Abstract
Purpose:
To describe the prevalence of various types of childhood glaucomas, their clinical features and treatment methods.
Methods:
We prospectively included consecutive children with glaucoma presenting to glaucoma clinic for the first time between March 2013 and May 2014. We classified childhood glaucomas as per the classification proposed by Congenital Glaucoma Research Network.
Results:
Of the 275 children (449 eyes) with glaucoma during this period, primary glaucomas constituted 56% (n = 252 eyes of 145 children), including 169 eyes (37.64%) of 97 children with primary congenital glaucoma (PCG), 16 eyes (3.56%) of 10 children with infantile glaucoma, and 67 eyes (14.9%) of 38 children with juvenile open angle glaucoma. Among these, 85% (214 eyes of 107 children) had bilateral involvement. Secondary glaucomas constituted 44%; they were glaucoma associated with ocular anomalies 18% (n = 80 eyes), glaucoma associated with acquired conditions (steroid induced and traumatic glaucoma) 14% (n = 61 eyes), glaucoma following congenital cataract surgery 7.6% (n = 34 eyes), and glaucoma associated with systemic or syndromic conditions 5% (n = 22 eyes). In primary glaucomas, boys and girls were equally affected (1:1), and more boys (3.8:1) had acquired glaucomas. Close to 80% PCG eyes were managed surgically with combined trabeculotomy and trabeculectomy (CTT). Majority of secondary glaucomas were managed medically.
Conclusion:
In our cohort, PCG was the most common childhood glaucoma and CTT was the most common surgery performed in these eyes. Steroid-induced and traumatic glaucomas were the most common acquired glaucomas; appropriate measures should be taken to avert these preventable glaucomas in children.
Keywords: Childhood glaucoma in India, childhood glaucomas, congenital glaucoma, prevalence of glaucoma in children, profile of glaucoma in children, South India
Childhood glaucoma affects >300,000 children worldwide, often associated with significant visual loss (two-third of these children are blind).[1] There is a higher prevalence with more severe disease phenotype in the developing countries.[2,3] The prevalence of primary congenital glaucoma (PCG) is one in 3,300 live births and PCG accounts for 4.2% of all childhood blindness in Indian population. The highest reported prevalence of PCG is in Slovakian gypsies (1 in 1,250),[4] followed by Saudi Arabia (1 in 2,500).[5,6] Compared with these, the prevalence in western populations is 1 in 10,000 to 1 in 68,254 live births.[7,8,9] The typical presentation of PCG in India is a severe disease phenotype with total or near-total corneal edema.[10] There are several types of secondary glaucomas in children, the prevalence of which is not reported. Understanding the disease pattern, their presenting features and proportion of different types of glaucoma is important to plan appropriate eye care and training, to create awareness and allocate resources, and to plan appropriate management strategies.
Due to lack of uniformity across studies to define glaucoma in children, age groups included, IOP cut offs and other criteria to diagnose and monitor response to treatment; there is a huge variability in reporting prevalence and outcomes. We know that the type of intervention and the outcomes vary based on the etiology of glaucoma.[11]
The main objective of our study is to understand the pattern of newly diagnosed pediatric glaucoma patients visiting our tertiary eye care service and to report the prevalence of each subtype of glaucoma in children <16 years of age. The Congenital Glaucoma Research Network (CGRN) proposed a classification system to classify children with glaucoma that is clinically meaningful,[12] which helps to recognize the etiology and mechanisms that cause glaucoma in children.[11,13] We used CGRN classification to describe the pediatric glaucoma profile in our cohort.
Methods
The study was conducted from March 2013 to May 2014. All consecutive children with glaucoma presenting to the institute for the first time were enrolled in the study. Informed consent was obtained from the guardians of all participants and the institutional review board approved the study. This study was a part of the International Pilot Survey of Childhood Glaucoma (IPSOCG). The study protocol adhered to the tenets of the Declaration of Helsinki for research involving human subjects. All children underwent a comprehensive examination including birth history, demographic data, laterality, visual acuity, refraction, anterior segment and posterior segment assessment, systemic evaluation, medication, and surgery. The visual acuity assessment was attempted in all children and age appropriate visual acuity assessment was performed. They were fixing and following light for nonverbal children, Teller acuity chart, or Snellen chart for verbal children. The details noted corneal clarity, corneal diameter, retinoscopy, IOP measurement, gonioscopy, and optic disc evaluation with direct or indirect ophthalmoscopy when corneal clarity permitted. Horizontal white-to-white corneal diameter was measured using calipers. Evaluation was done under general anesthesia (using sevofluorane anesthesia) or using slitlamp biomicroscope in the clinic. IOP was recorded using Goldmann Applanation Tonometer in older and cooperative children and with Perkins tonometer in children examined under anesthesia.
We defined childhood glaucoma with two or more of the following findings:
IOP > 21 mm Hg on repeated testing or when IOP was <21 in the presence of corneal changes or under medications for IOP lowering
Optic disc cupping: progressive increase in cup-disc ratio, cup-disc asymmetry of ≥0.2, or rim thinning
Corneal changes/findings: Haab's striae or corneal diameter ≥11 mm in newborn, >12 mm in child <1-year of age, >13 mm at any age
Progressive myopia or myopic shift coupled with increase in ocular dimensions beyond the normal growth
A reproducible visual field defect consistent with glaucomatous optic neuropathy (Humphrey visual field test).
