Abstract
Objective
To identify geographic disparities in access to opioid use disorder (OUD) treatment medications and county demographic and economic characteristics associated with access to buprenorphine and oral naltrexone prescribers in Medicare Part D.
Data Sources/Study Setting
We utilized data from the Medicare Part D Prescription Drug Event Standard Analytic File (2010‐2015).
Study Design/Data Collection
We used logistic regression to examine county‐level access to OUD medication prescribers.
Principal Findings
There was a 5.6 percentage point increase in counties with access to an OUD prescriber over the study period. However, in 2015, 60 percent of US counties lacked access to a Medicare Part D buprenorphine prescriber and over 75 percent lacked access to an oral naltrexone prescriber. Increased access to OUD prescribers was largely concentrated in urban counties. Results of logistic regression indicate regional differences and potential racial disparities in access to OUD prescribers.
Conclusions
To improve access to buprenorphine and naltrexone treatment for Medicare Part D enrollees, CMS may consider implementing educational and training initiatives focused on OUD treatment, offering training to obtain a buprenorphine waiver at no cost to providers, and sending targeted information to providers in low OUD treatment capacity areas.
Keywords: access, buprenorphine, medicare, naltrexone, opioid use disorder
1. INTRODUCTION
The United States is facing a growing public health crisis as opioid‐related overdose deaths continue to rise.1 Approximately 115 people a day are now dying from opioid‐related overdoses.2 Concurrent with this rise in deaths are increasing rates of opioid use disorder (OUD) and admissions for OUD treatment.3, 4 Central to addressing the opioid crisis is improving access to evidence‐based medications for the treatment for OUD—methadone, buprenorphine, and naltrexone. There are significant gaps in access to OUD medications across the United States. In 2012, there was an estimated OUD treatment gap of between 914 000 and 1.4 million, based on combined methadone and buprenorphine treatment capacity.3 This gap is due in part to the limited number and capacity of opioid treatment programs (OTPs), the sole providers of methadone for OUD treatment in the United States, and the geographic concentration of OTPs in urban areas.5
In an effort to expand access to schedule III‐V narcotics FDA‐approved for OUD treatment beyond OTPs, the federal government passed the Drug Addiction Treatment Act of 2000 (DATA 2000).6 DATA 2000 allows physicians to obtain a waiver to prescribe buprenorphine by completing an 8‐hour training course. There are, however, limits on the number of patients a physician can treat with buprenorphine at one time. Physicians can treat up to 30 patients at one time in the first year they hold a waiver and can apply to prescribe buprenorphine to up to 100 patients and then up to 275 patients in subsequent years.
1.1. Access to buprenorphine waivered prescribers
Several prior studies have examined potential access to buprenorphine waivered physicians.7, 8, 9, 10 Rosenblatt and colleagues found that in 2012 less than half of all US counties had at least one buprenorphine waivered physician and that only 2.2 percent of all US physicians held a waiver. Buprenorphine waivered physicians were highly concentrated in urban counties and in counties located on the east and west coasts.9 Stein and colleagues found that from 2008 to 2011 the percentage of US counties without a waivered physician significantly decreased from 50.1 percent to 43.4 percent and that counties in the South and Midwest had fewer waivered physicians than Northeastern counties.7 Similarly, Dick and colleagues found that the percentage of counties with a shortage of buprenorphine waivered physicians significantly decreased from 98.9 percent in 2002 to 46.8 percent in 2011. Results also indicated greater potential access to OUD treatment on the east and west coasts, with large sections of the Midwest identified as opioid treatment shortage areas.10
It is important to note that all of these studies used physician waiver status as a proxy for buprenorphine treatment. Waivered status does not ensure that physicians are prescribing buprenorphine. Estimates suggest that on average about 25 percent of physicians with a buprenorphine waiver do not currently prescribe the medication.11 In addition, these studies did not focus on a specific patient population, but rather on overall access to buprenorphine. Thus, it is not known how access to buprenorphine varies across different patient populations such as Medicaid and Medicare populations.
While the opioid crisis has received significant attention in both academic and public health circles, the specific experience of the Medicare population is not at the center of public discourse about the crisis. However, data indicate high rates of OUD among Medicare enrollees and high rates of opioid prescribing among Medicare Part D enrollees. For example, a 2014 study found that the 6‐month prevalence of OUD was 6.35 per 1000 Medicare enrollees, compared with 1.15 per 1000 patients with commercial insurance. The rate of OUD among Medicare enrollees doubled from 2008 to 2010.12 A recent report estimated that in 2016 approximately 500 000 Medicare Part D beneficiaries received high amounts of opioids, defined as an average morphine equivalent dose of greater than 120 milligrams a day for at least three months. Additionally, 90 000 beneficiaries were identified at serious risk of opioid misuse or overdose based on receiving extreme amounts of opioids (ie, an average daily morphine equivalent dose of greater than 240 milligrams for 12 months) or potentially engaging in doctor shopping.13
Only one prior study examined the use of buprenorphine in the Medicare population.14 Results showed that there were 6707 buprenorphine prescribers in 2013, which represented less than 2 percent of the 381575 prescribers of schedule II opioid painkillers. The study found a potential OUD treatment gap of 219 000, based on the estimated 81 000 of 300 000 Medicare Part D enrollees with OUD who received buprenorphine in 2013. In addition, findings indicated the six states with the highest ratio of buprenorphine claims were located in the Northeast. However, the study did not examine buprenorphine prescribing at the county level, was limited to a single year, and did not identify predictors of access to buprenorphine prescribers.
