Abstract
OBJECTIVE:
Both stereotactic radiosurgery (SRS) and fractionated radiation therapy (FRT) techniques are used for treatment of intracranial meningiomas with excellent local control (LC) rates. Although SRS techniques are convenient, toxicity including treatment-related edema can significantly impact patient quality of life. The long-term clinical outcomes of patients with magnetic resonance imaging (MRI)—defined meningiomas treated with radiation therapy (RT) alone are reported.
METHODS:
The charts of 211 patients with meningiomas diagnosed by contrast-enhanced MRI treated with either SRS or FRT between 1991 and 2012 at a single institution were reviewed. Actuarial rates for LC and development of treatment-related radiographic edema (TRE) were determined by the Kaplan-Meier method.
RESULTS:
There were 211 patients who received radiation therapy for 223 lesions. Median follow-up was 5.7 years. Eleven patients experienced a local failure; of these, 2 were ultimately found to have pathologically proven metastatic carcinoma. Two- and 5-year LC was 97.8% and 94.6%, respectively, with no significant difference based on modality of therapy. Actuarial rate for development of TRE at 1 and 2 years was 30.1% and 34.6% for the SRS group and 1.6% and 2.5% for the FRT group, respectively (P < 0.001).
CONCLUSIONS:
RT alone using a limited margin is an effective treatment option for MRI-defined meningiomas and should be considered even without biopsy if surgery will present significant morbidity. Although LC with SRS versus FRT was comparable, FRT was associated with a significantly decreased risk of TRE.
Keywords: Brain edema, Magnetic resonance imaging, Meningioma, Radiosurgery, Radiotherapy
INTRODUCTION
Meningiomas are the most common primary brain tumors, accounting for 36.4% of central nervous system tumors diagnosed between 2008 and 2012.1 The incidence of meningiomas rises with increasing age, with diagnosis being most common in the sixth and seventh decades of life.2 They are more common in women compared with men, at a ratio of 2—3:1.2,3 Most meningiomas are benign; however, the location of these tumors can be a cause of serious morbidity and mortality.3 Small asymptomatic meningiomas can be safely observed on active surveillance, especially in elderly patients, until the tumor grows significantly or becomes symptomatic.4–6 However, the standard of care for intracranial meningiomas diagnosed in healthy young patients and for those tumors which are symptomatic or enlarging is surgical resection with or without radiation therapy (RT). RT is often reserved for those lesions which are subtotally resected or for those which are unresectable given proximity to critical structures, patient comorbidities, or patient refusal. However, tumor locations and additional patient factors must be considered when determining the ideal treatment strategy.
Meningiomas can occur in areas not amenable to surgical resection or even biopsy. This has led to treatment of presumed meningiomas without a tissue diagnosis. Meningiomas are associated with characteristic imaging findings. On computed tomography (CT) scan, meningiomas are well-defined, homogenous, and displace normal brain tissue. They enhance brightly and are often associated with hyperostosis.7,8 Magnetic resonance imaging (MRI) is the preferred imaging modality because it can show the dural origin. Most meningiomas are also associated with the dural tail sign, which is a thickening of the meninges that tapers peripherally on T1-weighted postcontrast imaging.8–10 Although not exclusive to meningiomas, given these typical findings, it is common at our institution for meningioma tumors identified by MRI to be treated without a pathologic diagnosis when significant surgical resection is either not feasible or refused by the patient.
Treatment-related radiographic edema (TRE) is a known side effect of RT with potentially significant impact on patient quality of life.11-13 However, there have been few direct comparisons of the risk of edema when using stereotactic radiosurgery (SRS) versus fractionated radiation therapy (FRT) techniques. Herein, we report our more than 20 years’ experience at our institution of MRI-defined meningiomas treated with RT.
METHODS
Patient Population
After institutional review board approval, the charts of 879 patients seen in our department with a diagnosis of meningioma were reviewed. Patients were included if they were 18 years of age or older, had no prior histologic diagnosis of meningioma, were diagnosed by contrast-enhanced MRI, and were treated with radiation alone. A total of 211 patients treated between 1991 and 2012 at our institution met the criteria and were included. For all patients, there was agreement among the departments of radiation oncology, radiology, and neurosurgery that the tumor represented a meningioma based on MRI T1-weighted postcontrast imaging with characteristic findings, including homogeneous enhancement and a dural origin.