We classified childhood glaucomas in accordance with the recent CGRN classification:[13]
Primary glaucoma: The primary childhood glaucoma included PCG: glaucoma detected at birth to 1-year; infantile glaucoma: glaucoma detected >1–3 years of life; and juvenile open angle glaucoma (JOAG): glaucoma detected at age >3 and <16 years
-
Secondary glaucomas:
- Glaucoma associated with (nonacquired) ocular anomalies (e.g., anterior segment dysgenesis, etc.)
- Glaucoma associated with systemic disease or syndrome (e.g., Sturge–Weber syndrome)
- Glaucoma following congenital cataract surgery: postcataract surgery with aphakia or pseudophakia
- Glaucoma associated with acquired conditions included steroid-induced, traumatic, and uveitic glaucomas.
Statistical analysis
Normality of the data was analyzed using Shipro–Wilk test. Descriptive statistics included mean and standard deviation for normally distributed variables and median with interquartile range (IQR) for non-normally distributed variables. Categorical variables were summarized as percentages. For proportions, Pearson's Chi-squared test was used, and for means, one-way analysis of variance was used. All the calculations and charts were generated using Microsoft Excel 2016. Statistical analysis was performed using ‘R’ software (version 3.3.2).
Results
Demographic and clinical characteristics
In total, 275 children (449 eyes) were seen in the study period. Of the 275 children, 174 children (348 eyes) had bilateral involvement and remaining 101 children had unilateral involvement. The median age at presentation was 15 months (range: 0–190 months). Table 1 shows clinical characteristics of children with various subtypes of glaucoma. In our cohort, primary glaucoma was most common; accounting for 56% of patients, of this, PCG was highest at 38%. In children with primary glaucoma, 85% (214 eyes of 107 children) had bilateral involvement and 15% (38 eyes) had unilateral involvement. Male:female ratio was close to one in primary glaucoma as well as in glaucoma associated with ocular anomalies. The male:female ratio was 2:1 in children with glaucoma following cataract surgery and 3.8:1 in glaucoma associated with acquired conditions. The mean age at presentation was 3 months in PCG and was 10 years in children with glaucoma associated with acquired conditions. The mean IOP at presentation in different glaucoma subtypes is also given in Table 1. The mean presenting IOP was highest in JOAG group (28.40 ± 10.6 mm Hg). Posttreatment IOP was least in PCG group (13.27 ± 5.7 mm Hg) and was highest in glaucoma associated with congenital cataract surgery (21.26 ± 9.38 mm Hg).
Table 1.
Clinical Characteristics of children with various types of childhood glaucomas
| Diagnosis | Number of eyes (%) | Laterality | Gender | Age at presentation (months) | Age at surgery (months) | IOP at presentation (mean±SD) | IOP at last visit (mean±SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary childhood glaucoma (PCG + IG + JOAG) | 252 (56.12%) | OD | 20 | M:F | 77:68 | Mean | 35.31 | Mean | 18.44 | 26.20±7.45 | 14.60±7.24 |
| OS | 18 | Median | 5 | Median | 4 | ||||||
| OU | 107 | ||||||||||
| Congenital glaucoma (PCG) | 169 (37.64%) | OD | 14 | M:F | 49:48 | Mean | 3.09 | Mean | 3.56 | 25.52±5.68 | 13.27±5.76 |
| OS | 11 | Median | 2 | Median | 2 | ||||||
| OU | 72 | ||||||||||
| Infantile glaucoma | 16 (3.56%) | OD | 2 | M:F | 6:4 | Mean | 21.63 | Mean | 18.17 | 25.08±8.64 | 15.83±5.38 |
| OS | 2 | Median | 21 | Median | 16 | ||||||
| OU | 6 | ||||||||||
| Juvenile glaucoma (JOAG) | 67 (14.92%) | OD | 4 | M:F | 22:16 | Mean | 119.82 | Mean | 134.06 | 28.40±10.66 | 16.96±9.21 |
| OS | 5 | Median | 128 | Median | 132 | ||||||
| OU | 29 | ||||||||||
| Secondary glaucoma | 197 (43.88%) | ||||||||||
| Glaucoma following cataract Surgery | 34 (7.57%) | OD | 7 | M:F | 15:7 | Mean | 86.32 | Mean | 44.17 | 24.64±10.09 | 21.26±9.38 |
| OS | 3 | Median | 70 | Median | 44 | ||||||
| OU | 12 | ||||||||||
| Glaucoma associated with syndromes/systemic diseases | 22 (4.90%) | OD | 5 | M:F | 8:8 | Mean | 73.36 | Mean | 24.14 | 24.26±8.04 | 17.38±6.63 |
| OS | 5 | Median | 39 | Median | 1 | ||||||