The current study improves upon prior research by identifying potential geographic disparities in access to OUD medications in Medicare Part D. No prior studies have examined access to oral naltrexone, which is FDA‐approved for OUD treatment. In contrast to buprenorphine, naltrexone is not a scheduled narcotic and can be prescribed by any physician. This study also identifies county demographic and economic characteristics associated with access to OUD prescribers in Medicare Part D. Thus, study results can inform the development of targeted strategies to improve access to OUD medications among Medicare Part D enrollees.
2. METHODS
2.1. Data
Data on buprenorphine and oral naltrexone prescribing are taken from the Medicare Part D Prescription Drug Event Standard Analytic File (2010‐2015). This file includes information on all prescription drugs paid for under Medicare Part D including the total number of prescriptions dispensed for each provider and drug and total drug cost. For the purposes of this study, data are aggregated to the county level. The unit of analysis is the county‐year, and the final dataset includes 18 086 observations.
County demographic data are collected from a variety of public sources, including the Bureau of the Census and the Surveillance, Epidemiology, and End Results Program (SEER). Rural‐urban continuum codes are from the US Department of Agriculture Economic Research Service (https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx). Characteristics of the Medicare population are from the Centers for Medicare & Medicaid Services’ Geographic Variation Public Use File (https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF.html).15 County‐level opioid overdose deaths are from the Centers for Disease Control and Prevention National Center for Health Statistics’ National Vital Statistics System Multiple Cause of Death Mortality Files.
2.2. Measures
Two variables measure the use of buprenorphine‐naloxone and oral naltrexone treatment at the county level. First, we construct a dichotomous measure indicating if a county had at least one buprenorphine‐naloxone or oral naltrexone prescriber. Second, we measure the number of buprenorphine‐naloxone and oral naltrexone prescribers per 1000 Medicare enrollees in each county.
We include two measures of provider characteristics: provider sex and specialty (eg, general practitioner, internal medicine, pain medicine, emergency medicine, psychiatry). For consistency, nonphysicians were excluded from the analysis. To examine the geographic distribution of opioid prescribers in Medicare Part D, we include a set of dichotomous measures of census division (New England, Middle Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, and Pacific). The number of Medicare enrollees in census divisions ranges from about 2.6 million to 10.5 million, with an average of 5.7 million enrollees. We include a dichotomous measure of urbanicity based on rural‐urban continuum codes (1 = rural county, 0 = urban/metropolitan county). Rural counties are defined as those completely rural or less than 2500 urban population, either adjacent to or not adjacent to a metropolitan area. We also measure opioid overdose deaths per 100 000 persons in the county.
We control for several characteristics of the Medicare population including the percentage of Medicare enrollees who are black, Hispanic, and other race, the number of Medicare fee‐for‐service and Medicare Advantage enrollees (in 10 000s), percentage of dually eligible Medicare/Medicaid enrollees, average enrollee age, number of inpatient stays per 1000 Medicare beneficiaries, number of emergency department (ED) visits per 1000 Medicare beneficiaries, and Medicare beneficiary hierarchical condition category (HCC) score. All variables are measured at the county level.
Additional county demographic and economic variables are median household income in 10 000s, unemployment rate, percentage of the county population below the federal poverty level, and log of the total county population. To account for time trends, we include a set of dichotomous variables indicating the study year.
2.3. Analytic technique
Descriptive statistics were calculated for all study variables. Paired t tests were used to compare the percentage of counties with OUD prescribers and the number of OUD prescribers per 1000 Medicare enrollees in 2010 and 2015 and to compare rural and urban counties. Logistic regression with county‐level random effects was used to identify county characteristics associated with access to buprenorphine or oral naltrexone prescribers. Data were treated as a panel and analyzed using the xt command suite in STATA. Counties with missing data were excluded from multivariate analyses, resulting in a loss of 1.3 percent of counties over the study period. All analyses were conducted in STATA version 14.1 (College Station, TX, USA).
3. RESULTS
Over the study period, about 36.4 percent of US counties had at least one buprenorphine prescriber and 21.9 percent of counties had at least one oral naltrexone prescriber (Table 2). From 2010 to 2015, there was a 7.3 percent increase in counties with access to a buprenorphine prescriber (t = 5.86, P < 0.001) and a 3.8 percent increase in counties with access to an oral naltrexone prescriber (t = 3.47, P = 0.001) (see Table 1 and Figures 1 and 2).
