Abstract
Nitrosamines are well-known carcinogenic agents. Humans are exposed to nitrosamines in various ways, the most important of which is the diet. Pentoxifylline is a xanthine derivative, which is used as a drug that inhibits inflammatory factors, reduces blood viscosity, improves peripheral blood flow, and increases oxygenation of tissue. This study was designed to evaluate the effects of pentoxifylline against damage induced by nitrosamine to the kidneys of rats. In this study, 48 male rats were randomly assigned to 8 groups: control normal group and nitrosamine control treated group (40 mg/kg); pentoxifylline groups (25, 50, 100 mg/kg) and nitrosamine + pentoxifylline treated groups (25, 50, 100 mg/kg). Treatments were administered either intraperitoneally (nitrosamine) or orally (pentoxifylline) on a daily basis for 28 days. The normalized kidney weight, glomeruli characteristics, thiobarbituric acid reactive species, antioxidant capacity, kidney function indicators, and serum nitrite oxide levels were investigated. Nitrosamine administration increased kidney malondialdehyde (MDA) level, kidney weight, blood urea nitrogen (BUN), creatinine, and nitrite oxide levels and decreased significantly glomeruli number and tissue ferric reducing/antioxidant power (FRAP) level compared to the control normal group (P < 0.05). The pentoxifylline and pentoxifylline + nitrosamine treatments reduced BUN, kidney MDA level, creatinine, glomerular diameter, and nitrite oxide levels significantly at all doses and increased the glomeruli number, kidney weight, and tissue FRAP level compared to the nitrosamine control group (P < 0.05). It seems that pentoxifylline administration improved kidney injury induced by nitrosamine in rats.
Keywords: Kidney, Nitrosamine, Pentoxifylline
INTRODUCTION
Nitrosamines are specific carcinogenic compounds with high capability and function (1). Most of the injuries caused by nitrosamines are due to the nitrites and secondary amines in their compounds (2). Nitrite can also be converted to nitrosamine in the body and in acidic conditions of the stomach (3). Moreover, nitrosamines exist in foods containing nitrite (naturally or as a preservative or from the conversion of nitrate to nitrite by bacteria) and amines (4). Nitrosamine induces oxidative stress, DNA damage, lipid peroxidation, and the formation of excess protein in the body by generating reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (5).
The kidney is an organ that is highly vulnerable to damage caused by ROS, probably due to the abundance of polyunsaturated fatty acids in the composition of renal lipids (6). Pentoxifylline is a low-cost drug with antitumor necrosis factor α (TNF-α) properties (7). This drug is used in the treatment of many diseases such as cardiovascular disorders, diabetic nephropathy, alcoholic hepatitis, and venous leg ulcer (8). Pentoxifylline is a synthetic xanthine derivative with the chemical name 1-(5-oxohexyl)-3, 7-dimethylxanthine, which inhibits the effects of xanthine oxidase (9).
The body has a cellular defense system against ROS. This system consists of antioxidant enzymes and non-enzymatic low-weight molecular antioxidant compounds that protect the macromolecules and biological membranes against the oxidation by inhibiting reactive oxygen production (10). However, the body system is not fully capable of preventing damage caused by free radicals especially in acute conditions of the disease. Therefore, using antioxidant substances would reduce the damage and prevent the disease caused by free radicals (11). Antioxidants inhibit oxidation and protect the cells against the harmful effects of environmental factors (12). Pentoxifylline, as a noncompetitive inhibitor of phosphodiesterases, increases cyclic adenosine monophosphate (cAMP), activates protein kinase A, inhibits TNF and consequently reduces the production of leukotrienes and enhances innate immunity while reducing inflammation (13). Since pentoxifylline has diverse biochemical and antioxidant properties, it is capable of improving capillary blood flow and tissue oxygenation (14). The antioxidant effects of pentoxifylline on the neutrophils in response to superoxide are the result of NADPH oxidase (15). High level of nitrosamine inputs to the human body from foodstuffs and its damaging effects from one side and antioxidant effects of pentoxifylline from another side, the present study aimed to evaluate the effect of pentoxifylline on kidney tissue following damages induced by nitrosamine in male rats.
MATERIALS AND METHODS
Animals
To conduct this experimental study, 48 male Wistar rats (220-250 g) were purchased from the Pasteur Institute of Iran (Tehran, I.R. Iran) and transferred to the animal house of the school of medicine at Karmanshah University of Medical Sciences. During the study, the animals were kept under standard conditions for 12/12-h light/dark and 22 ± 2 °C in special cages and on a straw bed. Water and food were freely available to the animals. Animals were fed with standard pelletized food and treated municipal water. All investigations were conformed to the ethical principles of animal research and were approved by the Ethics Committee of Karmanshah University of Medical Sciences (No. 1396.424) (16).