| OU | 6 | ||||||||||
| Glaucoma associated with nonacquired ocular anomalies | 80 (17.82%) | OD | 11 | M:F | 27:21 | Mean | 55.63 | Mean | 32.67 | 25.83±10.16 | 18.71±8.66 |
| OS | 5 | Median | 34 | Median | 7.5 | ||||||
| OU | 32 | ||||||||||
| Glaucoma associated with acquired conditions | 61 (13.59%) | OD | 15 | M:F | 35:9 | Mean | 119.77 | Mean | 95.50 | 25.82±11.42 | 18.07±10.04 |
| OS | 12 | Median | 100 | Median | 65 | ||||||
| OU | 17 | ||||||||||
| Grand total | 449 (100.0%) | OD | 58 | M:F | 162:113 | Mean | 56.13 | Mean | 24.67 | 25.87±8.80 | 16.74±8.53 |
| OS | 43 | Median | 15 | Median | 4 | ||||||
| OU | 174 | ||||||||||
IOP=Intraocular pressure, SD=Standard deviation, PCG=Primary congenital glaucoma, IG=Infantile glaucoma, JOAG=Juvenile open angle glaucoma, OD=Right eye, OS=Left eye, OU=Both eyes, M=Male, F=Female
Prevalence of different subtypes of glaucoma in children
This is shown in Table 2; majority were primary childhood glaucomas; these were a total of 252 eyes (56.12%) of 145 children [Figs. 1 and 2]. Of these, majority were congenital glaucoma with 169 eyes (37.64%) of 97 children; infantile glaucoma was 16 eyes (3.56%) of 10 children followed by juvenile glaucoma with 67 eyes (14.92%) of 38 children. Glaucoma following cataract surgery was seen in 34 eyes (7.57%) of 22 children, of which 24 eyes of 14 children were aphakic (5.09%) and 10 eyes of eight children (2.23%) were pseudophakic [Fig. 3]. Glaucoma associated with syndromic/systemic conditions accounted to 22 eyes (4.9%) of 16 children. Phakomatosis was the major syndromic association seen in our cohort including Neurofibromatosis, Nevus of Ota, Phacomatosis Pigmentosa Vascularis, and Struge–Weber Syndrome [Fig. 4]. The others were Axenfeld-Rieger Syndrome (ARS) [Fig. 5], and Horlwis Syndrome. In glaucoma associated with nonacquired ocular anomalies 80 eyes (17.8%) of 48 subjects, majority were conditions associated with congenital corneal, lenticular, or retinal pathologies as shown in Table 2. In glaucoma associated with acquired conditions 61 eyes (13.59%) of 44 subjects, steroid-induced glaucoma was the most prevalent in 34 eyes (7.57%) of 17 subjects followed by traumatic glaucoma in 23 eyes (5.12%) of 23 subjects [Fig. 6].
Table 2.
Prevalence of different conditions in each category of diagnosis
| Diagnosis | Number of eyes | Percentage | Number of subjects | Percentage |
|---|---|---|---|---|
| Primary childhood glaucoma | 252 | 56.12 | 145 | 52.73 |
| Primary congenital glaucoma | 169 | 37.64 | 97 | 35.27 |
| Infantile glaucoma | 16 | 3.56 | 10 | 3.64 |
| Juvenile glaucoma | 67 | 14.92 | 38 | 13.82 |
| Glaucoma following congenital cataract surgery | 34 | 7.57 | 22 | 8.00 |
| Aphakia | 24 | 5.35 | 14 | 5.09 |
| Pseudophakia | 10 | 2.23 | 8 | 2.91 |
| Glaucoma associated with syndromes | 22 | 4.90 | 16 | 5.82 |
| Axenfeld--Rieger Syndrome | 6 | 1.34 | 3 | 1.09 |
| Horlwis Syndrome | 2 | 0.45 | 1 | 0.36 |
| Neurofibromatosis | 4 | 0.89 | 4 | 1.45 |
| Nevus of Ota | 3 | 0.67 | 2 | 0.73 |
| Phacomatosis Pigmentosa Vascularis | 2 | 0.45 | 2 | 0.73 |
| Struge-Weber Syndrome | 5 | 1.11 | 4 | 1.45 |
| Glaucoma associated with nonacquired ocular anomalies | 80 | 17.82 | 48 | 17.45 |
| Aniridia | 12 | 2.67 | 6 | 2.18 |
| Anterior segment dysgenesis (Peters anomaly and sclerocornea) | 17 | 3.79 | 9 | 3.27 |
| Microspherophakia | 14 | 3.12 | 7 | 2.55 |
| Congenital hereditary endothelial dystrophy | 14 | 3.12 | 7 | 2.55 |
| Ectopia lentis | 1 | 0.22 | 1 | 0.36 |
| Familial exudative vitreoretinopathy | 3 | 0.67 | 3 | 1.09 |
| Microphthalmos | 6 | 1.34 | 3 | 1.09 |
| Coats disease | 6 | 1.34 | 6 | 2.18 |
| Persistent hyperplastic primary vitreous | 3 | 0.67 | 2 | 0.73 |
| Retinoblastoma | 1 | 0.22 | 1 | 0.36 |
| Retinopathy of prematurity | 3 | 0.67 | 3 | 1.09 |
| Glaucoma associated with acquired conditions | 61 | 13.59 | 44 | 16.00 |
| Iris cyst | 1 | 0.22 | 1 | 0.36 |
| Steroid-induced glaucoma | 34 | 7.57 | 17 | 6.18 |
| Traumatic glaucoma | 23 | 5.12 | 23 | 8.36 |
| Uveitis | 3 | 0.67 | 3 | 1.09 |
| Grand total | 449 | 100.00 | 275 | 100.00 |
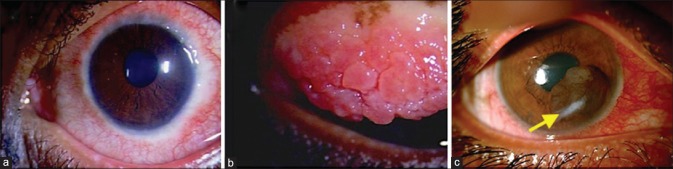
Figure 1.