Radiation Technique
Patients were treated with linear accelerator (LINAC)—based (n = 73) or gamma knife (n = 7) SRS or FRT (n = 131). SRS refers to radiation treatment in 5 or fewer fractions, whereas FRT refers to treatment in more than 5 fractions. Typically, SRS was preferred for tumors of3 cm or less in greatest dimension located a safe distance from critical normal structures such as the optic nerves/chiasm and brainstem. The FRT technique was used for patients not amenable to SRS based on the aforementioned criteria. The median prescription dose for tumors treated with SRS was 14 Gy (range, 10—20 Gy), and the median prescription dose for tumors treated with FRT was 50.4 Gy (range, 30—59.4 Gy). All patients undergoing SRS were treated in 1 fraction, whereas those receiving FRT were standardly treated to 1.8 Gy per fraction. For patients receiving FRT, CT simulation was performed using a thermoplastic head mask for immobilization. When treating with SRS, either a thermoplastic head mask (frameless technique) or a rigid headframe (framed technique) was used. A high-resolution, thin-slice, noncontrast CT scan was obtained for LINAC-based treatment planning. The planning CT scan was co-registered with a T1 postcontrast MRI scan. The MRI was typically obtained within 1—2 weeks of CT simulation. Once the registration was verified, the gross tumor volume (GTV) was delineated on the planning CT scan with the co-registered MRI used for reference. For gamma knife treatment, a high-resolution T1 postcontrast MRI scan was obtained on the day of planning with the headframe in place and subsequently used for treatment planning. The clinical target volume (CTV) was defined as GTV plus margin for microscopic extension, which cannot be seen on imaging. Use of a CTV margin varied, based on the treating physician, and was not used for SRS treatments. Either the GTV or CTV was uniformly expanded to create the planning treatment volume (PTV) at the discretion of the treating radiation oncologist.
Patients typically started treatment within 1 week of CT simulation. However, if the patient was treated using a rigid, stereotactic headframe, this was placed prior to simulation by a neurosurgeon, and treatment was delivered on the same day as the simulation. Radiation was typically prescribed to the 80% isodose line versus 90%—98% isodose lines for LINAC-based SRS and FRT cases, respectively. For gamma knife, radiation was prescribed to the 50% isodose line. Median GTV to PTV expansion, when available, was 1 mm (range, 1—2 mm) for SRS (n = 35) and 3 mm (range, 1—10 mm) for FRT (n = 117). Margin size varied with treatment era and bias of the treating physician. For LINAC-based treatment, daily image guidance was performed via cone beam CT scan for SRS and via daily kilovoltage planar orthogonal radiographs for FRT to confirm positioning. Treatment details are outlined in Table 1.
Table 1.
Treatment Characteristics (Lesion Level)
| Variable | Fractionated Radiation Therapy (n =135) |
SRS (n = 88) |
|---|---|---|
| Technique | ||
| IMRT | 123 | - |
| 3D Conformal | 10 | - |
| LINAC-based SRS | - | 81 |
| Gamma knife | - | 7 |
| Not available | 2 | - |
| Dose (Gy) | 50.4 (30–59.4) | 14 (10–20) |
| GTV to PTV expansion (mm) | 3 (1–10) | 1 (1–2) |
Values are number of participants or median (range).
SRS, stereotactic radiosurgery; IMRT, intensity-modulated radiation therapy; 3D, 3-dimensional; LINAC, linear accelerator; GTV, gross total volume; PTV, planning treatment volume.
Follow-Up Evaluation
Serial MRI or CT imaging was typically performed every 6 months for the first year, annually for years 1—5 after treatment, and biennially thereafter unless the clinical situation warranted earlier follow-up scans. Patients were considered to be controlled locally if the lesion was stable or decreased in size. A recurrence was considered to be in-field if 90% or more of the recurrent lesion was in the 100% prescription isodose line, out-of-field when the recurrent lesion was completely outside the prescription isodose line, and marginal when neither of the previous criteria was met. Patients were defined as having TRE if there was an increase in peritumor hyperintense T2 or fluid-attenuated inversion recovery signal compared with the co-registered pretreatment MRI.
