Abstract
Objective
Analogues of GDF15 (Growth Differentiation Factor 15) are promising new anti-obesity therapies as pharmacological treatment with GDF15 results in dramatic reductions of food intake and body weight. GDF15 exerts its central anorexic effects by binding to the GFRAL receptor exclusively expressed in the Area Postrema (AP) and the Nucleus of the Solitary Tract (NTS) of the hindbrain. We sought to determine if GDF15 is an indispensable factor for other interventions that cause weight loss and which are also known to act via these hindbrain regions.
Methods
To explore the role of GDF15 on food choice we performed macronutrient intake studies in mice treated pharmacologically with GDF15 and in mice having either GDF15 or GFRAL deleted. Next we performed vertical sleeve gastrectomy (VSG) surgeries in a cohort of diet-induced obese Gdf15-null and control mice. To explore the anatomical co-localization of neurons in the hindbrain responding to GLP-1 and/or GDF15 we used GLP-1R reporter mice treated with GDF15, as well as naïve mouse brain and human brain stained by ISH and IHC, respectively, for GLP-1R and GFRAL. Lastly we performed a series of food intake experiments where we treated mice with targeted genetic disruption of either Gdf15 or Gfral with liraglutide; Glp1r-null mice with GDF15; or combined liraglutide and GDF15 treatment in wild-type mice.
Results
We found that GDF15 treatment significantly lowered the preference for fat intake in mice, whereas no changes in fat intake were observed after genetic deletion of Gdf15 or Gfral. In addition, deletion of Gdf15 did not alter the food intake or bodyweight after sleeve gastrectomy. Lack of GDF15 or GFRAL signaling did not alter the ability of the GLP-1R agonist liraglutide to reduce food intake. Similarly lack of GLP-1R signaling did not reduce GDF15's anorexic effect. Interestingly, there was a significant synergistic effect on weight loss when treating wild-type mice with both GDF15 and liraglutide.
Conclusion
These data suggest that while GDF15 does not play a role in the potent effects of VSG in mice there seems to be a potential therapeutic benefit of activating GFRAL and GLP-1R systems simultaneously.
Keywords: GDF15, GLP-1, Bariatric surgery, Liraglutide
Abbreviations: AP, Area Postrema; CeA, Central Amygdala; GDF15, Growth Differentiation Factor 15; GFRAL, GDNF Family Receptor Alpha Like; NTS, Nucleus of the Solitary Tract; VSG, Vertical Sleeve Gastrectomy
Highlights
-
•
GDF15 acts in the hindbrain and holds great potential for anti-obesity therapy.
-
•
GDF15 lowers fat intake but is not necessary for weight loss after bariatric surgery in mice.
-
•
GLP-1 receptor neurons in the hindbrain are not activated by GDF15.
-
•
GDF15 drives synergistic weight loss when administered with a GLP-1R agonist.
1. Introduction
GDF15 (Growth Differentiation Factor 15) analogues hold great promise as pharmacological treatments for obesity as GDF15 decreases food intake by acting on distinct centers in the hindbrain involved in appetite control [1], [2]. However, little is known about the biology of this system that was first identified as a mediator of cancer anorexia and only recently was the receptor, GDNF Family Receptor Alpha Like (GFRAL), identified by several industry groups working in parallel [3], [4], [5], [6]. The CNS distribution of GFRAL is very limited with the highest expression levels in the Area Postrema (AP) and to a lesser extent in the Nucleus of the Solitary Tract (NTS) of the hindbrain. Both of these structures serve as an important gateway for satiety signals from the periphery to the CNS [7]. In the present study, our aim was to determine whether other anorexic treatments currently used for anti-obesity therapy might act via the GDF15/GFRAL system.
One such treatment is bariatric surgery. Bariatric procedures such as vertical sleeve gastrectomy (VSG) cause profound weight loss primarily as a result of reduced food intake [8]. The reduced food intake is associated with increased activation of brainstem circuitry in response to nutrients in the gastrointestinal tract [9]. In addition to changes in food intake, VSG-treatment in rodents also results in profound changes in food choice with a reduced preference for fat [10], [11]. Hence the goal of these experiments was to test the potential role for the GDF15/GFRAL system to mediate the anorectic effects of VSG and to alter food choice.
In addition to bariatric surgery, long acting agonists of the receptor for the gut hormone glucagon-like peptide-1 (GLP-1) also produce significant weight loss. GLP-1 receptor (GLP-1R) expression is found throughout the brain, including significant expression in the AP and NTS of the hindbrain [12]. A variety of evidence links anorectic actions of GLP-1 to the activation of these hindbrain areas, which are also the only areas shown to contain GFRAL-expressing neurons [3]. Consequently we sought to understand whether the anorexic effects of GLP-1R agonism depend on the GDF15/GFRAL system and whether combining GDF15 with a GLP-1R agonist would hold advantages to using them separately.
2. Methods
2.1. Human hindbrain tissue samples
Formalin-fixed paraffin embedded (FFPE) post-mortem human hindbrain tissue from one donor with no clinical evidence of neurologic disease (MRC data base number # BBN001.29529) was utilized in the study. The tissue was collected and archived at the Edinburgh Brain and Tissue Bank and sent for histological analysis at Novo Nordisk A/S. The Danish Research Ethics Committee (VEK) has approved the use of human tissues from the Edinburgh Brain and Tissue Bank (approval # H-17014257).