(a) Clinical photograph of a child with bilateral primary congenital glaucoma with acute hydrops in the left eye. (b) A magnified view of the left eye with acute hydrops. (c) Picture of clear corneas 1-month postoperative (after combined trabeculotomy and trabeculectomy). (d) The left eye with clear cornea and Habb's striae
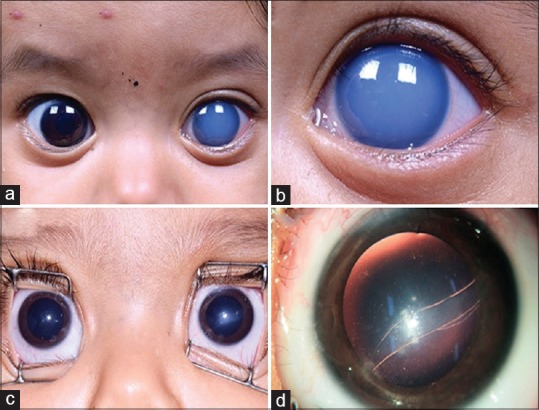
Figure 2.
Shows various grades of corneal edema in children with primary congenital glaucoma. Child with bilateral PCG with (a) grade 2 corneal edema, (b) grade 3 corneal edema, (c) grade 4 corneal edema, and (d) grade 5 corneal edema
Figure 3.
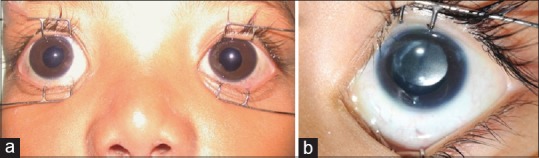
(a) Clinical photograph of postcongenital cataract surgery glaucoma: 3A: bilateral glaucoma in aphakia. (b) Picture of an eye with pseudophakia and secondary glaucoma
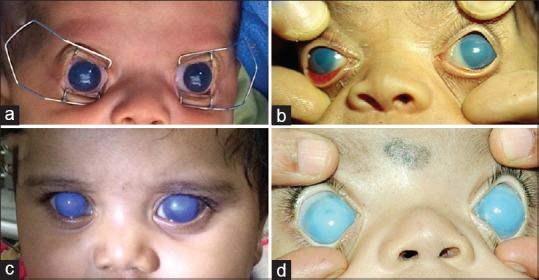
Figure 4.
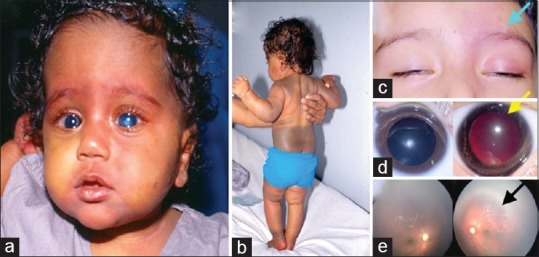
(a) Clinical photograph of a child with bilateral congenital glaucoma, bilateral vascular nevus on the face, and (b) extensive pigmentary nevus on the skin (Phacomatosis pigmentovascularis). (c) External photograph of a child with unilateral Sturge–Weber syndrome (Left-sided facial hemangioma). (d) Right and left eye photographs showing a dark red glow in the left eye suggestive of choroidal hemangioma. (e) Fundus photograph showing diffuse choroidal hemangioma in the left eye
Figure 5.
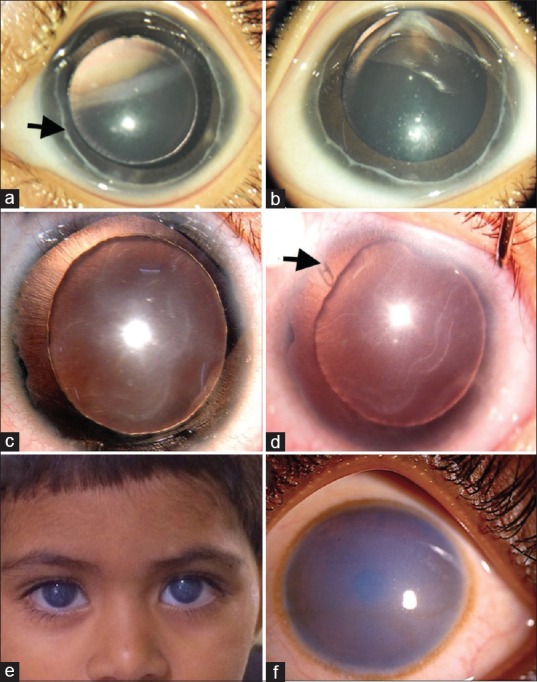
Postoperative photograph of bilateral Axenfeld-Rieger anomaly with congenital glaucoma (a and b), showing prominent Schwalbe's line in both eyes (arrow). Clinical photograph of Aniridia with congenital glaucoma (c), note the Habb's striae and corneal edema. Postoperatve (post-Ahmed glaucoma valve implantation) photograph (d), showing clear cornea with Habb's striae and tube visible superotemporally (arrow). Clinical photograph of bilateral congenital hereditary endothelial dystrophy showing diffuse corneal haze in both the eyes (e), higher magnification showing ground glass appearance of the corneal haze from limbus to limbus (f)
Figure 6.
Clinical picture of eye with severe vernal keratoconjunctivitis with limbal thickening (a) and palpebral giant papillae (b) with steroid induced glaucoma. (c) Clinical photograph of the right eye with post traumatic implantation iris cyst and secondary glaucoma. Note the corneal scar from penetrating injury (arrow)
Age distribution
At presentation, 126 children (45.8%) were <12 months of age, 35 children (12.73%) were between 1 and 5 years, and 114 children (41.46%) were aged between 5 and 16 years. Majority of them were diagnosed either <3 months of age (62 children) or >10 years of age (69 children) and the rest 144 children ranged between >3 months and <10 years. Majority of children with primary glaucomas were <1 year of age and those with secondary glaucomas were older. At presentation, 91% of children with glaucoma associated with acquired conditions, 62.5% of children with glaucoma associated with syndromes, 72.7% of children with glaucoma following cataract surgery were >3 years of age, and 67% of children with PCG were <1 year of age.