Statistical Analysis
Actuarial rates for local control (LC) and TRE were determined by the Kaplan-Meier method, and survival distributions were compared using log-rank tests. LC was defined as time from RT to disease recurrence or date of last radiographic follow-up, where those without disease recurrence were censored at last radiographic follow-up. TRE was defined as time from RT to treatment-related edema or last follow-up, where those without TRE were censored at last physical follow-up. We estimated lesion size as both the mean lesion size across all lesions for each patient and the sum of lesion sizes across all lesions for each patient; however, only 8 patients had more than one lesion. Lesion size was evaluated as a continuous variable; however, cut points also were explored using the median and third quartile. Univariate analysis (UVA) and multivariable analysis (MVA) were performed by the Cox proportional hazards method. The analysis was performed with and without atypical pathology. Model assumptions were checked and verified. The statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA), and the significance level was assessed at the 0.05 level.
RESULTS
Patient Characteristics
A total of 211 patients received RT for 223 lesions. Eighty patients were treated with SRS, whereas 131 underwent FRT. Median follow-up of living patients was 5.7 years. Median age at treatment was 58.5 years, and 84% of patients were women. Most patients (n = 203) were treated for a single lesion. The most common lesion location was base of skull (n = 136). The base of skull commonly refers to the ridge of bone posterior to the eyes and nose which extends to form the floor of the skull. Median lesion size was 5.3 cm3 (range, 0.01—200 cm3). Patient and tumor characteristics are outlined in Table 2.
Table 2.
Patient and Tumor Characteristics
| Treatment | |||||
|---|---|---|---|---|---|
| Covariate | Statistics | Level | SRS (n = 80) | FRT (n = 131) | P Value* |
| Number of lesions | Number of participants (%) | 1 | 75 (93.75) | 128 (97.71) | 0.169 |
| Number of participants (%) | 2 | 3 (3.75) | 3 (2.29) | ||
| Number of participants (%) | 4 | 2 (2.5) | 0(0) | ||
| Location | Number of participants (%) | Other | 48 (60) | 27 (20.61) | <0.001 |
| Number of participants (%) | Base of skull | 32 (40) | 104 (79.39) | ||
| XRT dose | Number of participants (%) | <30 | 80 (100) | 0(0) | <0.001 |
| Number of participants (%) | ≥30, <45 | 0(0) | 20 (15.27) | ||
| Number of participants (%) | 45–50.4 | 0(0) | 94 (71.76) | ||
| Number of participants (%) | >50.4 | 0(0) | 17 (12.98) | ||
| Pathology other than grade 1 meningioma | Number of participants (%) | No | 77 (96.25) | 127 (96.95) | >0.99 |
| Number of participants (%) | Yes | 3 (3.75) | 4 (3.05) | ||
| Median age at XRT (years) | Number of participants (%) | <58.5 | 45 (56.25) | 60 (45.8) | 0.141 |
| Number of participants (%) | ≥58.5 | 35 (43.75) | 71 (54.2) | ||
| Age at XRT (years) | Number of participants | 80 | 131 | 0.140 | |
| Mean | 56.53 | 59.43 | |||
| Median | 56.6 | 61.9 | |||
| Lesion size (average) | Number of participants | 65 | 117 | 0.002 | |
| Mean | 4.94 | 14.76 | |||
| Median | 2.36 | 7.81 | |||
| Lesion size (sum) | Number of participants | 65 | 117 | 0.002 | |
| Mean | 4.97 | 14.98 | |||
| Median | 2.36 | 7.9 | |||
SRS, stereotactic radiosurgery; FRT, fractionated radiation therapy; XRT, radiation therapy.
The P value is calculated by analysis of variance for numerical covariates and χ2 test or Fisher exact test for categorical covariates, where appropriate.
Tumor Control
Overall, 11 patients experienced a local failure, of whom 2 were ultimately found to have pathologically proven metastatic carcinoma. Two- and 5-year LC was 97.8% and 94.6%, respectively (Figure 1). On UVA, no significant difference in LC was noted with SRS versus FRT (P = 0.963) (Figure 2) or radiation dose (P = 0.941). However, lesion size was significantly associated with LC (P = 0.002) (Table 3). After excluding the cases of metastatic disease, 5 of 9 progressions had imaging available for review, with all recurrences being in-field. Of the patients with known treatment of their progression, 3 underwent craniotomy and 2 underwent re-irradiation with SRS. Of those receiving SRS, 1 was controlled at last follow-up. Overall, only 3.3% of patients were found to have a diagnosis other than typical grade I meningioma after additional testing was done because of atypical treatment response. These included cases of World Health Organization grade 2 meningioma (n = 1), metastatic disease (n = 2), prolactinoma (n = 1), neurosarcoidosis (n = 2), and nonspecific inflammation (n = 1). After eliminating the patients with atypical pathology, 2- and 5-year LC was 98.3% and 96.7%, respectively.