2.2. Animals
All animal experiments were approved by the Institutional Animal Care & Use Committee at University of Michigan (Animal Use Protocol # PRO00007908) or the Danish Animal Experiment Inspectorate in accordance with the Danish Act on Experiments on Animals, the Appendix A of ETS 123 and EU Directive 2010/63 (mice for histology in Figure 4). C57BL6/J mice (Jackson Laboratories) were single housed and fed a 60% high fat diet (Macronutrient study with GDF15 injections in Figure 1). For the generation of Gdf15- and Gfral- alleles in mice, we designed gRNAs flanking the second exon of Gdf15 and surrounding exons 2 and 5 of Gfral. Oligonucleotides corresponding to these gRNAs were cloned into px330 [13]. The plasmids were prepped, purified, and injected into fertilized embryos, which were implanted into pseudo-pregnant female mice. Potential founders were screened for the loss of the appropriate exons; PCR products from putative positives were confirmed by DNA sequencing at the founder stage and after transmission to first generation progeny. These mice were used for food intake, macronutrient preference, and VSG studies in Figure 1, Figure 2. Glp1r−/− mice were generated from homozygous breeding pairs, which were F1 offspring from Glp1r+/− heterozygote intercrosses. They were single housed throughout the studies. Glp1rcre/+;Rosa26eGFP-L10a/+ mice for the co-localization studies (Figure 3) were generated as described previously [14] and were group-housed on a chow diet. Tissue sections for histology in Figure 4 were derived from C57BL6/J mice (Jackson Laboratories) that were group-housed under standard conditions (12 h–12 h light–dark cycle, ∼21 °C, water and food ad libitum). Rodents were anesthetized with isoflurane and euthanized by transcardiac perfusion with 10 ml of heparinized saline followed by 10% neutral buffered formalin (NBF) prior to paraffin embedding.
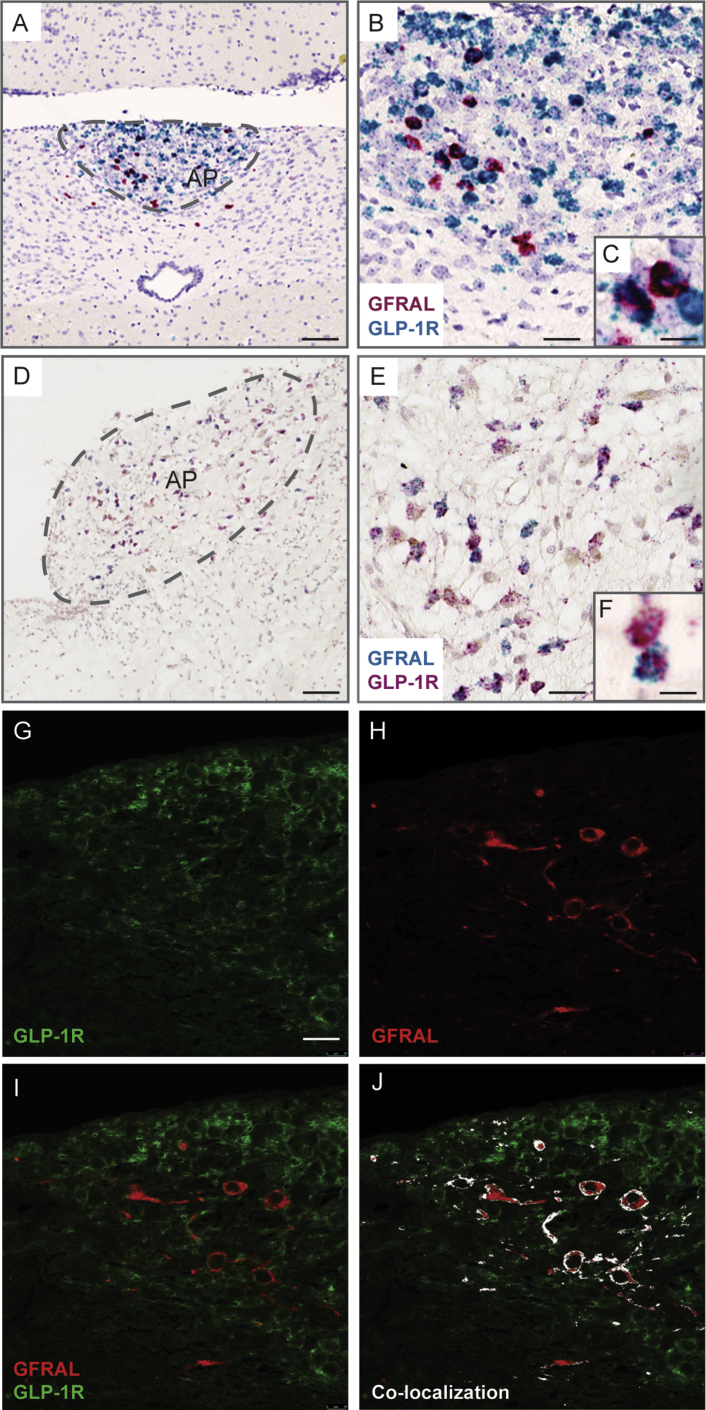
Figure 4.