Visual acuity
Of the 449 eyes, visual acuity could be assessed in 387 eyes and in 62 eyes visual acuity could not be assessed. The detail of visual acuity at presentation is provided in Table 3. In 204 eyes (52.7%), the recorded vision was fixing and following light, 10.8% eyes had VA better than 20/40, 11.3% eyes had VA between 20/50 and 20/200, 8% eyes had VA <20/200 and counting fingers, and 17% eyes had hand motions or less. Visual acuity better than 20/40 was seen in >40% eyes among glaucoma associated with acquired conditions. In PCG group, majority of the eyes had visual acuity recorded as fixing and following light.
Table 3.
Table showing visual acuity in eyes with various glaucoma subtypes in our cohort
| 20/20-20/40 | 20/50-20/200 | <20/200 to CF | HM or less | FFL | Total | Could not be assessed | |
|---|---|---|---|---|---|---|---|
| Primary childhood glaucoma (n=252) | 9 | 13 | 13 | 26 | 145 | 206 | 46 |
| Glaucoma associated with nonacquired ocular anomalies (n=80) | 4 | 13 | 3 | 17 | 40 | 77 | 03 |
| Glaucoma associated with syndromes (n=22) | 4 | 2 | 2 | 4 | 8 | 20 | 02 |
| Glaucoma following cataract surgery (n=34) | 0 | 6 | 6 | 9 | 6 | 27 | 07 |
| Glaucoma associated with acquired conditions (n=61) | 25 | 10 | 7 | 10 | 5 | 57 | 04 |
| Total | 42 (10.8%) | 44 (11.3%) | 31 (8.0%) | 66 (17%) | 204 (52.7%) | 387 | 62 |
CF=Counting finger, HM=Hand motions, FFL=Fixing following light, n=Number of eyes
Associated comorbidities
Associated comorbidities in eyes with primary developmental glaucomas were cataract in three eyes, congenital nasolacrimal duct obstruction in six eyes, congenital corneal opacity in two eyes, acute hydrops in two eyes, and retinal detachment in two eyes.
Treatment
Of the 449 eyes, 193 eyes (43%) required surgical treatment, and 256 eyes (57%) required medical treatment; the details of subtypes of glaucoma and the treatment details are given in Table 4. Of the 169 eyes, with PCG, 78.1% were surgically managed and the rest 21.9% were started on medical treatment. The PCG eyes that were on that were on medical treatment were awaiting surgery due to medical reasons; or had nasolacrimal duct obstruction underwent syringing and probing and were awaiting surgery or had poor visual prognosis hence were not operated on. About 62% of infantile glaucoma, 74.6% of JOAG, 85.3% eyes with glaucoma following cataract surgery, 68.2% eyes with syndromic glaucomas, 77.5% eyes with glaucoma associated with ocular anomalies, and 86.9% eyes with acquired glaucomas were managed medically. Surgical management was needed in 37.5% eyes with infantile glaucoma, 25.4% with juvenile glaucoma, 14.7% with glaucoma following cataract surgery, 31.8% eyes with syndromic glaucomas, 22.5% with glaucoma associated with ocular anomalies, and 13.1% with acquired glaucomas.
Table 4.
Distribution of medical and surgical treatment in eyes with paediatric glaucoma with various diagnoses
| Medical treatment, Number of eyes (%) | Surgical treatment, Number of eyes (%) | Total | |
|---|---|---|---|
| Primary congenital glaucoma | 37 (21.9) | 132 (78.1) | 169 |
| Infantile glaucoma | 10 (62.5) | 6 (37.5) | 16 |
| Juvenile glaucoma | 50 (74.6) | 17 (25.4) | 67 |
| Glaucoma following cataract surgery | 29 (85.3) | 5 (14.7) | 34 |
| Glaucoma associated with syndromes/systemic conditions | 15 (68.2) | 7 (31.8) | 22 |
| Glaucoma associated with non-acquired ocular anomalies | 62 (77.5) | 18 (22.5) | 80 |
| Glaucoma associated with acquired conditions | 53 (86.9) | 8 (13.1) | 61 |
| Total | 256 (57) | 193 (43) | 449 |
Glaucoma surgery
Majority of the eyes (76.6%) were operated at the age of <1 year, 5.4% children between >1 and 3 years, and 18% children >3 years. Among the 193 eyes that underwent surgery, combined trabeculotomy with trabeculectomy (CTT) was performed in 152 eyes (78.8%), trabeculectomy in 17 (8.8%) eyes, trabeculectomy with mitomycin C (MMC) in 12 (6.2%) eyes, combined trabeculectomy with MMC and lens aspiration in 2 eyes (1%), and transscleral cyclophotocoagulation in 10 eyes (5.2%).
The type of intervention, the IOP at presentation and final follow-up, amount of IOP reduction, and duration of follow-up are given in Table 5. In primary glaucoma group, there was significant reduction in mean IOP postsurgery but not significantly with medications in PCG and infantile glaucoma. Juvenile glaucoma had significant reduction in mean IOP with both medications and surgery. In the eyes with postcongenital cataract surgery glaucoma, the mean IOP pre- and posttreatment with surgery or medications were not significantly different. In the other types of glaucoma as well, the mean IOP following surgical treatment was significantly less than the medical treatment except in eyes with acquired glaucomas. At final follow–up, 135 eyes were on antiglaucoma medications (AGM).