Figure 1.
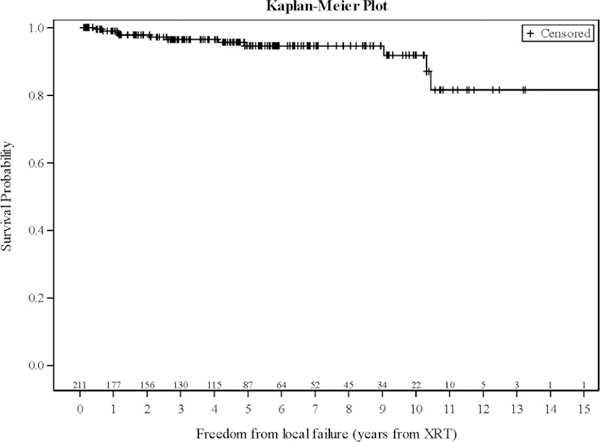
Actuarial local control curve for all patients. XRT,radiation therapy.
Figure 2.
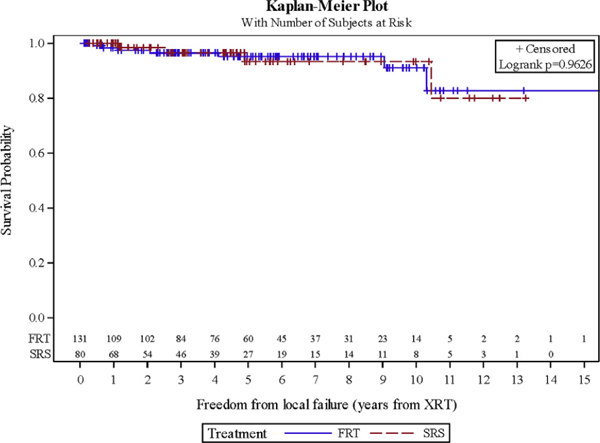
Local control for fractionated radiation therapy versus stereotactic radiosurgery. FRT, fractionated radiation therapy; SRS, stereotactic radiosurgery; XRT, radiation therapy.
Table 3.
Local Contral: Univariate Analysis
| Freedom from Local Failure (years from XRT) | |||||
|---|---|---|---|---|---|
| Covariate | Level | Number of Patients | HR (95% CI) | HR P Value | Log-Rank P Value |
| Number of lesions | >1 | 8 | 0.00 (0.00) | 0.993 | 0.488 |
| 1 | 203 | - | - | ||
| Location | Base of skull | 136 | 0.69 (0.21–2.26) | 0.541 | 0.539 |
| Other | 75 | - | - | ||
| XRT dose | ≥45 | 111 | 0.91 (0.28–3.00) | 0.879 | 0.879 |
| <45 | 100 | - | - | ||
| Treatment | FRT | 131 | 1.03 (0.30–3.53) | 0.963 | 0.963 |
| SRS | 80 | - | - | ||
| Median age at XRT | ≥58.5 | 106 | 1.03 (0.31–3.42) | 0.967 | 0.967 |
| <58.5 | 105 | - | - | ||
| Upper quartile age at XRT | ≥69.6 | 53 | 1.23 (0.25–5.94) | 0.799 | 0.798 |
| <69.6 | 158 | - | - | ||
| Median lesion size (average) | ≥5.30 | 92 | 1.68 (0.47–5.96) | 0.421 | 0.416 |
| <5.30 | 90 | - | - | ||
| Upper quartile lesion size (average) | ≥11.62 | 46 | 2.12 (0.60–7.52) | 0.246 | 0.235 |
| <11.62 | 136 | - | - | ||
| Median lesion size (sum) | ≥5.33 | 91 | 1.80 (0.51–6.40) | 0.365 | 0.358 |
| <5.33 | 91 | - | - | ||
| Upper quartile lesion size (sum) | ≥11.70 | 46 | 2.09 (0.59–7.41) | 0.256 | 0.245 |
| <11.70 | 136 | - | - | ||
| Age at XRT | 211 | 0.99 (0.95–1.04) | 0.754 | - | |
| Lesion size (average) | 182 | 1.03 (1.01–1.05) | 0.002 | - | |
| Lesion size (sum) | 182 | 1.03 (1.01–1.05) | 0.002 | - | |
HR, hazard ratio; CI, confidence interval; SRS, stereotactic radiosurgery; FRT, fractionated radiation therapy; XRT, radiation therapy.