Co-localization of GFRAL and GLP-1R in the hindbrain. Coronal sections of mouse (4A, 4B, and 4C) and human hindbrain (4D, 4E, and 4F) at the level of the area postrema (AP) were co-stained for GFRAL and GLP-1R mRNA using the RNAscope in situ hybridization technology. Coronal sections of mouse hindbrain (4G-4J) at the level of the AP were co-stained with antibodies for GFRAL and GLP-1R 1R using immunofluorescent staining. Scale bars: A: 50 mm, B: 30 mm, C and F: 20 mm, D: 100 mm, E: 50 mm, G–J: 40 mm.
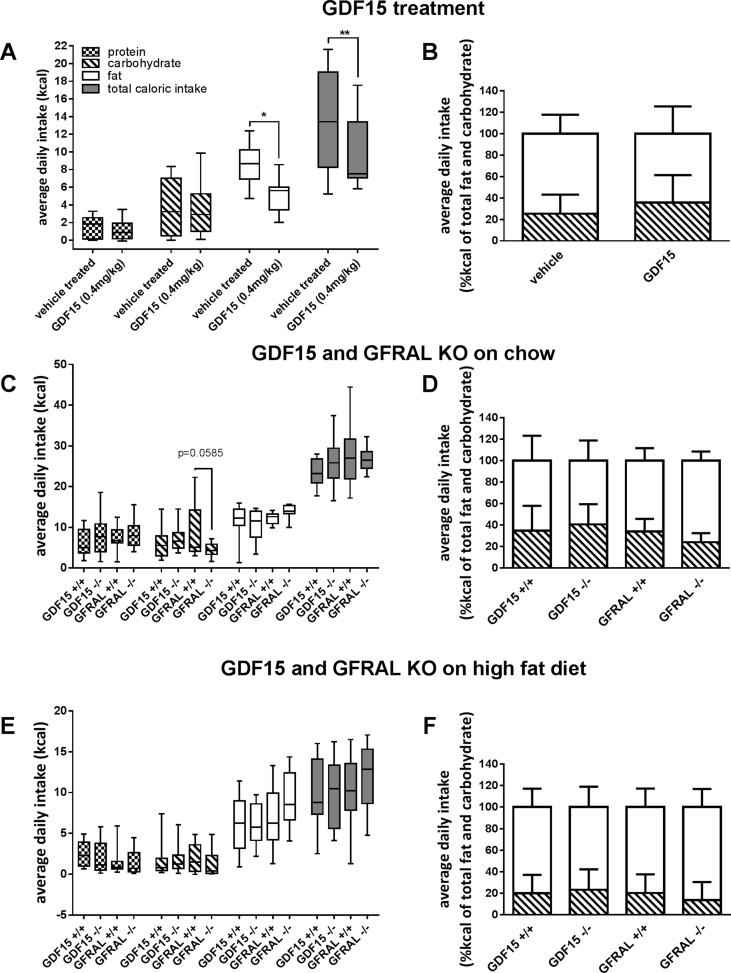
Figure 1.
Macronutrient preference test in mice treated with GDF15, or in mice lacking GDF15 or GFRAL. In all experiments mice were presented with pure fat, carbohydrate (starch), or protein in their home cages, and intake was measured daily. 1A and 1B: regular mice treated with GDF15, 1C and 1D: GDF15 deleted and GFRAL deleted mice fed chow diets prior to test. 1E and 1F: GDF15 deleted and GFRAL deleted mice fed with high fat diet prior to test. The first column (1A, 1C, and 1E) represents crude intake from each macronutrient group in kcal, where the second column (1B, 1D, and 1F) represents the ratio between fat and carbohydrate intake. All bars on the right side (1A, 1C, and 1D) are represented as box and whiskers plot with error bars spanning the lowest and highest value within each bar. All graphs on the left side (1B, 1D, and 1F) are represented as stacked bars displaying the average value with error bars showing standard deviations.
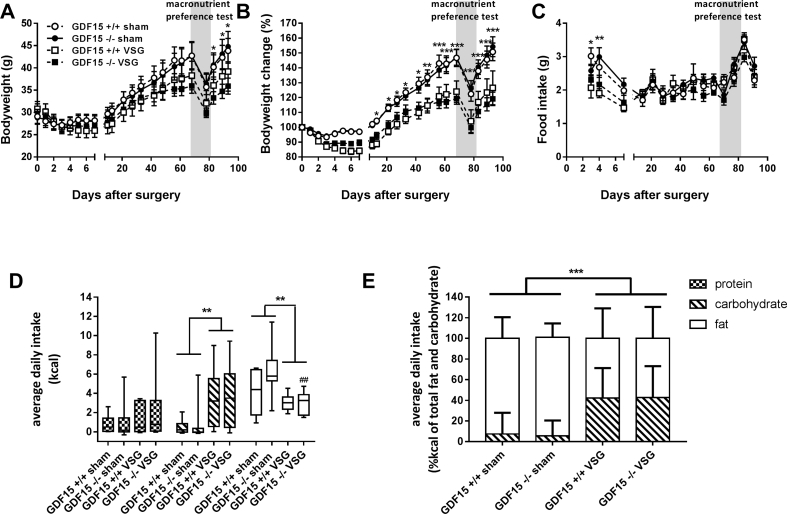
Figure 2.