Table 2.
Descriptive statistics for counties (n = 17 849)
| Mean (SD) | |
|---|---|
| Any buprenorphine prescribers in county | 0.37 (0.44) |
| Any naltrexone prescribers in county | 0.22 (0.36) |
| Number of physicians prescribing buprenorphine in county | 0.21 (0.18) |
| Number of physicians prescribing naltrexone in county | 0.12 (0.15) |
| Opioid overdose deaths per 100 000 in county | 6.85 (7.08) |
| Medicare fee‐for‐service enrollees in county | 1.12 (2.54) |
| Medicare Advantage enrollees in county | 0.50 (1.82) |
| Percentage Medicare/Medicaid dually eligible | 21.26 (8.55) |
| Average age of Medicare beneficiaries | 71.13 (1.94) |
| Percentage of male physicians in the county | 76.90 (21.08) |
| Inpatient stays per 1000 Medicare beneficiaries | 285.52 (52.28) |
| Emergency department visits per 1000 Medicare beneficiaries | 661.37 (137.06) |
| Average hierarchical condition category (HCC) score for Medicare beneficiaries | 0.95 (0.09) |
| Percentage Medicare beneficiaries who are black | 7.33 (9.69) |
| Percentage Medicare beneficiaries who are Hispanic | 3.25 (6.69) |
| Percentage Medicare beneficiaries who are other race | 2.72 (4.03) |
| Median county income (in 10 000s) | 4.57 (1.15) |
| County unemployment rate | 7.57 (2.50) |
| Percentage county population in poverty | 16.95 (6.28) |
| Log of county total population | 11.26 (1.39) |
| General practitioner | 0.34 (0.27) |
| Internal medicine | 0.11 (0.15) |
| Pain medicine | 0.002 (0.02) |
| Emergency medicine | 0.05 (0.11) |
| Psychiatry | 0.02 (0.07) |
| Rural county | 0.18 (0.38) |
| New England | 0.02 (0.15) |
| Middle Atlantic | 0.05 (0.22) |
| East North Central | 0.14 (0.35) |
| West North Central | 0.19 (0.39) |
| South Atlantic | 0.19 (0.40) |
| East South Central | 0.12 (0.32) |
| West South Central | 0.15 (0.36) |
| Mountain | 0.09 (0.28) |
| Pacific | 0.05 (0.22) |
Table 1.
Change in county‐level access to buprenorphine and oral naltrexone prescribers, 2010‐2015
| 2010 | 2015 | Change from 2010 to 2015 | t statistic, P‐value | |
|---|---|---|---|---|
| Total number of buprenorphine prescribers per 1000 Medicare enrollees in the county, mean | 0.19 | 0.23 | 0.04 | 5.03, P < 0.001 |
| Total number of oral naltrexone prescribers per 1000 Medicare enrollees in the county, mean | 0.12 | 0.11 | −0.01 | 1.29, P = 0.20 |
| Percentage of counties with a buprenorphine prescriber, n (%) | 955 (32.7%) | 1200 (40.0%) | 7.3% | 5.86, P < 0.001 |
| Percentage of counties with an oral naltrexone prescriber, n (%) | 598 (20.5%) | 727 (24.3%) | 3.8% | 3.47, P < 0.001 |
| Percentage of rural counties with a buprenorphine prescriber, n (%) | 40 (7.5%) | 49 (9.4%) | 1.9% | 1.13, P = 0.26 |
| Percentage of rural counties with an oral naltrexone prescriber, n (%) | 8 (1.5%) | 6 (1.2%) | −0.3% | 0.49, P = 0.63 |
| Percentage of urban counties with a buprenorphine prescriber, n (%) | 915 (38.4.%) | 1151 (46.5%) | 8.1% | 5.72, P < 0.001 |
| Percentage of urban counties with an oral naltrexone prescriber, n (%) | 590 (24.7%) | 721 (29.1%) | 4.4% | 3.43, P = 0.001 |
| Number of buprenorphine physicians per 1000 Medicare enrollees in rural counties, mean | 0.52 | 0.56 | 0.04 | 0.53, P = 0.60 |
| Number of oral naltrexone physicians per 1000 Medicare enrollees in rural counties, mean | 0.27 | 0.48 | 0.22 | a |
| Number of buprenorphine physicians per 1000 Medicare enrollees in urban counties, mean | 0.17 | 0.22 | 0.04 | 5.66, P < 0.001 |
| Number of oral naltrexone physicians per 1000 Medicare enrollees in urban counties, mean | 0.12 | 0.11 | −0.01 | 1.49, P = 0.137 |
Due to small cell sizes, we were unable to perform a t test.