Treatment-Related Edema
In total, TRE developed in 32 cases at a median of 7.6 months post-RT. This occurred in 28 patients treated with SRS and 3 patients treated with FRT. Actuarial rate for freedom from TRE at 1 and 2 years for the SRS and FRT groups was 69.9% and 65.4% and 98.4% and 97.5%, respectively (P < 0.001) (Figure 3). After eliminating the patients with atypical pathology, 1- and 2-year freedom from TRE was 70% and 65.3% and 98.3% and 97.4%, respectively (P < 0.001). On MVA, treatment group remained a significant predictor for development of TRE (P < 0.001) when controlling for location and lesion size (Table 4). However, tumor location (P = 0.359) and lesion size (P = 0.473) were not significant. Within each treatment group, radiation dose did not reach significance on UVA (P > 0.5). A representative image of TRE is shown in Figure 4. In 19 of the SRS patients (68%), TRE was symptomatic. Fourteen of these patients were treated with steroids alone, and 5 underwent craniotomy after failing medical management. Craniotomy was complicated by catastrophic hemorrhage and death in 1 patient. Two of 3 patients receiving FRT were symptomatic and treated with a course of steroids. There was no statistically significant difference in average tumor size between those with edema and those without on MVA (P = 0.473). New posttreatment cranial nerve damage was not observed in any of the patients.
Figure 3.
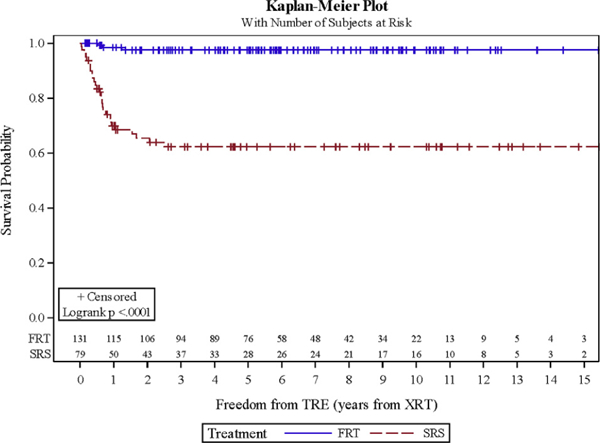
Freedom from treatment-related radiographic edema: stereotactic radiosurgery versus fractionated radiation therapy. FRT, fractionated radiation therapy; SRS, stereotactic radiosurgery; XRT, radiation therapy.
Table 4.
Treatment-Related Edema: Multivariable Analysis
| Freedom from TRE (years from XRT) | ||||
|---|---|---|---|---|
| Covariate | Level | HR (95% CI) | HR P Value | Type 3 P Value |
| Treatment | FRT | 0.04 (0.01–0.20) | <0.001 | <0.001 |
| SRS | - | - | ||
| Location | Base of skull | 0.67 (0.29–1.57) | 0.359 | 0.359 |
| Other | - | - | ||
| Lesion size (average) | 1.01 (0.98–1.05) | 0.473 | 0.473 | |
XRT, radiation therapy; TRE, treatment-related radiographic edema; HR, hazard ratio; CI, confidence interval; FRT, fractionated radiation therapy; SRS, stereotactic radiosurgery.
Number of observations in the original data set was 211, and the number of observations used was 181.
Backward selection with an alpha level of removal of 0.05 was used. No variables were removed from the model.
Figure 4.