Bodyweight, food intake, and macronutrient preference after VSG in mice lacking GDF15. GDF15 deleted mice and their wild type littermates were fed high fat diet for 8 weeks prior to having VSG or sham operations. Their body weight (1A and 1B) was monitored for 13 weeks after surgery together with their food intake (1C). White circles are GDF15+/+ sham, black circles are GDF15−/− sham, white squares are GDF15+/+ VSG, and black squares are GDF15−/− VSG. 10 weeks post-surgery, the mice were given the choice of different macronutrients in the form of fat, carbohydrate (starch), and protein; the crude kcal intake from each of these components was measured (1D), and the ratio between carbohydrate and fat intake as a percent of the total intake of those two macronutrients combined was calculated (1E). Graphs 1A, 1B, and 1C display average values with error bars representing SEM. 1D is a box and whiskers plot with error bars spanning the lowest and highest value within each bar. 1E is represented as stacked bars displaying the average value with error bars showing standard deviations. In Figure 2A, B: * = statistically significant effect of VSG versus sham as revealed by Tukey post hoc analysis following repeated measurement 2 way ANOVA.
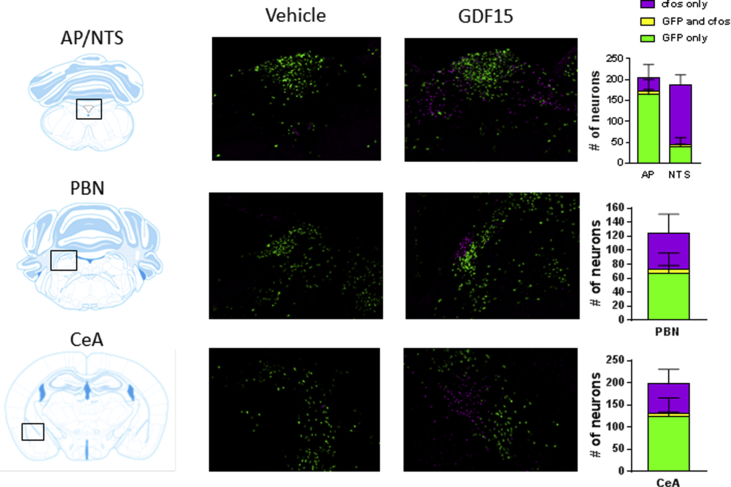
Figure 3.
Co-localization of neurons activated by GDF15 and neurons carrying the GLP1R in AP/NTS, PBN, and CeA. GLP1Rcre/+; Rosa26eGFP-L10a/+ mice expressing GFP in GLP-1 receptor carrying neurons were treated with GDF15 or vehicle (n = 3 per group) and stained for either c-fos or GFP in coronal sections corresponding to Area Postrema/Nucleus of the Solitary Tract (AP/NTS), the Parabrachial Nucleus (PBN), or the Central Amygdala (CeA). Anatomical guides and pictures are included. In each area the number of GFP positive neurons, c-fos activated neurons, or both types of neurons co-localized were counted and represented as bar graphs.
2.3. VSG
Gdf15−/− and Gdf15+/+ littermates were given 60% high fat diet after weaning and maintained on this diet for 8 weeks before surgery. 2 weeks prior to surgery the mice were split into single housing and the day before surgery they were stratified based on bodyweight. VSG and sham surgeries were performed under isoflurane anesthesia as described previously [15]. Briefly, the lateral 80% of the stomach was excised, leaving a tubular gastric remnant in continuity with the esophagus proximally and the pylorus distally. The sham procedure involved the opening of the peritoneal cavity and the application of manual pressure on the stomach with blunt forceps along a vertical line between the esophageal sphincter and the pylorus. Mice consumed a liquid diet (Osmolite 1 Cal) for the first 4 postoperative days and were reintroduced to solid food (high-fat diet) on day 4. Postoperative deaths, mainly within the first week, yielded final group numbers of n = 6 for Gdf15+/+ sham, n = 9 for Gdf15−/− sham, n = 6 for Gdf15+/+ VSG, and n = 8 for Gdf15−/− VSG. The mice were maintained on high-fat diet after surgery except during the immediate postoperative period. Body weights after surgery were measured daily for the first 2 weeks and then weekly.
2.4. Macronutrient preference
In all studies, food was removed from the cage and the three pure macronutrient diets (TD.02521 (3.3 kcal/g carbohydrate, 0.1% calories protein, 99.9% calories carbohydrate, and 0% calories fat), TD.02522 (6.9 kcal/g fat, 0.1% calories protein, 1.3% calories carbohydrate, and 98.6% calories fat), and TD02523 (3.2 kcal/g protein, 96.1% calories protein, 1.4% calories carbohydrate, and 2.6% calories fat); Harlan-Teklad) were presented in separate containers simultaneously. In all studies, mice were allowed 3 days to acclimatize to the containers before intake was measured. With GDF15 therapy this acclimatization period was extended with baseline saline injections for 2 additional days before GDF15/control injections began. At the beginning of the study period, all containers were replaced and weighed daily for 4 days. At the end of the study period the macronutrient containers were removed and food was added back to the cages.
2.5. Food intake studies
The food intake studies were performed in mice fed regular chow diet, and the mice were allowed at least one week of resting in between injections. All studies took place over a 3-day period. On day 1, the mice received a saline injection and were provided with a clean cage and fresh chow 2 h prior to lights out. The following day, the mice were weighed and food was removed and weighed 2 h prior to lights out. One hour later, injections took place, and at lights out the food was placed back in the cage. Food intake was then measured 1 h, 4 h, and 24 h. All injection solutions were freshly prepared from sterile frozen stock solutions immediately before administration (GDF15 and Liraglutide were provided by Novo Nordisk A/S).