Figure 1.
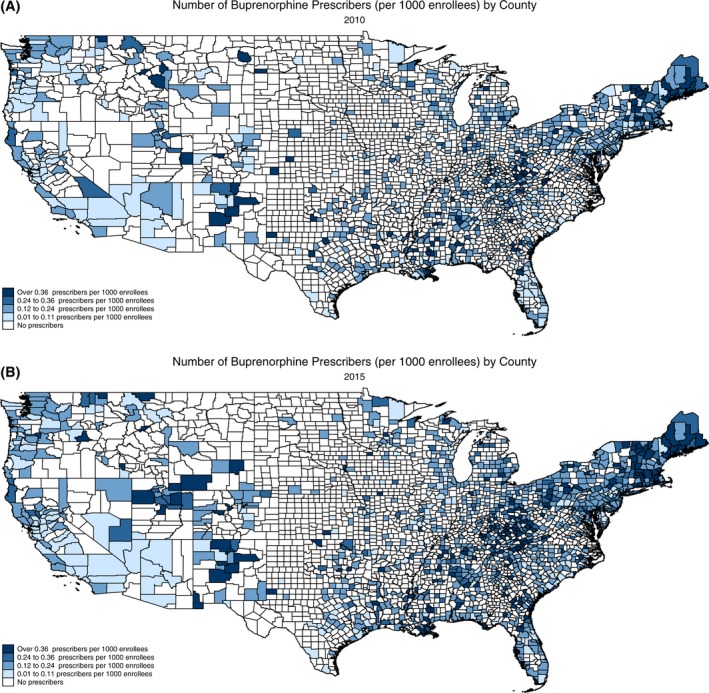
Number of buprenorphine prescribers per 1000 Medicare enrollees, 2010 and 2015. A, Number of buprenorphine prescribers (per 1000 enrollees) by county 2010. B, Number of buprenorphine prescribers (per 1000 enrollees) by county 2015 [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
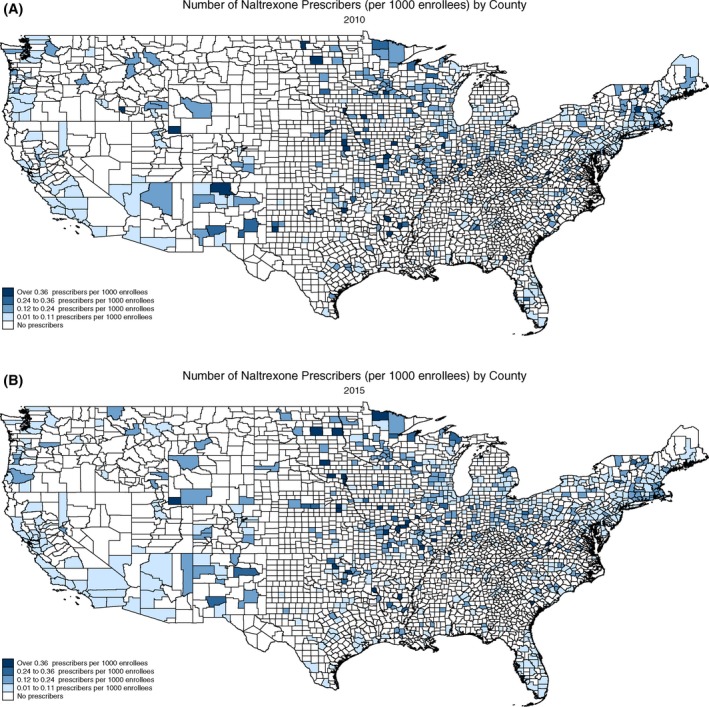
Number of oral naltrexone prescribers per 1000 Medicare enrollees, 2010 and 2015. A, Number of naltrexone prescribers (per 1000 enrollees) by county 2010. B, Number of naltrexone prescribers (per 1000 enrollees) by county 2015 [Color figure can be viewed at wileyonlinelibrary.com]
A larger percentage of urban counties had access to OUD prescribers over the study period compared to rural counties. In 2015, approximately 46.5 percent of urban counties had access to a buprenorphine prescriber, while only 9.4 percent of rural counties had access to a buprenorphine prescriber (t = 16.43, P < 0.001). The increase in access to oral naltrexone prescribers occurred exclusively in urban counties, increasing from 24.7 percent in 2010 to 29.1 percent in 2015 (t = 3.43, P = 0.001). The percentage of rural counties with at least one oral naltrexone prescriber decreased from 1.5 percent to 1.2 percent over the study period, but the decrease was not statistically significant (t = 0.49, P = 0.63).