Preparation of chemicals, drugs, and measuring kits
Nitrosamines and pentoxifylline were bought from Merck (Germany). Ether, Formalin, FRAP, sodium acetate, ferric chloride, Iron sulfate, hematoxylin-eosin, and zinc sulfate powder were purchased from Sigma (USA). Commercial kits were obtained from Pars Azmoon (I.R. Iran). All other buffer additives and solvents were obtained from Merck (Germany). Pentoxifylline was dissolved in distilled water and diluted by normal saline (0.9%) to produce a stock solution from which different doses were prepared. The potency of the drug was confirmed by the manufacturer just prior to the initiation of this study. The stock solution was passed through a 0.45-mm pore size filter (Lida Manufacturing, Kenosha, Wis. USA). The drug was further diluted to the desired concentrations with normal saline. Nitrosamines, 200 μL from 1 g/mL stock solution in normal saline, was added to 19.8 mL of PBS to prepare 10 mg/mL nitrosamines solution for the injection.
Study groups and treatment of animals
Rats were randomly divided into 8 groups of 6 animals each. Normal control group received normal saline (orally or as injection); Nitrosamine control group received 40 mg/kg a single dose of nitrosamine intraperitoneally (17); Pentoxifylline groups received 25, 50, and 100 mg/kg of pentoxifylline orally for 28 consecutive days (18); Nitrosamine + pentoxifylline groups received a single dose of 40 mg/kg nitrosamine in order to induce kidney damage followed by 25, 50 and 100 mg/kg of pentoxifylline orally for 28 days.
Dissection and sampling
At the end of the treatment period, all rats were deeply anesthetized with ether. Blood was collected from the right ventricle of the heart, serum separated and stored at -80 °C for measurement of the biochemical parameters, nitrite oxide, and total antioxidant levels. The animals were then sacrificed. The kidneys were removed and was maintained in 10% formalin solution for histological and morphometric investigations (16).
Ferric reducing/antioxidant power and malondialdehyde assays
In order to assess oxidative stress, the thiobarbituric acid reactive species were measured using MDA as the last product of lipid peroxidation in kidney tissue by performing colorimetric analysis. The antioxidant capacity of the kidney was measured using FRAP assay. The FRAP substance was consisted of 30 mL of acetate buffer (Sigma, USA) and 1.5 mL ferric chloride (Sigma, USA). Sequential concentrations of ferric sulfate (Sigma, USA) were used as an external standard (19).
Normalized kidney weight
In order to calculate the normalized kidney weight, the body weight of the animals was first determined at the end of the study. After the dissection of animals, the kidneys were removed and weighed on a microbalance with sensitivity up to 0.001 mg (Precisa 125A, Switzerland) and the average weights of the kidneys were calculated and recorded. The normalized kidney weight was obtained by dividing the total left and right kidneys weight into the body weight of the animal (20).
Assessment of renal function
The serum samples were kept in a freezer at -20 °C. Serum and urine creatinine were measured photometrically without removing protein by Humastar 600 analyzer and commercial kits (Pars Azmoon, Tehran, I.R. Iran) (coefficients of variation = 2.38%) and using Jaffe method. Serum urea nitrogen was measured by enzymatic UV method using Humastar 600 analyzer and laboratory kits (coefficients of variation = 3.3%) (16).
Preparation of tissues and measuring the number and diameter of renal glomeruli
The kidney was divided into two equal parts from the middle by a cross-sectional cut after fixing the samples with 10% formalin solution and washing.
The process of tissue preparation was based on the conventional histology method. Serial sections were prepared by microtome (5 μm) from the tissues after molding of each sample. Five sections were selected from each sample for analysis in order to avoid cell recounting. Following hematoxylin-eosin staining and preparing microscopic slides, three fields of view were selected from each slide for analysis and then were subjected to analysis using an Olympus BX- 51T- 32E01 research microscope connected to a DP12 camera with 3.34-million pixel resolution and Olysia Bio software (Olympus Optical, Tokyo, Japan). For each glomerulus, the total glomerulus area was measured (the distance between the basal membranes of the Bowman capsule to the corresponding point).
The outline of each glomerulus was measured after taking an image with a 40× objective. The longest and shortest axes were measured in the drawing of each glomerulus in order to estimate the mean diameter (mean axis). Twenty samples of each structure were investigated from each animal; totally, 120 structures per group (16).
Nitrite oxide measurement
Griess technique uses zinc sulfate powder to eliminate the serum protein of the samples. Accordingly, zinc sulfate powder (6 mg) was mixed with serum samples (400 μL), and vortexed for 1 min. The samples were centrifuged at 4 °C for 10 min at 12,000 rpm and supernatant was used to measure the nitrite oxide (11).
Statistical analysis
Kolmogorov-Smirnov test was first conducted to confirm the data compliance of the normal distribution. One-way analysis of variance (one-way ANOVA) was used for statistical analysis and Tukey post hoc test was used to determine the difference between the groups. SPSS Software (Ver.16) was used for data analysis and the results were expressed as mean ± standard error. P values < 0.05 was considered significant.
