity of Fumaria vaillantii extracts on different cancer cell lines. Res Pharm Sci. 2016;11(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- 21.Chakraborty A, Bodipati N, Demonacos MK, Peddinti R, Ghosh K, Roy P. Long term induction by pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Mol Cell Endocrinol. 2012;355(1):25–40. doi: 10.1016/j.mce.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao PC, Chou YE, Tan P, Lee WJ, Yang SF, Chow JM, et al. Pterostilbene simultaneously induced G0/G1-phase arrest and MAPK-mediated mitochondrial-derived apoptosis in human acute myeloid leukemia cell lines. PLoS One. 2014;9(8):e105342,1–12. doi: 10.1371/journal.pone.0105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, et al. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosisinducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Bio. 2005;37(8):1709–1726. doi: 10.1016/j.biocel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Dellinger RW, Garcia AM, Meyskens FL. Differences in the glucuronidation of resveratrol and pterostilbene: altered enzyme specificity and potential gender differences. Drug Metab Pharmacokinet. 2014;29(2):112–119. doi: 10.2133/dmpk.dmpk-13-rg-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nutakul W, Sobers HS, Qiu P, Dong P, Decker EA, McClements DJ, et al. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: a side-by-side comparison. J Agric Food Chem. 2011;59(20):10964–10970. doi: 10.1021/jf202846b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zandi K, Tajbakhsh S, Nabipour I, Rastian Z, Yousefi F, Sharafian S, et al. In vitro antitumor activity of Gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. Afr J Biotechnol. 2010;9(40):6787–6790. [Google Scholar]
- 27.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–4711. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 28.Pan MH, Chang YH, Badmaev V, Nagabhushanam K, Ho CT. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J Agric Food Chem. 2007;55(19):7777–7785. doi: 10.1021/jf071520h. [DOI] [PubMed] [Google Scholar]
- 29.Siedlecka-Kroplewska K, Jozwik A, Boguslawski W, Wozniak M, Zauszkiewicz-Pawlak A, Spodnik JH, et al. Pterostilbene induces accumulation of autophagic vacuoles followed by cell death in HL60 human leukemia cells. J Physiol Pharmacol. 2013;64(5):545–556. [PubMed] [Google Scholar]
- 30.Roslie H, Chan KM, Rajab NF, Velu SS, Kadir SA, Bunyamin I, et al. 3,5-dibenzyloxy-4’-hydroxystilbene induces early caspase-9 activation during apoptosis in human K562 chronic myelogenous leukemia cells. J Toxicol Sci. 2012;37(1):13–21. doi: 10.2131/jts.37.13. [DOI] [PubMed] [Google Scholar]
- 31.Riche DM, McEwen CL, Riche KD, Sherman JJ, Wofford MR, Deschamp D, et al. Analysis of safety from a human clinical trial with pterostilbene. J Toxicol. 2013;2013 doi: 10.1155/2013/463595. Article ID: 463595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghasemi-Pirbaluti M, Pourgheysari B, Shirzad H, Sourani Z, Beshkar P. The inhibitory effect of Epigallocatechin gallate on the viability of T lymphoblastic leukemia cells is associated with increase of caspase-3 level and Fas expression. Indian J Hematol Blood Transfus. 2018;34(2):253–260. doi: 10.1007/s12288-017-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahbub AA, Le Maitre CL, Haywood-Small SL, McDougall GJ, Cross NA, Jordan-Mahy N. Differential effects of polyphenols on proliferation and apoptosis in human myeloid and lymphoid leukemia cell lines. Anticancer Agents Med Chem. 2013;13(10):1601–1613. doi: 10.2174/18715206113139990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, et al. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995;2(4):341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]