The number of buprenorphine prescribers per 1000 Medicare enrollees in the county also increased significantly over the study period (t = 5.03, P < 0.001). However, the number of oral naltrexone prescribers per 1000 Medicare enrollees did not significantly change over the study period (t = 1.29, P = 0.20). The number of buprenorphine prescribers per 1000 Medicare enrollees in urban counties increased significantly from 0.17 to 0.22 prescribers per 1000 enrollees (t = 5.72, P < 0.001) over the study period. The number of buprenorphine prescribers in rural areas also increased from 0.52 to 0.56. However, the increase was not statistically significant (t = 0.534, P = 0.595).
While the number of oral naltrexone prescribers per 1000 enrollees in urban counties did not significantly change over the study period (t = 1.49, P = 0.14), the number of oral naltrexone prescribers increased in rural counties, from 0.27 per 1000 enrollees to 0.48 per 1000 enrollees. However, there were less than 10 rural counties with access to a naltrexone prescriber so we were unable to perform a t test.
It is important to note that the number of buprenorphine and oral naltrexone prescribers per 1000 Medicare enrollees was greater in rural counties compared to urban counties. Thus, although only 9.3 percent of rural counties had access to a buprenorphine prescriber and 1.2 percent of rural counties had access to an oral naltrexone prescriber in 2015, there was a higher density of OUD prescribers in these counties.
3.1. Logistic regression results
The odds of having an OUD prescriber were greater in counties with a higher number Medicare fee‐for‐service enrollees, and the odds of having an oral naltrexone prescriber were greater in counties with a higher percentage of dual enrollees (see Table 3). The odds of having an oral naltrexone prescriber were greater in counties with a higher opioid overdose death rate.
Table 3.
Results of logistic regression predicting county‐level access to buprenorphine and oral naltrexone prescribers
| Buprenorphine prescribers | Oral naltrexone prescribers | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Opioid overdose deaths per 100 000 | 1.008 (0.996, 1.020) | 1.017** (1.005, 1.029) |
| Number FFS Medicare | 8.00** (4.256, 15.047) | 1.477** (1.190, 1.832) |
| Number MA Medicare | 1.76 (0.768, 4.042) | 1.051 (0.803, 1.375) |
| Percentage Medicare/Medicaid dually eligible | 1.01 (0.974, 1.054) | 1.041* (1.008, 1.076) |
| Average age of Medicare beneficiaries | 0.90 (0.772, 1.048) | 1.049 (0.930, 1.185) |
| Prescriber sex | 1.002 (1.000, 1.004) | 0.999 (0.998, 1.001) |
| Inpatient stays per 1000 Medicare beneficiaries | 1.001 (0.996, 1.004) | 1.001 (0.998, 1.005) |
| Emergency department visits per 1000 Medicare beneficiaries | 1.00 (0.999, 1.003) | 0.999 (0.998, 1.001) |
| Average hierarchical condition category (HCC) score for Medicare beneficiaries | 0.090 (0.004, 2.112) | 0.521 (0.035, 7.755) |
| Percentage Medicare beneficiaries who are black | 0.971* (0.948, 0.995) | 1.001 (0.981, 1.021) |
| Percentage Medicare beneficiaries who are Hispanic | 0.934** (0.899, 0.969) | 0.974 (0.949, 1.001) |
| Percentage Medicare beneficiaries who are other race | 1.031 (0.976, 1.088) | 1.028 (0.992, 1.065) |
| Median county income (in 10 000s) | 0.972 (0.735, 1.285) | 0.906 (0.736, 1.116) |
| Unemployment rate | 0.914 (0.845, 0.989) | 0.903** (0.844, 0.966) |
| Percentage county population in poverty | 1.07** (1.025, 1.125) | 1.018 (0.977, 1.062) |
| Log of total county population | 28.209** (16.123, 49.354) | 10.836** (7.980, 14.713) |
| Rural county | 1.014 (0.536, 1.919) | 0.434* (0.228, 0.828) |
| Middle Atlantic | 0.022** (0.003, 0.163) | 0.171** (0.055, 0.532) |
| East North Central | 0.001** (0.0001, 0.004) | 0.364* (0.129, 1.032) |
| West North Central | 0.00004** (0.0000, 0.0003) | 0.505 (0.176, 1.452) |
| South Atlantic | 0.001** (0.000, 0.005) | 0.048** (0.016, 0.143) |
| East South Central | 0.004** (0.001, 0.027) | 0.031** (0.010, 0.096) |
| West South Central | 0.0002** (0.00004, 0.002) | 0.035** (0.011, 0.108) |
| Mountain | 0.001** (0.0002, 0.009) | 0.119** (0.037, 0.394) |
| Pacific | 0.004** (0.001, 0.029) | 0.048** (0.014, 0.160) |
| New England (reference category) | ||
| 2010 | 0.007** (0.003, 0.018) | 0.051** (0.029, 0.093) |
| 2011 | 0.389** (0.254, 0.598) | 0.759 (0.532, 1.082) |
| 2012 | 0.451** (0.318, 0.639) | 0.719* (0.532, 1.081) |
| 2013 | 0.643* (0.473, 0.874) | 0.774 (0.596, 1.005) |
| 2014 | 0.782* (0.603, 1.013) | 0.865 (0.691, 1.083) |
| 2015 (reference category) | ||
| Number of Observations | 17 849 | 17 849 |
Note: Data are aggregated to all prescriptions in drug category by county.