RESULTS
Ferric reducing/antioxidant power and malondialdehyde levels
The results of the oxidative stress examinations showed that the kidney MDA levels increased significantly in the nitrosamine control group compared to the normal control group (P < 0.05). The kidney MDA levels decreased significantly in all pentoxifylline + nitrosamine groups compared to the nitrosamine control group (P < 0.05). Similarly, nitrosamine decreased significantly the renal tissue FRAP levels in the nitrosamine control group compared to the normal control group (P < 0.05). Administration of pentoxifylline significantly increased the FRAP level in the kidney tissue in all pentoxifylline + nitrosamine groups compared to the nitrosamine control group (P < 0.05). Treatment with pentoxifylline in all groups had no significant effect on the renal tissue FRAPs and kidney MDA levels compared to the normal control group (P > 0.05) (Fig. 1).
Fig. 1.
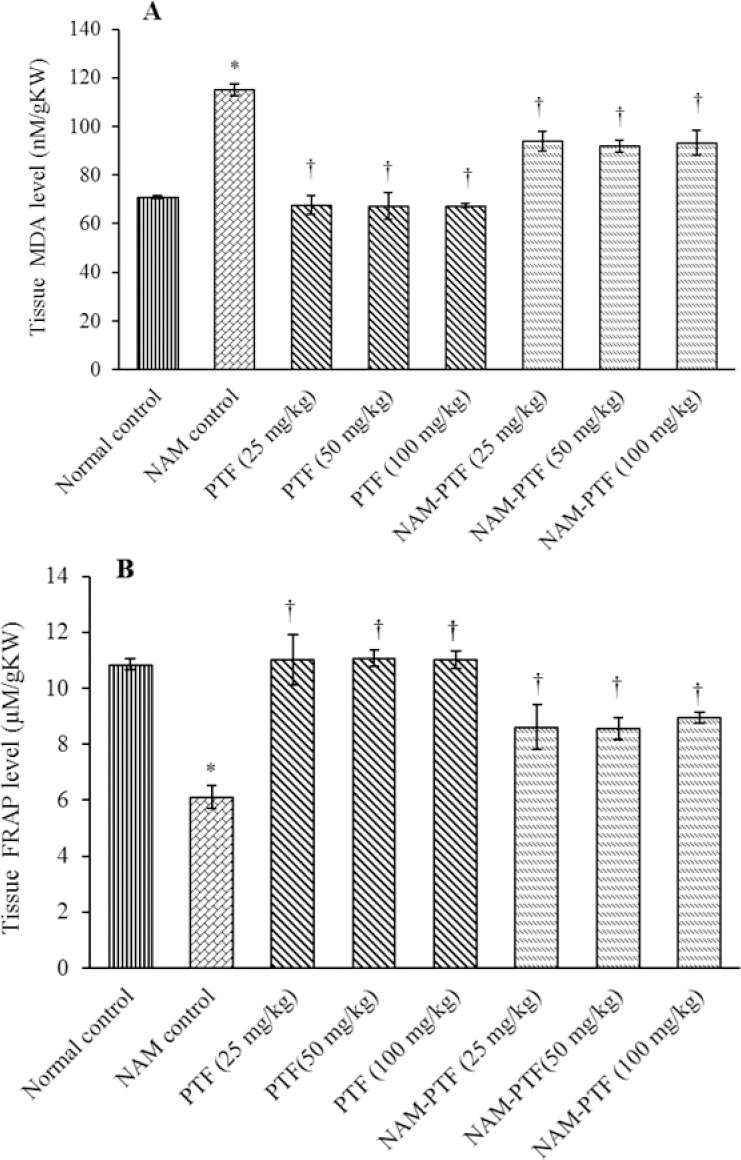
Comparison of nitrosamine, saline, and pentoxifylline groups of (A) kidney MDA level, (B) tissue FRAP level. *P < 0.05 compared to the control normal group. †P < 0.05 compared to the nitrosamine control group. MDA, malondialdehyde; FRAP, ferric reducing/antioxidant power; NAM, nitrosamine; PTF, pentoxifylline.
Normalized kidney weight
Pentoxifylline improved the normalized kidney weight in animals treated with all doses compared to the nitrosamine control group (P < 0.05). The normalized kidney weight was not significant in pentoxifylline-treated animals in all doses compared to the normal control group (P > 0.05). The normalized kidney weight increased significantly in animals that were treated with pentoxifylline and pentoxifylline + nitrosamine at all doses compared to the nitrosamine control group (P < 0.05). Moreover, the normalized kidney weight was significantly decreased following administration of the effective dose of nitrosamine compared to the normal control group (P < 0.05) (Fig. 2).
Fig. 2.