Table 5.
Table showing change in IOP with medical and surgical intervention with duration of follow-up
| Diagnosis | Type of intervention | IOP at presentation, mean±SD | IOP at final visit, mean±SD | Difference between initial and final IOP, mean±SD | P | Age at presentation in months median (IQR) | Duration of follow-up in months median (IQR) |
|---|---|---|---|---|---|---|---|
| Primary glaucoma | Total | 27.17±7.73 | 14.55±7.30 | 12.61±9.70 | <0.0001 | 5 (1,52.5) | 5 (1,11) |
| Surgery | 28.35±6.85 | 13.95±7.10 | 14.40±9.33 | <0.0001 | 3 (1,6) | 7.5 (4,12) | |
| Medical | 23.97±9.08 | 16.19±7.67 | 7.78±9.11 | <0.0001 | 44 (4,120) | 1 (0,5) | |
| primary congenital glaucoma | Total | 25.91±5.44 | 13.29±5.85 | 12.62±7.75 | <0.0001 | 2 (1,5) | 7 (2,11) |
| Surgery | 26.58±4.74 | 13.10±5.86 | 13.48±7.19 | <0.0001 | 2 (1,5) | 9 (5,12.5) | |
| Medical | 18.29±7.34 | 15.43±5.74 | 2.86±7.56 | 0.4173 | 4 (1,5) | 0 (0,6) | |
| Infantile glaucoma | Total | 30.33±5.75 | 15.83±5.38 | 14.50±8.83 | <0.0001 | 21 (15,25) | 6 (1.75,7.75) |
| Surgery | 30.75±5.85 | 13.00±3.83 | 17.75±7.41 | <0.0001 | 15 (15,15.75) | 6 (3,6.75) | |
| Medical | 29.50±7.78 | 21.50±2.12 | 8.00±9.90 | 0.1605 | 25 (21,25) | 4 (0.5,9) | |
| Juvenile glaucoma | Total | 29.16±10.73 | 16.80±9.31 | 12.36±12.8 | <0.0001 | 128 (93,165.8) | 3 (0,8) |
| Surgery | 36.00±9.74 | 18.12±10.9 | 17.88±16.01 | <0.0001 | 128 (90,181) | 4 (2,12) | |
| Medical | 25.00±9.16 | 16.00±8.29 | 9.00±9.30 | 0.0001 | 128 (94,157.5) | 2 (0,4.5) | |
| Glaucoma following cataract surgery | Total | 25.39±10.19 | 21.09±9.83 | 4.30±6.70 | 0.1447 | 70 (39,139.5) | 0 (0,5) |
| Surgery | 25.25±10.44 | 17.75±5.56 | 7.50±7.68 | 0.2046 | 44 (2,44) | 14 (14,14) | |
| Medical | 25.42±10.43 | 21.79±10.4 | 3.63±6.50 | 0.2843 | 96.5 (63,150) | 0 (0,1.5) | |
| Glaucoma associated with syndromes | Total | 26.20±7.07 | 17.73±6.70 | 8.47±7.57 | 0.0007 | 39 (1,131.5) | 5 (1,10) |
| Surgery | 28.67±4.32 | 13.83±2.56 | 14.83±4.40 | <0.0001 | 1 (0.5,17) | 8 (4.5,8.5) | |
| Medical | 24.56±8.26 | 20.33±7.45 | 4.22±6.12 | 0.2549 | 90 (29.5,142.5) | 4 (0,12) | |
| Glaucoma associated with nonacquired ocular anomalies | Total | 24.77±9.63 | 18.54±9.37 | 6.23±8.47 | 0.0037 | 34 (7,133) | 1 (0,8) |
| Surgery | 27.00±5.31 | 15.67±4.27 | 11.33±5.90 | <0.0001 | 6.5 (3,55.75) | 8 (6.5,9) | |
| Medical | 23.78±10.97 | 19.81±10.7 | 3.96±8.54 | 0.1796 | 50 (12,141) | 1 (0,4) | |
| Glaucoma associated with acquired conditions | Total | 26.21±11.42 | 18.36±10.3 | 7.85±13.43 | 0.0002 | 100 (43,148) | 3 (0,9) |
| Surgery | 22.00±6.03 | 19.67±14.8 | 2.33±17.14 | 0.7211 | 64.5 (3,106) | 7.5 (6,15) | |
| Medical | 26.74±11.86 | 18.19±9.82 | 8.55±12.94 | 0.0001 | 109 (50,158.5) | 1 (0,8.5) | |
| Grand total | Total | 26.42±9.02 | 16.63±8.72 | 9.79±10.48 | <0.0001 | 15 (2,116) | 4 (0,10) |
| Surgery | 27.84±6.77 | 14.49±7.24 | 13.35±9.63 | <0.0001 | 3 (1,10) | 8 (4,12.25) | |
| Medical | 25.11±10.53 | 18.60±9.50 | 6.50±10.17 | <0.0001 | 76 (12,142) | 1 (0,6) |
IOP=Intraocular pressure, SD=Standard deviation
Discussion
In this study, we describe different glaucoma subtypes, and their demographic and clinical profile in children presenting with glaucoma to a tertiary eye care center. We classified childhood glaucoma using the CGRN classification, proposed by an international consortium of glaucoma specialists, based on the clinical findings, timing, and context in which a diagnosis of glaucoma is made.[12]
During the study period, there were 275 children (449 eyes) new patients with glaucoma. Of these, primary glaucoma constituted 56% and secondary glaucoma constituted 44%. Majority of primary glaucomas were PCG (37.6%), infantile glaucoma was 3.56%, and JOAG was 14.9%. Among the secondary glaucomas, majority were glaucomas associated with nonacquired ocular anomalies (18%) followed by glaucoma associated with acquired conditions such as trauma and steroid-induced glaucoma (13.5%). The glaucoma associated with congenital cataract surgery was at 8% and glaucoma associated with syndromic conditions was at 5%.