*P < 0.05, **P < 0.01.
The odds of having a buprenorphine prescriber were lower in counties with a higher percentage of black Medicare Part D enrollees and Hispanic enrollees, compared to white Medicare Part D enrollees. County unemployment rate was negatively associated with access to both buprenorphine and oral naltrexone prescribers, while county poverty rate was positively associated with access to buprenorphine prescribers. The odds of having access to a prescriber were greater in counties with larger populations.
Compared to counties in the New England census division, the odds of having access to a buprenorphine prescriber were lower in all other census divisions. The odds of having access to an oral naltrexone prescriber were lower in rural counties and counties located in the Middle Atlantic, South Atlantic, East South Central, West South Central, Mountain, and Pacific census divisions, compared to the New England division.
4. DISCUSSION
Consistent with prior research examining access to buprenorphine waivered physicians, this study found an overall increase in potential access to OUD prescribers. However, in 2015, 60 percent of US counties lacked access to a buprenorphine prescriber and over 75 percent lacked access to an oral naltrexone prescriber. Overall growth in the percentage of counties with access to an OUD prescriber was modest, about a 5.6 percentage point increase over the five‐year period.
Growth in access to OUD prescribers was concentrated largely in urban counties over the study period. The percentage of urban counties with access to buprenorphine and oral naltrexone prescribers significantly increased from 2010 to 2015. In contrast, the percentage of rural counties with access to OUD prescribers did not significantly change. However, there was a higher density of OUD prescribers in rural counties. While the higher density of OUD prescribers in rural counties is positive, relatively few rural counties had access to an OUD prescriber. Less than 10 percent of rural counties had at least one buprenorphine prescriber, and less than 2 percent of rural counties had at least one oral naltrexone prescriber in 2015.
Although there was a greater density of OUD prescribers in the few rural counties with an OUD prescriber, they continue to lag behind urban counties in overall access to OUD prescribers. Given the challenges of establishing OTPs in rural areas, increasing access via office‐based prescribing of buprenorphine and naltrexone is likely a more viable option for Medicare enrollees. However, numerous barriers remain including a general lack of health care infrastructure in rural areas, lack of behavioral health services, travel distance to receive treatment, stigma, general provider willingness to treat opioid use disorder, provider's negative perceptions about medications, cost of medications, reimbursement concerns, and concerns about diversion.3, 4, 16, 17, 18
Consistent with prior research, counties in New England were more likely to have access to a buprenorphine prescriber.7, 9, 10 This geographic disparity is particularly concerning given the high rate of OUD and opioid overdose deaths in many Midwestern (eg, Ohio, Indiana) and Southern states (eg, Arkansas, Kentucky, Tennessee, Oklahoma, West Virginia). This pattern was similar for oral naltrexone prescribers.
Counties with a higher percentage of black and Hispanic Medicare Part D enrollees were less likely to have access to a buprenorphine prescriber, indicating potential racial disparities in access to buprenorphine treatment. This finding aligns with prior studies, which show that availability of SUD treatment is more limited in areas with higher percentage of minorities.19, 20 This disparity may be exacerbated in Southern states with large rural and minority populations.21 Consistent with prior studies, we also found that poverty rate, a proxy measure of OUD treatment demand, was positively associated with access to buprenorphine prescribers.7, 10 Unemployment rate was negatively associated with access to OUD prescribers, suggesting that even in Medicare, enrollees are not getting increased access to OUD treatment when transient economic stressors increase.
Counties with a higher percentage of Medicare FFS enrollees were more likely to have access to an OUD prescriber, suggesting that Medicare Advantage Plans are able to achieve some positive selection by setting rules to attract healthier patients. Counties with a higher percentage of dually eligible enrollees were more likely to have access to an oral naltrexone prescriber. This finding is encouraging given that dual enrollees have higher rates of OUD.
While we found an increase in access to OUD prescribers, we do not have data on the number of patients treated by each physician. However, using the patient limits established by DATA 2000 and subsequent prescribing regulations, we can estimate the number of patients receiving buprenorphine. In 2010, the total number of buprenorphine physicians was 4425. Assuming that all physicians prescribed at the 30 patient limit and that average duration of treatment was 12 months, for example, at least 132 750 patients could have been treated with buprenorphine in 2010. Assuming that all physicians prescribed at the 100 patient limit, 442 500 patients could have been treated with buprenorphine. In 2015, if all 7935 buprenorphine physicians prescribed at the 30 patient limit, 238 050 patients could have potentially received buprenorphine and at the 100 patient limit, 793 500 patients could have potentially received the medication.