2.6. Co-localization studies
2.6.1. Immunohistochemistry
Glp1rcre/+;Rosa26eGFP-L10a/+ mice were group-housed on a chow diet. 4 days prior to GDF15 injections the mice received daily injections of vehicle. On the day of the study, each mouse received 4 mg/kg SQ of GDF15 or a vehicle injection 4 h prior to brain extraction. For the extraction, the mice were anesthetized with a single dose of 150ul Euthasol IP and perfused with rinse solution followed by fixative (4% paraformaldehyde in PBS). The brain was extracted, left overnight in 10% paraformaldehyde, and then transferred to 30% sucrose in PBS for 2 days. The brains were frozen and sectioned at 30um thickness on a cryostat. These sections were stored in cryoprotectant at −20 °C until use. Sections were washed 10 × 5 minutes in PBS, incubated for 20 min in 1% H2O2 with 0.3% NaOH in PBS, 10 min in 0.3% glycine in PBS, and 10 min in 0.03% SDS in PBS before blocking for one hour in blocking buffer (3% Normal Donkey Serum and 2.5% triton X100). The sections were then incubated overnight in 1:2000 chicken anti-cfos (Milipore, MA, USA) and 1:1000 rabbit antiGFP (Aveslab, OR, USA) antibodies. The following day, the sections were washed 10 × 5 minutes in PBS, incubated for 2 h with 1:200 of biotinylated anti chicken antibody (Jackson Immuno Research, PA, USA), washed 6 × 5 minutes in PBS before being transferred to ABC solution (Vectorlabs, CA, USA) for 1 h. After washing, the 10 × 5 minutes in PBS stain took place by adding sections to DAB reagent (Thermofisher, MA, USA) for no more than 10 min. Sections were checked regularly for stain development. The sections were then washed 10 × 5 minutes in PBS before incubation in 1:200 donkey anti chick fluorescent antibody (Jackson Immuno Research, PA, USA). After 3 × 5 minutes of wash in PBS, the sections were mounted on gelatin coated slides (Fischer Scientific). Once they were almost dry they were cover-slipped using prolong gold (Thermofisher, NH, USA). Images were obtained using either regular light or fluorescence microscopy. These images were then overlaid to check for co-localization. GFP containing neurons and c-fos positive nuclei were counted using Image J and the Franklin and Paxinos mouse brain atlas for anatomical reference.
2.6.2. For immunofluorescent detection of GFRAL and GLP-1R in mouse hindbrain, FFPE mouse brain was sectioned into 4.5 μm thick sections using a microtome (Leica RM 2255, Leica Biosystems)
Sections were dewaxed, hydrated, and incubated in 0.1% pronase in TBS-T buffer for 10 min. Sections were then blocked with avidin-biotin (004303, Thermo Fisher Scientific, Waltham, MA) prior to incubation with 5 μg/ml rabbit anti-GLP-1R antibody (7F38, Novo Nordisk A/S, Denmark) for 1 h at RT. Following washing in TBS-T, sections were incubated with biotinylated donkey anti-rabbit antibody (1:200, 711-165-152, Jackson ImmunoResearch, Cambridgeshire, UK) for 30 min. Sections were washed in TBS-T and signal amplification was performed with ABC elite kit (PK6100, Vector Laboratories, Burlingame, CA) followed by incubation in 1:50 TSA-Flourscein-Cy2 (SAT701001EA, Perkin Elmer, Waltham, MA) for 40 min. Next, the sections were incubated in TEG buffer (pH 9.0) for 15 min, washed in TBS-T, blocked with avidin-biotin, and incubated in TNB for 30 min prior to incubation with 0.2 mg/ml sheep-anti GFRAL antibody (PA547769, Thermo Fisher Scientific) over night at 4 °C. Following washing in TBS-T, sections were incubated in 1:1000 biotinylated donkey anti-sheep antibody (713-065-147, Jackson Immuno Research) for 30 min at RT. Signal amplification was performed with 1:100 TSA Tyramide-Cy3 (SAT704A001EA, Perkin Elmer). Sections were washed in dH2O and mounted with fluorescent mounting media (S3023, Dako). Confocal images were acquired using the Leica Confocal Imaging System TCS SP8.
2.6.3. RNAscope in situ hybridization (ISH) technology
Duplex ISH was performed on the Leica system platform (IL, USA) using the RNAscope 2.5 LS Duplex Reagent kit (322440, Advanced Cell Diagnostics (ACDbio), Newark, CA) and the RNAscope 2.5 LS Green accessory pack (322550, ACDbio) according to manufacturer's instructions. For staining of mouse tissue sections, the following probes from ACDbio were used; Gfral 439149-C1, Glp1r 418858-C2, bacterial Dihydrodipicolinate reductase (Dapb) 312039-C1 (negative control probe), or Ppib 313918-C2 (positive control probe). For staining of human tissue sections the following probes from ACDbio were used; GFRAL 435678-C1, GLP1R 519828-C3, DAPB 312038-C2, or PPIB 313908-C1. Slides were counterstained in hematoxylin/eosin, washed in dH2O, mounted, and coverslipped. Bright field images were obtained with a Hamamatsu NanoZoomer 2.0 HT.