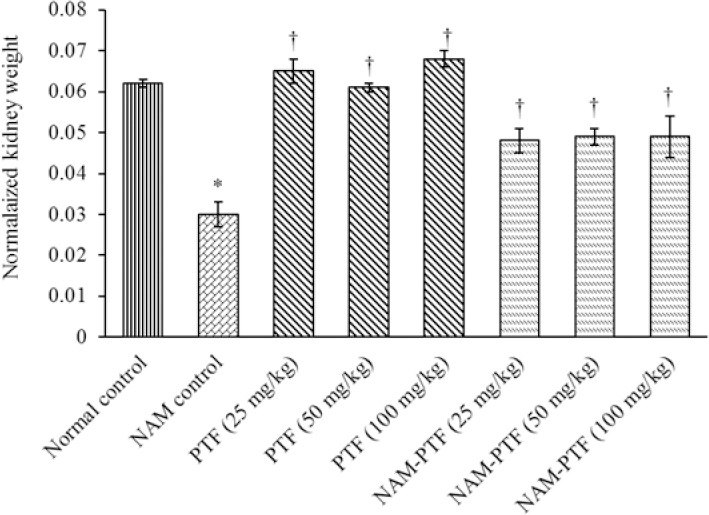
Effect of nitrosamine, pentoxifylline, and nitrosamine + pentoxifylline on normalized kidney weight. *P < 0.05 compared to the normal control group . †P < 0.05 compared to the nitrosamine control group. NAM, nitrosamine; PTF, pentoxifylline.
Renal function
The mean plasma BUN and creatinine concentration were increased significantly following nitrosamine administration as compared to the normal control group (P < 0.05). The mean plasma BUN and creatinine concentration did not differ significantly in all pentoxifylline-treated groups as compared to the normal control group (P > 0.05). The mean plasma BUN and creatinine concentration decreased significantly in all pentoxifylline and pentoxifylline + nitrosamine groups compared to the nitrosamine control group (P < 0.05) (Fig. 3).
Fig. 3.
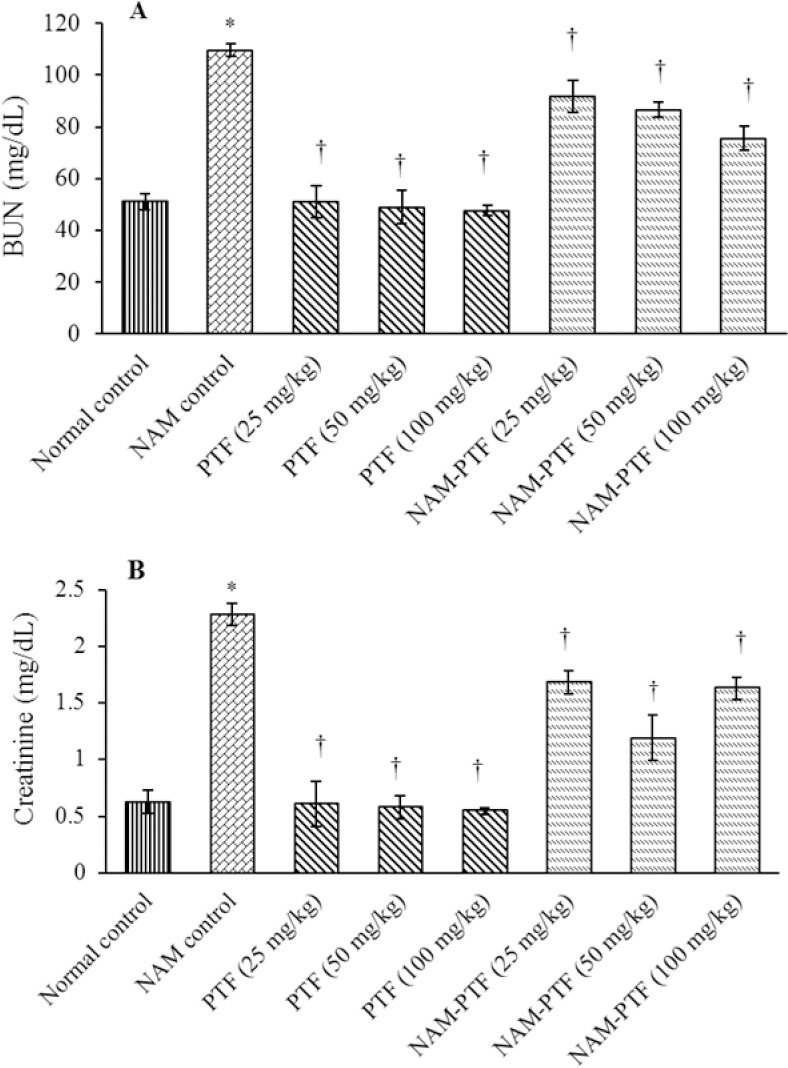
Effect of nitrosamine, pentoxifylline and nitrosamine + pentoxifylline on the mean kidney biochemical factors. (A) Blood urea nitrogen and (B) creatinine. *P < 0.05 compared to the normal control group. †P < 0.05 compared to the nitrosamine control group. BUN, blood urea nitrogen; NAM, nitrosamine; PTF, pentoxifylline.