However, recent data suggest that physicians are prescribing well below the 30 patient limit.22 In the seven states with the highest number of buprenorphine physicians, the median monthly patient census was 13 patients. Thus, if physicians prescribed buprenorphine to 13 patients on average, about 57 525 patients in 2010 and 103 116 patients in 2015 could have received the medication. This estimate is consistent with a prior study, which found that 6707 physicians prescribed buprenorphine to 81 000 of the 300 000 Medicare Part D enrollees with OUD in 2013.14 Our findings suggest that if physicians prescribed buprenorphine closer to the 30 patient limit, the OUD treatment gap could be significantly reduced.
There are no such limitations on the number of patients who can be prescribed naltrexone. However, our findings show there were substantially fewer oral naltrexone prescribers in Medicare Part D (1523 in 2010 and 2270 in 2015). This is likely due in part to the effectiveness of oral naltrexone, which has been hampered by patient noncompliance. Oral naltrexone is recommended for patients who are highly motivated and/or are regularly monitored for compliance.23 Additionally, naltrexone is an opioid antagonist, and patients must be opioid‐free for seven to ten days prior to beginning a naltrexone treatment regimen. As a result, oral naltrexone may not be the first choice for many patients with OUD. However, along with the injectable formulation of naltrexone, it is the only opioid antagonist medication available to treat OUD.
4.1. Limitations
This study has several limitations. First, we do not know whether buprenorphine prescribers have a DATA 2000 waiver. It is possible that physicians are prescribing buprenorphine‐naloxone off‐label for conditions other than OUD. Second, oral naltrexone is FDA‐approved for the treatment of relapse to opioids and alcohol use disorder (AUD). Therefore, it is possible physicians are prescribing oral naltrexone for AUD. However, an analysis of access to prescribers of the two medications FDA‐approved solely for the treatment of AUD—disulfiram and acamprosate—showed no significant change in either the percentage of counties with AUD prescribers or the number of AUD prescribers per 1000 Medicare enrollees.
Third, the Medicare Part D Prescription Drug Event Standard Analytic File does not include diagnostic codes, measures of patient or practice setting characteristics, or measures of treatment quality and duration. Thus, we are unable to account for these factors in our analyses. Fourth, one measure of treatment demand, county‐level OUD rates, is not currently available. However, we did include a county‐level measure of opioid overdose deaths in our models.
Fifth, we were unable to examine methadone because it is not a covered benefit under Medicare Part D. Methadone prescriptions represented in Medicare Part D data are prescribed only for pain. Sixth, we were also unable to examine extended‐release injectable naltrexone due to the small number of prescriptions in Medicare Part D data.
5. CONCLUSIONS
Preliminary estimates of drug overdose deaths in 2016 indicate the largest annual increase in drug overdose deaths in the United States—a 19 percent increase from 2015 to 2016. With no indication that the opioid crisis is waning, increasing access to OUD treatment is critical. While treatment with OUD medications may not be the preferred or recommended course of treatment for all patients, medications used in conjunction with psychosocial therapy are considered the gold standard in care for OUD.24, 25, 26
As federal, state, and local governments work to develop and implement policies to address the opioid crisis, targeted efforts may be needed to expand access to OUD prescribers in Medicare. This may be particularly important in areas with large numbers of dually eligible enrollees, in Southern and Midwestern regions, and in rural counties.12 While expanding OUD treatment is part of Centers for Medicare & Medicaid Services’ (CMS) recently issued Opioid Misuse Strategy, the specific strategies to increase access to medications in Medicare are not clear.27
To increase OUD treatment capacity, CMS may consider implementing educational and training initiatives focused on OUD treatment, offering training to obtain a buprenorphine waiver at no cost to providers, sending targeted information to providers in low OUD treatment capacity areas, increasing reimbursement rates for OUD treatment services, and covering methadone under Medicare Part D. More specifically, CMS could collaborate with the FDA to develop an OUD treatment module and integrate the module into the FDA's Opioid Risk Evaluation and Mitigation Strategy (REMS). CMS could also identify areas with low OUD treatment capacity and mail letters to prescribers to increase awareness about the need for OUD treatment, encourage them to obtain training in addiction medicine, and offer OUD treatment to their patients. Other strategies to increase access to buprenorphine and oral and injectable naltrexone among Medicare Part D enrollees may include eliminating prior authorization, and reducing or eliminating cost sharing and other utilization control mechanisms that may limit access to OUD medications.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: The authors would like to acknowledge the support of the University of Georgia and Indiana University. Disclosures: None.
Abraham AJ, Adams GB, Bradford AC, Bradford WD. County‐level access to opioid use disorder medications in medicare Part D (2010‐2015). Health Serv Res. 2019;54:390–398. 10.1111/1475-6773.13113
REFERENCES
- 1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid‐involved overdose deaths – United States, 2010‐2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445‐1452. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Wide‐ranging online data for epidemiologic research (WONDER). 2018; https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed May 1, 2018.