3. Results
3.1. GDF15 treatment lowers the preference for fat whereas deletion of Gdf15 or Gfral does not alter food choice
To test the effect of GDF15 on macronutrient intake in a diet induced obese mouse model, C57BL/6 mice were maintained on a high-fat diet for 9 weeks before being introduced to the macronutrients. Daily subcutaneous injections of GDF15 (0.4 mg/kg) over 4 days significantly decreased the intake of fat, whereas there was no difference in protein or carbohydrate intake (Figure 1A). Due to the reduced overall caloric intake (Figure 1A), the reduction in fat intake did not significantly shift the overall relationship between the percentages of calories obtained via fat versus carbohydrate (Figure 1B).
Next we tested if deletion of either Gdf15 or Gfral would affect the preference for macronutrients in mice that were fed either regular chow (Figure 1C,D) or high fat diet (Figure 1E,F). In both experiments, we found that fat preference was not affected by the lack of GDF15 or GFRAL signaling. However there was a trend towards a decreased carbohydrate intake in the chow-fed Gfral−/− mice (Figure 1C). Similar to what was observed in GDF15-treated mice, none of these changes caused the relationship between the percentages of calories obtained from fat versus carbohydrate to change (Figure 1D,F).
3.2. Body weight, food intake, and macronutrient preference after VSG in Gdf15−/−mice
Mice were fed high-fat diets for 8 weeks prior to surgery and were monitored for food intake and body weight for up to 13 weeks post-surgery. After an initial drop in body weight in the recovery period (Figure 2A,B) during which the food intake was suppressed (Figure 2C) the mice resumed to consuming the same number of calories as in the sham-treated groups. This is consistent with what we have observed in other experiments as well [16], [17]. The food intake was significantly reduced with VSG surgery during the first few weeks after putting the mice back on high fat diet and the mice undergoing VSG gained less weight throughout the majority of the study – an effect that was not altered by loss-of-function for Gdf15 (Figure 2B). During the macronutrient preference test (indicated by the grey bar across Figure 2A–C), the mice ate fewer calories (data not shown) causing weight loss. This was followed by a compensatory increase in food intake upon high-fat diet re-feeding. These changes in bodyweight and caloric intake did not differ with Gdf15 deletion. In the macronutrient preference test, VSG caused mice to substitute fat calories for carbohydrates (Figure 2D,E). Sham animals ingested small amounts of carbohydrates relative to fat, whereas the ratio between carbohydrates and fat intake was significantly altered with VSG (Figure 2E). However, only in the Gdf15−/− mice was there a significant effect of surgery on fat intake within genotype such that fat intake in these mice was reduced to a larger extent with VSG than in the WT mice (Figure 2D).
3.3. Anatomical co-localization of GLP-1R expressing neurons and neurons activated by GDF15
As GDF15 acts on hindbrain areas known to also express the GLP-1R, we used a GLP-1R reporter mouse line expressing GFP in Glp1r-expressing neurons to test if the neurons activated by GDF15 express the GLP-1R. We treated Glp1rcre/+;Rosa26eGFP-L10a/+ mice with 0.4 mg/kg of GDF15 4 h prior to brain extraction. Tissue sections were stained for FOS and GFP, and the numbers of GFP positive neurons, FOS activated neurons, or both, were counted in the AP/NTS, the PBN, and CeA region, which are all known to be activated by GDF15 [5]. But only few GLP-1R positive neurons were also activated by GDF15 (Figure 3).
3.4. Anatomical co-localization of GLP-1R and GFRAL in mouse and human hindbrain
In situ hybridization analysis was utilized in order to identify hindbrain neurons carrying mRNA for Gfral, Glp1r, or both. In the mouse hindbrain (Figure 4A–C), we found that many Gfral neurons within the AP area also contained Glp1r mRNA, albeit in varying amounts. However, the majority of the Glp1r neurons in this hindbrain area did not express Gfral mRNA. A similar expression pattern of GFRAL and GLP1R was observed in the human hindbrain (Figure 4D–F) with many GFRAL neurons expressing GLP1R mRNA to varying degrees, whereas only few of the neurons expressing GLP1R mRNA also expressed GFRAL mRNA. Immunoflourescent staining of mouse hindbrain confirmed the co-localization of GFRAL and GLP-1R protein in many neurons localized within the AP area (Figure 4G–J).