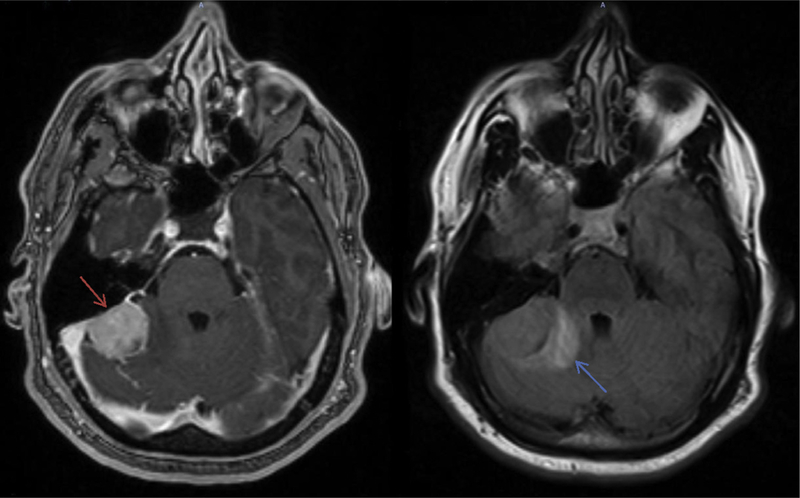
T1 postcontrast magnetic resonance imaging (left image) demonstrating the treated meningioma (red arrow) and corresponding fluid-attenuated inversion recovery (right image) imaging showing treatment-related edema (blue arrow).
DISCUSSION
In our series, 2- and 5-year LC for MRI-defined meningiomas was 98.3% and 96.7%, respectively. Said LC rates were achieved in the setting of a median GTV to PTV expansion of 1 mm for patients treated with SRS and 3 mm for patients treated with FRT. Further, only 3.3% of patients were ultimately found to have a diagnosis other than grade I meningioma. However, SRS was associated with significantly higher rates of TRE, with 32 cases of TRE in patients receiving SRS versus 3 cases when FRS was delivered. Of the SRS patients, 68% with TRE were symptomatic, with 26% of symptomatic patients requiring craniotomy for medically refractory symptoms.
For meningiomas, MRI is the preferred imaging modality because it can show the dural origin of the tumor. Most meningiomas demonstrate the dural tail sign, which is a dural thickening that tapers peripherally on T1-weighted imaging.8–10 In a prospective study by Rokni-Yazdi et al.,10 the dural tail sign had a sensitivity of 58.6% and a specificity of 94% for the diagnosis of meningioma. Modern series have supported the use of MRI in diagnosing meningiomas, which are not easily accessible for surgical resection or biopsy.14–16 In our study, 3.3% of patients were ultimately found to have a diagnosis other than a grade I meningioma. Given the risk of surgical resection or biopsy of meningiomas in certain locations, along with other patient factors that can preclude a tissue diagnosis, we feel that with an educated patient this risk is acceptable. Further, all local meningioma recurrences in our study were in-field. In addition, median GTV to PTV expansion was 1 mm (range, 1—2 mm) for SRS (n = 35) and 3 mm (range, 1—10 mm) for FRT (n = 117). This supports the accuracy of MRI for tumor delineation and daily image-guided radiation therapy for localization. Flickinger et al.14 reported the outcomes of 219 meningiomas diagnosed by imaging alone and treated with gamma knife radiosurgery to a median marginal tumor dose of 14 Gy. LC rates at 5 and 10 years were 93.2% ± 2.7%. At 5 and 10 years, the actuarial rate of identifying a diagnosis other than meningioma was 2.3% ± 1.4%, which is comparable with our findings.
In our series, the risk of treatment-related edema at 1 and 2 years was 30.1% and 34.6% for the SRS group and 1.6% and 2.5% for the FRT group (P < 0.001). Hasegawa et al.13 reported on 112 patients with convexity, parasagittal, or falcine meningiomas treated with gamma knife surgery between 1991 and 2008. The median tumor volume was 8 cm3, and the median maximum and marginal doses were 30 and 16 Gy, respectively. Twenty-eight percent of patients experienced new or worsened peritumoral edema 3—12 months after treatment. The incidence of treatment-related edema was significantly higher in those patients undergoing gamma knife as the initial treatment for their tumor. In a series of 182 meningiomas treated with SRS for a median dose of 13.6 ± 2.6 Gy, Cai et al.11 found 24.7% of patients developed peritumoral edema. Notably, increasing tumor-brain contact interface area was a significant risk factor for developing peritu-moral edema, with the odds of its development increasing by 17.2% (P = 0.0107) for every 1-unit (cm2) increase in surface area. Although increased size was not found to be significant in our study, this could be because of the heterogeneity of the sample. Because of development of significant symptoms, posttreatment edema can require treatment with prolonged steroids, or even craniotomy, which may detrimentally impact a patient’s quality of life. As seen in our study, craniotomy led to the death of 1 patient because of massive hemorrhage. Our data suggest that use of FRT, particularly in larger tumors and those with pretreatment edema, may help mitigate this risk.