Histological and morphometric analysis
Histological analysis showed normal kidney structure in the normal control and pentoxifylline-treated groups. After treatment with nitrosamine, the kidneys showed evident changes and injuries. These anomalies included an increase in the Bowman’s capsule, decrease in the number of glomeruli, intertubular bleeding, and enlarged diameters of the distal and proximal tubules. Treatment with nitrosamine along with pentoxifylline with all doses reduced the kidney damage caused by nitrosamine toxicity (Fig. 4). Morphometric analysis revealed that nitrosamine treatment significantly increased the mean diameter of the glomerulus tubule and considerably decreased the glomeruli number compared to the normal control group (P < 0.05).
Fig. 4.
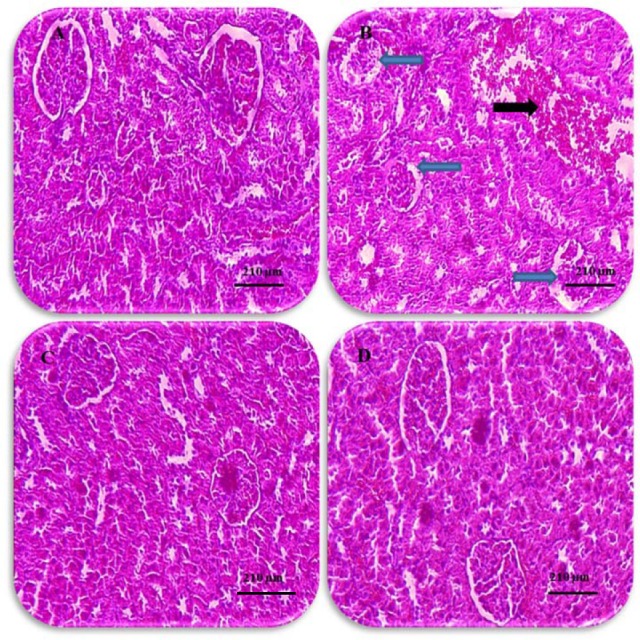
Histological changes in kidneys (hematoxylin-eosin, × 100). (A) Normal kidney, saline group, (B) nitrosamine control group, increased Bowman’s capsule space and glomerular shrinkage (blue arrow) and bleeding in the space between the tubules (black arrow), (C) normal kidney, pentoxifylline 100 mg/kg group showing glomeruli with Bowman’s capsule and distal and proximal tubules, (D) normal kidney structure in nitrosamine + pentoxifylline 100 mg/kg group (scale bars: black = 210 μm).
Treatment with pentoxifylline significantly increased the diameter of the glomeruli (P < 0.05) while had no significant effect on the number of glomeruli in all treatment groups compared to the normal control group (P > 0.05). Treatment with pentoxifylline or nitrosamine plus pentoxifylline significantly decreased the diameter of the glomeruli and significantly increased the number of glomeruli in all treatment groups compared to the nitrosamine control group (P < 0.05) (Fig. 5).
Fig. 5.
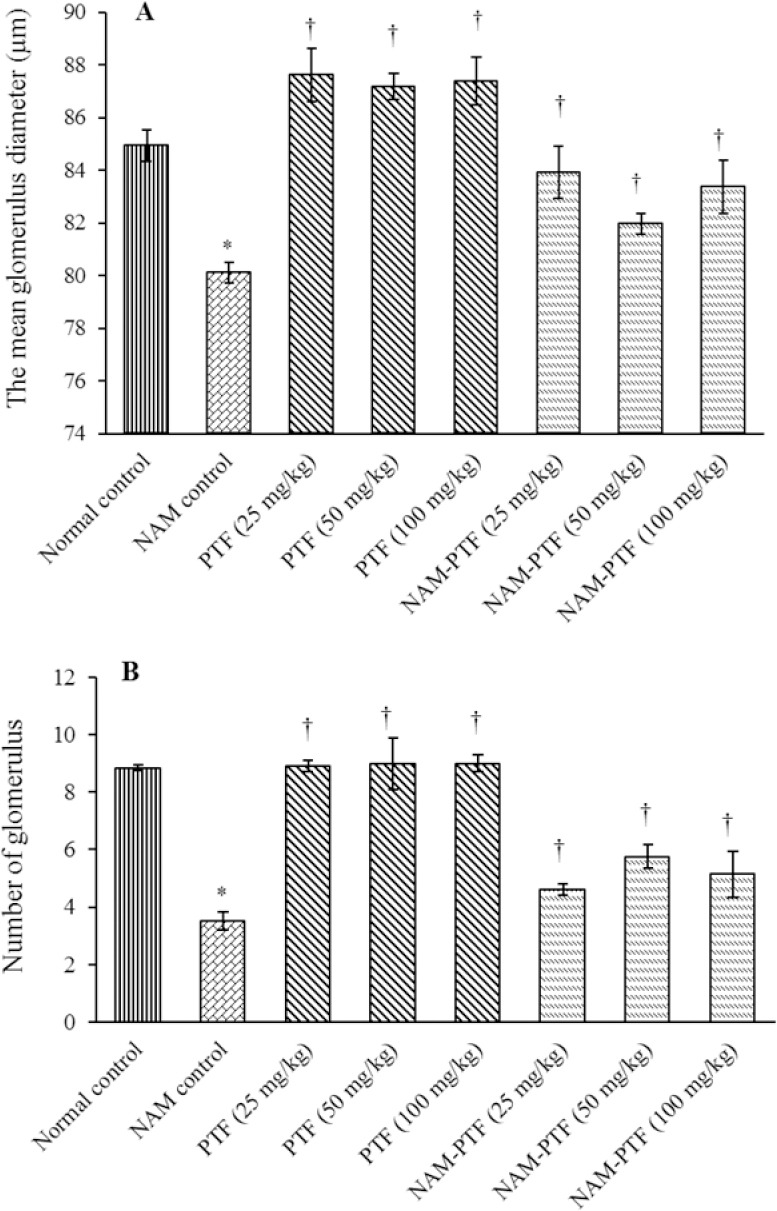
Morphometrical changes in kidneys (A) glomerular diameter and (B) glomeruli number. *P < 0.05 compared to the normal control group. †P < 0.05 compared to nitrosamine control group. NAM, nitrosamine; PTF, Pentoxifylline.