3.5. Food intake after GLP-1R agonist (liraglutide) treatment in mice with targeted genetic deletion of Gdf15 or Gfral; GDF15 treatment in mice with targeted genetic deletion of Glp1r; combined GDF15 and liraglutide treatment
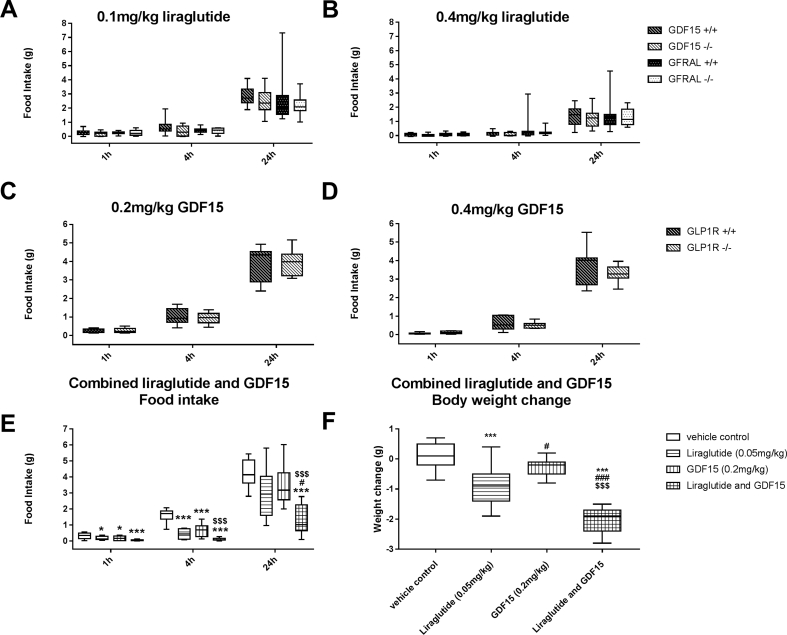
Given the overlap of GLP-1R and GFRAL expression in the AP (Figure 4) but also the minimal activation of GLP-1R neurons by GDF15 (Figure 3), we sought to test the necessity of GLP-1R signaling to the anorexic effects of GDF15 and vice versa. We found that liraglutide-induced anorexia was not altered by lack of either Gdf15 or Gfral (Figure 5A,B). Similarly GDF15-induced reductions in food intake were not altered by lack of Glp1r (Figure 5C,D). Lastly, we measured food intake in mice treated either with low doses of liraglutide or GDF15 alone or in combination (Figure 5E). At the doses chosen, the effect on food intake by either of the two peptides alone was modest, and the food intake recovered to control levels within 24 h after dosing. However, the combined GDF15 and liraglutide treatment resulted in significantly greater reductions in food intake significant at 4 h and 24 h after dosing. The combination of liraglutide and GDF15 resulted in a synergistic weight loss effect with the weight loss observed in GDF15 and liraglutide co-treated animals (−2.01 ± 0.42 g) being significantly greater than the combined weight loss of mice with either GDF15 or liraglutide alone (−1.20 ± 0.97 g, p = 0.0304 Tukey's multiple comparison post-hoc one-way ANOVA) (Figure 5F).
Figure 5.
Food intake after liraglutide in mice lacking GDF15 or GFRAL, in GLP-1R deleted mice treated with GDF15, and combined GDF15 and liraglutide treatment. Chow fed GDF15 or GFRAL deleted mice and their wild type litter mates were injected with either 0.1 mg/kg (5A) or 0.4 mg/kg (5B) liraglutide and the food intake was measured after 1, 4, and 24 h. Chow fed mice lacking the GLP-1 receptor were treated with 0.2 mg/kg GDF15 (5C) or 0.4 mg/kg GDF15 (5D) and their food intake was measured for 1, 4, and 24 h. Chow fed regular C57BL/6 mice were injected with vehicle, 0.05 mg/kg liraglutide, 0.2 mg/kg GDF15 or a combination of liraglutide and GDF15, respectively. Food intake (5E) and bodyweights (5F) were recorded after the injections. All graphs are displayed as box and whiskers plots where error bars represent the highest and lowest value within each bar.
Note added in proof: As per the recommendation of David Polidori, PhD, and as described in Slinker [18], we performed a 2-way ANOVA of the 4 groups arranged in a 2×2 factorial design and have found that p = 0.016 for an interaction between liraglutide and GDF15.
4. Discussion
While GDF15-based therapies are promising new avenues for the treatment of obesity, little is known about this system outside of the context of cancer-related anorexia. First, we examined the role that GDF15 plays not only to alter caloric intake but also to alter food choice. Neither Gdf15-nor Gfral-null mice had significant differences in their relative food choice when given jars of pure macronutrients as compared to their WT controls. This outcome indicates that endogenous GDF15 does not play a major role in food choice in this paradigm. However, GDF15 administration selectively reduced preference for fat (see Figure 1A). This is reminiscent of what is observed in both rats and mice after bariatric surgery including VSG and RYGB [10], [11]. Consequently, we hypothesized that the VSG may increase GDF15/GFRAL signaling in the brainstem as an important regulatory component on food intake, body weight and food choice. The current data replicate the effect of VSG to alter food choice, but the effect was similar in Gdf15−/− and control mice. This extended to the effects of VSG on food intake, body weight, and body fat as well. Consequently, it would appear that GDF15 is not necessary for the potent effects of VSG on food intake, body weight and food choice.
Another important therapeutic advance in the treatment of obesity has come in the form of long-acting GLP-1R agonists. GLP-1R is found in the same hindbrain regions as GFRAL, and GFRAL is expressed in a subset of GLP-1R neurons in both human and mouse (see Figure 4). However, GDF15 did not appear to activate GLP-1R neurons in the AP (see Figure 3), which most likely reflects that, whereas the majority of GFRAL neurons contained highly varying degrees of GLP-1R mRNA and protein expression, only a small fraction of the GLP-1R neurons also contained GFRAL. Moreover, GDF15 was equally effective in Glp1r-null and control mice. The reverse was also true for the GLP-1R agonist liraglutide, which was equally effective in Gdf15-and Gfral-null mice as in controls. Interestingly, when low doses of liraglutide and GDF15 were combined, the reductions in food intake were additive, and the effect on body weight loss was statistically synergistic. This has two important implications. First, it implies that despite some overlap between GFRAL and GLP-1R expression in the hindbrain, GDF15 and GLP-1R agonists engage separate anorexic neuronal systems. Second, activating both the GFRAL and GLP-1R simultaneously may make it possible to produce greater weight loss than can be safely achieved by activating either of these systems alone.