Limitations to our study include its retrospective design, limited follow-up time, and heterogeneity of the patients included. However, our findings support the continued treatment of MRI-defined meningiomas without a pathologic diagnosis. Further, our study is the largest to our knowledge comparing the risk of TRE with SRS versus FRS techniques. Careful consideration must be taken into account for those offered treatment with SRS.
In conclusion, radiation alone using limited margin is an effective treatment option for MRI-defined meningiomas and should be considered even without biopsy if surgery will present significant risks to the patient. Although LC with SRS versus FRT was comparable, FRT was associated with a significantly decreased risk of TRE. Work is ongoing to determine treatment and dosimetric and imaging factors predictive for TRE in our patient cohort.
Abbreviations and Acronyms
- CT
Computed tomography
- CTV
Clinical target volume
- FRT
Fractionated radiation therapy
- GTV
Gross tumor volume
- LC
Local control
- LINAC
Linear accelerator
- MRI
Magnetic resonance imaging
- MVA
Multivariable analysis
- PTV
Planning treatment volume
- RT
Radiation therapy
- SRS
Stereotactic radiosurgery
- TRE
Treatment-related radiographic edema
- UVA
Univariate analysis
Footnotes
Conflict of interest statement: This research was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and the NIH/NCI (P30CA138292). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, et al. Meningioma. Crit Rev Oncol Hematol. 2008;67:153–171. [DOI] [PubMed] [Google Scholar]
- 3.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095 [discussion: 1088–1095]. [DOI] [PubMed] [Google Scholar]
- 4.Go RS, Taylor BV, Kimmel DW. The natural history of asymptomatic meningiomas in Olmsted County, Minnesota. Neurology. 1998;51:1718–1720. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, Roser F, Michel J, Jacobs C, Samii M. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62–70 [discussion: 70–71]. [DOI] [PubMed] [Google Scholar]
- 6.Yano S, Kuratsu J, Kumamoto Brain Tumor Research Group. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105:538–543. [DOI] [PubMed] [Google Scholar]
- 7.Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742–746 [discussion: 746–747]. [DOI] [PubMed] [Google Scholar]
- 8.Wilms G, Lammens M, Marchal G, Van Calenbergh F, Plets C, Van Fraeyenhoven L, et al. Thickening of dura surrounding meningiomas: MR features. J Comput Assist Tomogr. 1989;13: 763–768. [DOI] [PubMed] [Google Scholar]
- 9.Goldsher D, Litt AW, Pinto RS, Bannon KR, Kricheff II. Dural “tail” associated with meningiomas on Gd-DTPA-enhanced MR images: characteristics, differential diagnostic value, and possible implications for treatment. Radiology. 1990;176:447–450. [DOI] [PubMed] [Google Scholar]
- 10.Rokni-Yazdi H, Sotoudeh H. Prevalence of “ dural tail sign” in patients with different intracranial pathologies. Eur J Radiol. 2006;60:42–45. [DOI] [PubMed] [Google Scholar]
- 11.Cai R, Barnett GH, Novak E, Chao ST, Suh JH. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010;66:513–522. [DOI] [PubMed] [Google Scholar]
- 12.Conti A, Pontoriero A, Siddi F, Iati G, Cardali S, Angileri FF, et al. Post-treatment edema after meningioma radiosurgery is a predictable complication. Cureus. 2016;8:e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa T, Kida Y, Yoshimoto M, Iizuka H, Ishii D, Yoshida K. Gamma Knife surgery for convexity, parasagittal, and falcine meningiomas. J Neurosurg. 2011;114:1392–1398. [DOI] [PubMed] [Google Scholar]
- 14.Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801–806. [DOI] [PubMed] [Google Scholar]
- 15.Korah MP, Nowlan AW, Johnstone PA, Crocker IR. Radiation therapy alone for imaging-defined meningiomas. Int J Radiat Oncol Biol Phys. 2010;76:181–186. [DOI] [PubMed] [Google Scholar]
- 16.Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Single-fraction radiosurgery for presumed intracranial meningiomas: efficacy and complications from a 22-year experience. Int J Radiat Oncol Biol Phys. 2012;83:1414–1418. [DOI] [PubMed] [Google Scholar]