In our cohort, PCG was the most common type of childhood glaucoma that constituted 37.6%. Majority was bilateral (85%) with a male to female ratio close to 1. In several studies, PCG was the most common childhood glaucoma, similar to our study.[7,14,15,16] The incidence of PCG in published reports ranged from 19% to 47%.[7,9,13,17] and were bilateral in 62%–82%.[7,14,15]
In a study by Aponte et al., the acquired glaucomas constituted 63%.[9] In a study by Barsoum-Homsay et al., there were higher number of subjects with glaucoma associated with congenital anomalies (46%).[18] In a study by Fung et al., most common was secondary glaucoma, constituting 45%; of these, trauma and aphakia were most common glaucomas.[17] The incidence of acquired glaucomas are higher in developed countries compared with PCG.[9,17,18]
The definitions used for PCG and JOAG were different across the studies; this could possibly affect the percentage of children diagnosed in these categories. PCG was defined as at birth, up to 3 months, 1 year or 3 years,[7,9,13,14,15] and the age range for JOAG was from 2 to 20 years.[7,9,14,15,17]
In our study, the mean age at presentation was 3 months in PCG and was 10 years in acquired glaucoma group. Majority of children with PCG (80%) presented at age <6 months in our series. Most studies have reported early age at presentation for children with PCG ranging from 3 months to 2 years.[7,14,15,16] In the British Infantile glaucoma study, the age at presentation for >50% children from Asian background was <3 months, compared with Caucasian children where 52% presented within 6 months of age. Majority of the PCG children with Asian ethnicity were presented to the emergency services. Early age at presentation and self-reporting by the parents possibly signify more severe disease phenotype in children with Asian ethnicity. Hence, creating awareness to help early diagnosis and prompt referral by pediatricians is important.
There was no difference in the gender ratio in children with PCG. Similar results were seen in some groups.[4,5,19] Male female ratio is found to be equal in patients with consanguinity and in familial cases. However, greater number of male children was affected in certain populations with ratio of 2.5:1 and 3:2.[16,20,21]
The gender distribution for secondary glaucomas had males more commonly affected with a ratio of 2:1 with glaucoma following cataract surgery and 3.8:1 in acquired glaucoma group (trauma and steroid induced).
Phakomatosis was the major syndromic association seen in our cohort. Steroid-induced glaucoma was the most prevalent acquired glaucoma (7.57%) followed by traumatic glaucoma (5.12%). The mean presenting IOP was highest in JOAG group. Posttreatment IOP was least in PCG group and was highest in glaucoma associated with congenital cataract surgery.
Trauma was the most common cause of secondary glaucoma; males and older children are more affected.[7,9,14,15,22] The ratio was as high as 8:1 in Yuan et al. series.[14] Preventing trauma, advising protective eye wear, encouraging best or safe practices, and educating parents to prevent access to sharp objects/toys can help to decrease the incidence of traumatic glaucomas. Monitoring IOP in posttraumatic glaucomas over long time and educating parents about the likelihood of glaucoma development and encouraging follow-up can help screen children at risk of secondary glaucoma.
In our series, glaucoma following congenital cataract surgery was seen in 7.32%; of this, 5.09% were aphakic and 2.23% were pseudophakic. In the published studies, glaucoma in aphakia and pseudophakia ranged from 9.19% to 20% aphakic glaucoma.[7,13,14,15] The incidence of glaucoma in these children is high and mostly asymptomatic; hence, awareness and appropriate screening is important. In our series, the IOP control in this group with both medical and surgical treatment was not adequate. Although we have not looked at the reasons for this, tube implants would be a good option in eyes with postcataract surgery glaucoma.
In our series, 43% eyes required surgical treatment and 57% required medical treatment; the eyes with PCG were surgically managed (78.1%) and operated <1 year of age. The primary surgical procedure for majority of eyes with pediatric glaucoma was CTT (78.5%). There was significant reduction in mean IOP postsurgery in eyes with PCG. Juvenile glaucoma had significant reduction in mean IOP with both medications and surgery. In the eyes with postcongenital cataract surgery glaucoma, the mean IOP pre- and posttreatment with surgery or medications were not significantly different. In western reports, the most common surgery for PCG was goniotomy with success rates ranging from 50% to 71% with first goniotomy and 93% with multiple goniotomies at 1 year.[7,15,23,24] CTT was the most common procedure in two studies published from China.[14,16]
The limitations of our study are, it is not a population-based study but conducted at a tertiary referral hospital; hence, there would be referral bias. Although we looked at the disease profile, disease severity was not studied. Ours was not a longitudinal study; hence, only interim data were presented and treatment outcomes were not evaluated.