Nitrite oxide
The mean nitrite oxide in the blood serum increased significantly in the nitrosamine control group compared to the control normal group (P < 0.05). The mean nitrite oxide in the blood serum did not change significantly in all pentoxifylline groups compared to the normal control group (P > 0.05). The mean nitrite oxide in the blood serum decreased significantly in all pentoxifylline and pentoxifylline plus nitrosamine groups compared to the nitrosamine control group (P < 0.05) (Fig. 6).
Fig. 6.
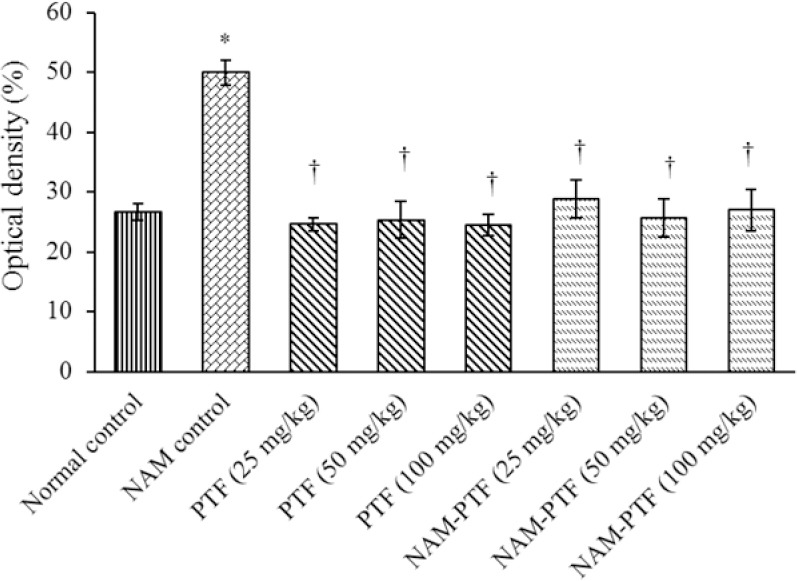
Effects of pentoxifylline, nitrosamine, and pentoxifylline + nitrosamine on mean nitrite oxide levels. *P < 0.05 compared to the control normal group. †P < 0.05 compared to the nitrosamine control group. NAM, nitrosamine; PTF, Pentoxifylline.
DISCUSSION
Pentoxifylline seems to improve capillary blood flow and tissue oxygenation due to its diverse biochemical and antioxidant properties (8). In the present study, the effect of pentoxifylline on the oxidative stress induced by nitrosamine was investigated. The results of normalized kidney weight indicated that there was a significant decrease in the index of animals’ kidney in nitrosamine control group compared to the normal control group. The nitrosamines present in the foodstuffs seem to produce N-nitroso in the stomach after consumption. Nitrosamines ultimately reduce the oxygen transport to the tissues leading to weight loss of body organs (21). The antioxidant properties of pentoxifylline increases blood circulation to body tissues (13). The results of the present study are consistent with the findings of Davila-Esqueda et al. indicating that the prescription of pentoxifylline improved the kidney weight (22). Our findings also showed a decrease in the number and an increase in the glomerular diameter in nitrosamine-treated animals. The treatment of animals with pentoxifylline at all doses increased the number of glomeruli and reduced their diameter compared to the nitrosamine control group. Rupture in the glomerular order, diminished glomerular size, and damage of proximal tubules are some of the changes that can indicate the pathological effects of nitrosamine on glomeruli (23). It is likely that nitrosamines increase the expression of xenobiotic metabolism through the induction of P450-dependent cytochrome, thereby enhancing the formation of ROS (3). Presumably, pentoxifylline reduces oxidative stress through cAMP (14). Previous findings on the pentoxifylline role in amikacin-induced kidney toxicity were consistent with the results of the present study (24).