- 3. Jones CM, Campopiano M, Baldwin G, McCance‐Katz E. National and state treatment need and capacity for opioid agonist medication‐assisted treatment. Am J Public Health. 2015;105(8):e55‐e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication‐assisted therapies–tackling the opioid‐overdose epidemic. N Engl J Med. 2014;370(22):2063‐2066. [DOI] [PubMed] [Google Scholar]
- 5. Substance Abuse and Mental Health Services Administration . National Survey of Substance Abuse Treatment Services. Bethesda, MD: Substance Abuse and Mental Health Services Administration; 2015. [Google Scholar]
- 6. Center for Substance Abuse Treatment . The Determinations Report: A Report On the Physician Waiver Program Established by the Drug Addiction Treatment Act of 2000 (“DATA”). Rockville, MD: Substance Abuse and Mental Health Services Administration & U.S. Department of Health and Human Services; 2006. [Google Scholar]
- 7. Stein BD, Gordon AJ, Dick AW, et al. Supply of buprenorphine waivered physicians: the influence of state policies. J Subst Abuse Treat. 2015;48(1):104‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stein BD, Pacula RL, Gordon AJ, et al. Where is buprenorphine dispensed to treat opioid use disorders? The role of private offices, opioid treatment programs, and substance abuse treatment facilities in urban and rural counties. Milbank Q. 2015;93(3):561‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dick AW, Pacula RL, Gordon AJ, et al. Growth in buprenorphine waivers for physicians increased potential access to opioid agonist treatment, 2002‐11. Health Aff (Millwood). 2015;34(6):1028‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arfken CL, Johanson CE, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office‐based treatment with buprenorphine: National surveys of physicians. J Subst Abuse Treat. 2010;39(2):96‐104. [DOI] [PubMed] [Google Scholar]
- 12. Dufour R, Joshi AV, Pasquale MK, et al. The prevalence of diagnosed opioid abuse in commercial and Medicare managed care populations. Pain Pract. 2014;14(3):E106‐E115. [DOI] [PubMed] [Google Scholar]
- 13. Office of Inspector General . Opioids in Medicare Part D: Concerns about Extreme Use and Questionable Prescribing In: Services USDoHH, ed. Vol HHS OIG Data Brief. Washington, DC: U.S. Department of Health & Human Services; 2017:3‐5. [Google Scholar]
- 14. Lembke A, Chen JH. Use of opioid agonist therapy for Medicare patients in 2013. JAMA Psychiatry. 2016;73(9):990‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geographic Variable Public Use File: State/county Report of all Beneficiaries. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF.html.
- 16. Lu N, Samuels ME, Kletke PR, Whitler ET. Rural‐urban differences in health insurance coverage and patterns among working‐age adults in Kentucky. J Rural Health. 2010;26(2):129‐138. [DOI] [PubMed] [Google Scholar]
- 17. Roman PM, Abraham AJ, Knudsen HK. Using medication‐assisted treatment for substance use disorders: evidence of barriers and facilitators of implementation. Addict Behav. 2011;36(6):584‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliva EM, Maisel NC, Gordon AJ, Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psychiatry Rep. 2011;13(5):374‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummings JR, Wen H, Ko M, Druss BG. Race/ethnicity and geographic access to Medicaid substance use disorder treatment facilities in the United States. JAMA Psychiatry. 2014;71(2):190‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guerrero EG, Kao D. Racial/ethnic minority and low‐income hotspots and their geographic proximity to integrated care providers. Subst Abuse Treat Prev Policy. 2013;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McAuliffe WE, Dunn R. Substance abuse treatment needs and access in the USA: interstate variations. Addiction. 2004;99(8):999‐1014. [DOI] [PubMed] [Google Scholar]
- 22. Stein BD, Sorbero M, Dick AW, Pacula RL, Burns RM, Gordon AJ. Physician capacity to treat opioid use disorder with buprenorphine‐assisted treatment. JAMA. 2016;316(11):1211‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Society of Addiction Medicine . The ASAM National Practice Guideline For the Use of Medications in the Treatment of Addiction Involving Opioid Use. Chevy Chase, MD: American Society of Addiction Medicine; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amass L, Ling W, Freese TE, et al. Bringing buprenorphine‐naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field experience. Am J Addict. 2004;13(Suppl 1):S42‐S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev 2011;(10):Cd004147. [DOI] [PubMed] [Google Scholar]
- 26. Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2011(9):Cd005031. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Medicare & Medicaid Services . Opioid Misuse Strategy 2016 In: CfMM Services, Washington, DC: Centers for Medicare & Medicaid Services; 2017. https://www.cms.gov/outreach-and-education/outreach/partnerships/downloads/cms-opioid-misuse-strategy-2016.pdf. Accessed May 1, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