Conclusion
The profile of pediatric glaucoma in our cohort included 56% primary glaucomas and 44% secondary glaucomas. PCG was the most common at 37.6% and 85% of these were bilateral; male: female ratio was 1:1 for PCG and was 3.8:1 in glaucoma associated with acquired conditions. Glaucoma associated with ocular anomalies was the most common secondary glaucoma; steroid-induced and traumatic glaucomas were the most common acquired glaucomas. Majority of primary glaucomas were surgically managed and secondary glaucomas were managed medically.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Hyderabad eye research institute
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020--the right to sight. Bull World Health Organ. 2001;79:227–32. [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert CE, Rahi JS, Quinn GE. 2 ed. London: Edward Arnold Ltd; 2003. Visual Impairment and Blindness in Children. [Google Scholar]
- 3.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gencik A. Epidemiology and genetics of primary congenital glaucoma in Slovakia. Description of a form of primary congenital glaucoma in gypsies with autosomal-recessive inheritance and complete penetrance. Dev Ophthalmol. 1989;16:76–115. [PubMed] [Google Scholar]
- 5.Sarfarazi M, Stoilov I. Molecular genetics of primary congenital glaucoma. Eye (Lond) 2000;14:422–8. doi: 10.1038/eye.2000.126. [DOI] [PubMed] [Google Scholar]
- 6.Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, et al. Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet. 1998;62:325–33. doi: 10.1086/301725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulos M, Cable N, Rahi J, Khaw PT BIG Eye Study Investigators. The British Infantile and Childhood Glaucoma (BIG) eye study. Invest Ophthalmol Vis Sci. 2007;48:4100–6. doi: 10.1167/iovs.06-1350. [DOI] [PubMed] [Google Scholar]
- 8.Bermejo E, Martinez-Frias ML. Congenital eye malformations: Clinical-epidemiological analysis of 1,124,654 consecutive births in Spain. Am J Med Genet. 1998;75:497–504. [PubMed] [Google Scholar]
- 9.Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: A population-based study. Arch Ophthalmol. 2010;128:478–82. doi: 10.1001/archophthalmol.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandona L, Williams JD, Williams BC, Rao GN. Population-based assessment of childhood blindness in southern India. Arch Ophthalmol. 1998;116:545–6. [PubMed] [Google Scholar]
- 11.Biglan AW. Glaucoma in children: Are we making progress? J AAPOS. 2006;10:7–21. doi: 10.1016/j.jaapos.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Beck A, Chang TC, Freedman S. Definition, classification, differential diagnosis. In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J, Freedman S, editors. Childhood Glaucoma. Amsterdam: Kugler Publications; 2013. pp. 3–10. [Google Scholar]
- 13.Hoguet A, Grajewski A, Hodapp E, Chang TC. A retrospective survey of childhood glaucoma prevalence according to Childhood Glaucoma Research Network classification. Indian J Ophthalmol. 2016;64:118–23. doi: 10.4103/0301-4738.179716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao CY, Wang LH, Tang X, Wang T, Yang DY, Wang NL. Epidemiology of hospitalized pediatric glaucoma patients in Beijing Tongren Hospital. Chin Med J (Engl) 2009;122:1162–6. [PubMed] [Google Scholar]
- 15.Taylor RH, Ainsworth JR, Evans AR, Levin AV. The epidemiology of pediatric glaucoma: The Toronto experience. J AAPOS. 1999;3:308–15. doi: 10.1016/s1091-8531(99)70028-5. [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Long Q, Guo W, Sun X. Profile of pediatric glaucoma patients in Shanghai Eye, Ear, Nose and Throat Hospital. Chin Med J (Engl) 2014;127:1429–33. [PubMed] [Google Scholar]
- 17.Fung DS, Roensch MA, Kooner KS, Cavanagh HD, Whitson JT. Epidemiology and characteristics of childhood glaucoma: Results from the Dallas Glaucoma Registry. Clin Ophthalmol. 2013;7:1739–46. doi: 10.2147/OPTH.S45480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barsoum-Homsy M, Chevrette L. Incidence and prognosis of childhood glaucoma. A study of 63 cases. Ophthalmology. 1986;93:1323–7. doi: 10.1016/s0161-6420(86)33569-3. [DOI] [PubMed] [Google Scholar]
- 19.Turacli ME, Aktan SG, Sayli BS, Akarsu N. Therapeutical and genetical aspects of congenital glaucomas. Int Ophthalmol. 1992;16:359–62. doi: 10.1007/BF00917991. [DOI] [PubMed] [Google Scholar]
- 20.deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma) Surv Ophthalmol. 1983;28:1–19. doi: 10.1016/0039-6257(83)90174-1. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer RN. Genetics and the congenital glaucomas. Am J Ophthalmol. 1965;60:981–94. doi: 10.1016/0002-9394(65)92805-9. [DOI] [PubMed] [Google Scholar]
- 22.MacEwen CJ, Baines PS, Desai P. Eye injuries in children: The current picture. Br J Ophthalmol. 1999;83:933–6. doi: 10.1136/bjo.83.8.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell-Eggitt IM, Rice NS, Jay B, Wyse RK. Relapse following goniotomy for congenital glaucoma due to trabecular dysgenesis. Eye (Lond) 1992;6:197–200. doi: 10.1038/eye.1992.38. [DOI] [PubMed] [Google Scholar]
- 24.Chen TC, Bhatia LS, Walton DS. Ahmed valve surgery for refractory pediatric glaucoma: A report of 52 eyes. J Pediatr Ophthalmol Strabismus. 2005;42:274. doi: 10.3928/0191-3913-20050901-09. [DOI] [PubMed] [Google Scholar]