The results of this study indicated that there was a significant increase in the level of BUN and creatinine in the nitrosamine control group compared to the normal control group. Also a significant decrease in the level of BUN and creatinine in all groups receiving pentoxifylline was observed. Increased BUN and creatinine suggests a glomerular damage (19). Oxidative stress induces an increase in the production of free radicals, causes glomerular necrosis, and then affects the capacity of renal filtration (16). Nitrosamine would increase the level of creatinine and BUN by enhancing the activity of lipid peroxidation (25).
Chen et al. indicated that prescription of pentoxifylline in proteinuric patients could decrease the level of creatinine, which is in line with the findings of the present study (26). We also showed that pentoxifylline is able to reduce lipid peroxidation and increase anti-oxidant capacity of kidney tissue, consequently, reduces oxidative stress. Thus, it appears that pentoxifylline with its anti-oxidant properties could reduce MDA and increase FRAPS in the treatment groups by inhibiting the production of ROS. Measurements of nitrite oxide level indicated a significant increase in level of nitrite oxide in the nitrosamine control group compared to the normal control group. In this study, administration of pentoxifylline significantly decreased the nitrite oxide level in the study groups. It appears that nitrosamine stimulates the generation of nitrite oxide by stimulating the release of noradrenaline in the paraventicular and amygdala nucleus (27). Oliveira Garcia et al. reported that treatment with different doses of pentoxifylline in diabetic rats reduced the expression level of nitrite oxide (28) being in agreement with the results of the present study. Treatment with pentoxifylline in this study significantly reduced the amount of damage caused by nitrosamine through inhibition of oxidative damage in the kidney tissues.
CONCLUSION
The results of this study indicated that pentoxifylline might recover some kidney function in rats treated with nitrosamine. This could help individuals who have been exposed to nitrosamine by protecting their kidneys. The antioxidant properties of pentoxifylline may be a main reason for its positive effect on kidney parameters; however, additional studies are required to define its exact mechanism of action.
ACKNOWLEDGEMENTS
This work was conducted in partial fulfillment of the requirements of MD degree of Delnia Moradi which was financially (Grant No. 96469) supported by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran.
REFERENCES
- 1.Piazzoli A, Breider F, Aquillon CG, Antonelli M, von Gunten U. Specific and total N-nitrosamines formation potentials of nitrogenous micropollutants during chloramination. Water Res. 2018;135:311–321. doi: 10.1016/j.watres.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60(2):131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann SS, Granby K, Duedahl-Olesen L. Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chem. 2015;174:516–526. doi: 10.1016/j.foodchem.2014.11.101. [DOI] [PubMed] [Google Scholar]
- 4.Ndwberne PM, Shank RC. Induction of liver and lung tumours in rats by the simultaneous administration of sodium nitrite and morpholine. Food Cosmet Toxicol. 1973;11(5):819–825. doi: 10.1016/0015-6264(73)90140-5. [DOI] [PubMed] [Google Scholar]
- 5.Moreira AJ, Ordoñez R, Cerski CT, Picada JN, García-Palomo A, Marroni NP, et al. Melatonin activates endoplasmic reticulum stress and apoptosis in rats with diethylnitrosamine-induced hepatocarcinogenesis. PLoS One. 2015;10(12):e0144517. doi: 10.1371/journal.pone.0144517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigo R, Rivera G. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radic Biol Med. 2002;33(3):409–422. doi: 10.1016/s0891-5849(02)00908-5. [DOI] [PubMed] [Google Scholar]
- 7.el-Mofty M, el-Darouti M, Rasheed H, Bassiouny DA, Abdel-Halim M, Zaki NS, et al. Sulfasalazine and pentoxifylline in psoriasis: a possible safe alternative. J Dermatolog Treat. 2011;22(1):31–37. doi: 10.3109/09546630903460260. [DOI] [PubMed] [Google Scholar]
- 8.Navarro-González JF, Sánchez-Niño MD, Donate-Correa J, Martín-Núñez E, Ferri C, Pérez-Delgado N, et al. Effects of pentoxifylline on soluble klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care. 2018;41(8):1817–1820. doi: 10.2337/dc18-0078. [DOI] [PubMed] [Google Scholar]
- 9.Hammerman C, Goldschmidt D, Caplan MS, Kaplan M, Schimmel MS, Eidelman AI, et al. Amelioration of ischemia-reperfusion injury in rat intestine by pentoxifylline-mediated inhibition of xanthine oxidase. J Pediatr Gastroenterol Nutr. 1999;29(1):69–74. doi: 10.1097/00005176-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Jalili C, Kamani M, Roshankhah S, Sadeghi H, Salahshoor MR. Effect of Falcaria vulgaris extracts on sperm parameters in diabetic rats. Andrologia. 2018;50(10):e13130. doi: 10.1111/and.13130. [DOI] [PubMed] [Google Scholar]