While it is clear that the GDF15/GFRAL system plays an important role in regulating food intake under certain pathophysiological conditions such as cancer-associated weight loss and toxic chemotherapies [5], [19], key questions remain as to whether there are other circumstances where this system plays a vital role in regulating energy balance. The current data indicate that the GDF15/GFRAL system is not critical to the effects of VSG or GLP-1R agonists. With the identification of GFRAL as the target for the anorexic effects of GDF15, it will now be possible to reveal how these neurons are connected to the circuitry that serves to regulate food intake under a wide range of circumstances.
Acknowledgements
The authors would like to thank the Edinburgh Brain and Tissue Bank (Edinburgh, Scotland), funded by the UK MRC, for providing human hindbrain samples. We also thank the surgeons carrying out the VSG procedure: Alfor Lewis, Andriy Myronovych, Mouhamadoul Toure, and Diane Farris (all at University of Michigan), and Jeanette Bannebjerg Johansen (Novo Nordisk A/S) for technical assistance with ISH and IHC analyses. H.F.-S. designed, performed, and analyzed all experiments (except the ISH and IHC data) and drafted the manuscript including editing and revising. K.H. designed and analyzed the ISH and IHC experiments and helped drafting, editing, revising, and approving the manuscript. J.G. developed the key methodologies for identifying activated neurons. S.B.J., M.G.M., and R.J.S. designed the studies, interpreted the results and edited, revised, and approved the final version of the manuscript. M.G.M. designed the Gdf15−/− and Gfral−/− mouse lines and provided the Glp1rcre/+;Rosa26eGFP-L10a/+ mice. R.J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
We declare that the studies were funded NIH grant R01DK107652 (R.J.S. and H.F.-S.) and by Novo Nordisk through which the authors SBJ and KH are employees and stakeholders. This does not alter our adherence to all the Molecular Metabolism policies on sharing data and materials. All Intellectual Property Rights of the current study are owned by University of Michigan, and there has been no compromise of the objectivity or validity of the data in the manuscript. RJS receives financial support from Novo Nordisk, Janssen, Zafgen, Kallyope, and Medimune. He has also served as a paid consultant for Novo Nordisk, Janssen, Kallyope, and Scohia. MGM receives research support from Novo Nordisk and MedImmune.
References
- 1.Tsai V.W., Zhang H.P., Manandhar R., Lee-Ng K.K.M., Lebhar H., Marquis C.P. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. International Journal of Obesity. 2017 doi: 10.1038/ijo.2017.258. [DOI] [PubMed] [Google Scholar]
- 2.Mullican S.E., Rangwala S.M. Uniting GDF15 and GFRAL: therapeutic opportunities in obesity and beyond. Trends in Endocrinology and Metabolism. 2018;29(8):560–570. doi: 10.1016/j.tem.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Yang L., Chang C.C., Sun Z., Madsen D., Zhu H., Padkjaer S.B. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nature Medicine. 2017;23(10):1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 4.Mullican S.E., Lin-Schmidt X., Chin C.N., Chavez J.A., Furman J.L., Armstrong A.A. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nature Medicine. 2017;23(10):1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 5.Hsu J.Y., Crawley S., Chen M., Ayupova D.A., Lindhout D.A., Higbee J. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550(7675):255–259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 6.Emmerson P.J., Wang F., Du Y., Liu Q., Pickard R.T., Gonciarz M.D. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nature Medicine. 2017;23(10):1215–1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 7.Price C.J., Hoyda T.D., Ferguson A.V. The area postrema: a brain monitor and integrator of systemic autonomic state. The Neuroscientist. 2008;14(2):182–194. doi: 10.1177/1073858407311100. [DOI] [PubMed] [Google Scholar]
- 8.Stefater M.A., Wilson-Perez H.E., Chambers A.P., Sandoval D.A., Seeley R.J. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocrine Reviews. 2012;33(4):595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers A.P., Wilson-Perez H.E., McGrath S., Grayson B.E., Ryan K.K., D'Alessio D.A. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. American Journal of Physiology Endocrinology and Metabolism. 2012;303(8):E1076–E1084. doi: 10.1152/ajpendo.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson-Perez H.E., Chambers A.P., Sandoval D.A., Stefater M.A., Woods S.C., Benoit S.C. The effect of vertical sleeve gastrectomy on food choice in rats. International Journal of Obesity. 2013;37(2):288–295. doi: 10.1038/ijo.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pressler J.W., Haller A., Sorrell J., Wang F., Seeley R.J., Tso P. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2015;64(2):498–507. doi: 10.2337/db14-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cork S.C., Richards J.E., Holt M.K., Gribble F.M., Reimann F., Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular Metabolism. 2015;4(10):718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison M.B., Patterson C.M., Krashes M.J., Lowell B.B., Myers M.G., Jr., Olson D.P. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Molecular Metabolism. 2015;4(4):299–309. doi: 10.1016/j.molmet.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson-Perez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D.A., Stoffers D. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myronovych A., Salazar-Gonzalez R.M., Ryan K.K., Miles L., Zhang W., Jha P. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity (Silver Spring) 2014;22(11):2301–2311. doi: 10.1002/oby.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slinker B.K. The Statistics of Synergism. Journal of Molecular Cellular Cardiology. 1998;30:723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 19.Johnen H., Lin S., Kuffner T., Brown D.A., Tsai V.W., Bauskin A.R. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nature Medicine. 2007;13(11):1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]