- 11.Salahshoor MR, Roshankhah Sh, Hosseni P, Jalili C. Genistein improves liver damage in male mice exposed to morphine genistein improves liver damage in male mice exposed to morphine. Chin Med J (Engl) 2018;131(13):1598–604. doi: 10.4103/0366-6999.235117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roshankhah Sh, Salahshoor MR, Jalili F, Karimi F, Sohrabi M, Jalili C. Crocin effects on the nicotine-induce ovary injuries in female rat. Int J Life Sci Pharma Res. 2017;7(4):1–8. [Google Scholar]
- 13.Khorami S, Farrokhi F, Tukmechi A, Nowrozi R. Effect of pentoxifylline and vitamin E on ovarian follicles in Rats. J Shahrekord Univ Med Sci. 2013;15(3):64–73. [Google Scholar]
- 14.Oliveira TR, Oliveira GF, Simões RS, Tikazawa EH, Monteiro HP, Fagundes DJ, et al. The role of ischemic preconditioning and pentoxifylline in intestinal ischemia/reperfusion injury of rats. Acta Bras Cir. 2017;32(7):559–567. doi: 10.1590/s0102-865020170070000007. [DOI] [PubMed] [Google Scholar]
- 15.Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxifylline and its mechanism. Atherosclerosis. 2007;192(2):313–322. doi: 10.1016/j.atherosclerosis.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Jalili C, Salahshoor MR, Hoseini M, Roshankhah Sh, M Sohrabi, A Shabanizadeh. Protective effect of thymoquinone against morphine injuries to kidneys of mice. Iran J Kidney Dis. 2017;11:142–150. [PubMed] [Google Scholar]
- 17.Saricicek E, Celik A, Uremis N, Kilinc M. Protective effects of simvastatin, Nigella sativa oil and thmoquinone against dimethylnitrosamine-induced oxidative stress in rat kidney. Biomed Res. 2016;27(3):854–859. [Google Scholar]
- 18.Albersen M, Fandel TM, Zhang H, Banie L, Lin G, De Ridder D, et al. Pentoxifylline promotes recovery of erectile function in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2011;59(2):286–296. doi: 10.1016/j.eururo.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najafi H, Changizi Ashtiyani S, Madani SH, Fakhri S, Mohamadi Yarijani Z, Hazem M. Therapeutic effects of curcumin on renal tissue damages induced by ischemia/reperfusion in rats. Koomesh. 2015;16(2):273–281. [Google Scholar]
- 20.Jalili C, Makalani F, Roshankhah Sh, Sohrabi Kh, Salahshoor MR. Protective effect of resveratrol against morphine damage to kidneys of mice. Int J Morphol. 2017;35(4):1409–1415. [Google Scholar]
- 21.Verhaegen A, Van Gaal L. Do E-cigarettes induce weight changes and increase cardiometabolic risk? A signal for the future. Obes Rev. 2017;18(10):1136–1146. doi: 10.1111/obr.12568. [DOI] [PubMed] [Google Scholar]
- 22.Davila-Esqueda ME, Martinez-Morales F. Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res. 2004;5(4):245–251. doi: 10.1080/154386090897974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma A, Ahmed B, Anwar F, Rahman M, Patel DK, Kaithwas G, et al. Novel glycoside from Wedelia calendulacea inhibits diethyl nitrosamineinduced renal cancer via downregulating the COX-2 and PEG 2 through nuclear factor-κB pathway. Inflammopharmacology. 2017;25(1):159–175. doi: 10.1007/s10787-017-0310-y. [DOI] [PubMed] [Google Scholar]
- 24.Ozer MK, Asci H, Oncu M, Yesilot S, Savran M, Bayram D, et al. Effects of pentoxifylline on amikacin-induced nephrotoxicity in rats. Ren Fail. 2009;31(2):134–139. doi: 10.1080/08860220802595492. [DOI] [PubMed] [Google Scholar]
- 25.Khan N, Sharma S, Alam A, Saleem M, Sultana S. Tephrosia pursuer ameliorates N-diethyl nitrosamine and potassium bromated-mediated renal oxidative stress and toxicity in Wistar rats. Pharmacol Toxicol. 2001;88(6):294–299. [PubMed] [Google Scholar]
- 26.Chen YM, Lin SL, Chiang WC, Wu KD, Tsai TJ. Pentoxifylline ameliorates proteinuria through suppression of renal monocyte chemoattractant protein-1 in patients with proteinuric primary glomerular diseases. Kidney Int. 2006;69(8):1410–1415. doi: 10.1038/sj.ki.5000302. [DOI] [PubMed] [Google Scholar]
- 27.Hebels DG, Briedé JJ, Khampang R, Kleinjans JC, de Kok TM. Radical mechanisms in nitrosamine-and nitrosamide-induced whole-genome gene expression modulations in Caco-2 cells. Toxicol Sci. 2010;116(1):194–205. doi: 10.1093/toxsci/kfq121. [DOI] [PubMed] [Google Scholar]
- 28.Garcia FA, Rebouças JF, Balbino TQ, da Silva TG, de Carvalho-Júnior CH, Cerqueira GS, et al. Pentoxifylline reduces the inflammatory process in diabetic rats: relationship with decreases of pro-inflammatory cytokines and inducible nitric oxide synthase. Int J Inflam (Lond) 2015;12:33–42. doi: 10.1186/s12950-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


