Abstract
Bacterial biofilms are surface-attached communities of non-replicating bacteria innately tolerant to antibiotics. Biofilms display differential gene expression profiles and physiologies as compared to their planktonic counterparts; however, their biology remains largely unknown. In this study, we used a halogenated phenazine (HP) biofilm eradicator in transcript profiling experiments (RNA-seq) to define cellular targets and pathways critical to biofilm viability. WoPPER analysis with time–course validation (RT-qPCR) revealed that HP-14 induces rapid iron starvation in MRSA biofilms, as evident by the activation of iron-acquisition gene clusters in 1 hour. Serine proteases and oligopeptide transporters were also found to be up-regulated, whereas glycolysis, arginine deiminase, and urease gene clusters were down-regulated. KEGG analysis revealed that HP-14 impacts metabolic and ABC transporter functional pathways. These findings suggest that MRSA biofilm viability relies on iron homeostasis.
Keywords: antibiotics, biofilm eradication, chemical biology, halogenated phenazine, RNA sequencing
Bacteria exist in two distinct lifestyles: 1) free-floating, planktonic bacteria and 2) surface-attached communities encased in a matrix of biomolecules, known as biofilms.[1,2] Planktonic bacteria secrete organic signaling molecules in a communication process (quorum sensing), which enables bacterial cells to monitor population density and coordinate group behavior, including surface attachment and biofilm formation.[3] Biofilm cells grow at a dramatically slower rate as compared to their planktonic counterparts, or are metabolically dormant persister cells.[4,5]
The non-replicative nature of persister cells in conjunction with the polymeric matrix acting as a bacterial fortress enables biofilms to thrive in hostile environments (e.g., host immune responses). Biofilms demonstrate tolerance to all classes of conventional antibiotics, which were initially discovered as bacterial-growth-inhibiting agents. Therefore, it should be no surprise that biofilms have led to devastating consequences to human health, as these bacterial communities of enriched persister-cell populations are credited as the underlying cause of persistent and recurring infections.[6] Biofilms occur in approximately 80% of bacterial infections[7] and result in more than 500000 deaths each year.[8] Innovative therapeutic strategies are required to address the many clinical problems associated with biofilm infections.[1]
Despite a detailed understanding of the molecular biology of planktonic cells over decades of bacteriological investigations, we have a limited knowledge of bacterial biofilms. Understanding the biological underpinnings of biofilm survival will enable innovative strategies to eradicate biofilm-associated infections in a clinical setting. Biofilm communities are highly complex and consist of heterogeneous cell populations exposed to diverse microenvironments, complicating biological studies. Despite these challenges, gene expression profiles obtained for pathogenic biofilms by the use of DNA microarrays have enabled the study of differential gene expression between planktonic and biofilm cells,[9] and gene expression profiles of biofilms subjected to innate immune cells.[10]
As NextGen sequencing has become more affordable, RNA-sequencing (RNA-seq)[11] is now the method of choice for the transcriptomic study of biofilms (Figure 1).[12] RNA-seq has increased sensitivity and dynamic range as compared to microarrays, which is ideal for biofilms and persister cells that may synthesize low levels of RNA.[12] Transcriptomic analyses of bacterial biofilms utilizing RNA-seq technology have been reported for the following: 1) comparative transcriptomic analysis of biofilms versus planktonic cultures,[13] 2) microbe–microbe interactions,[14] and 3) the impact of biofilm inhibitors (ursolic acid, resveratrol; note: biofilm inhibitors typically operate on quorum sensing machinery or other targets that do not eradicate biofilms) on Staphylococcus aureus biofilms.[15,16]
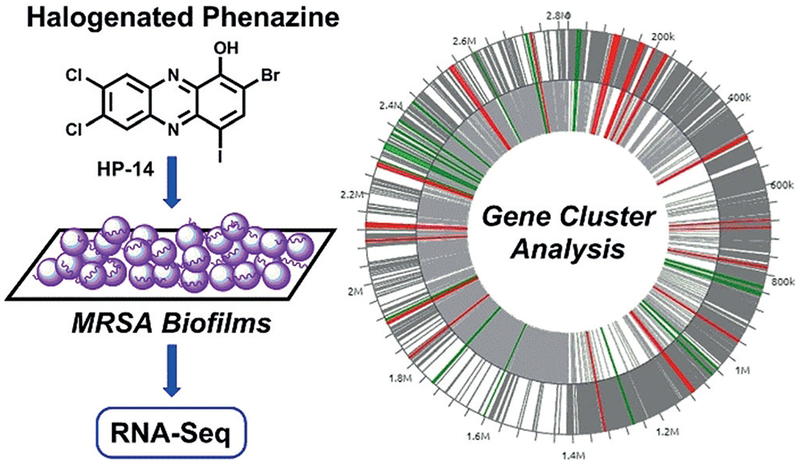
Figure 1.
Transcript profiling (RNA-seq) of MRSA biofilms treated with HP-14 to define cellular targets and pathways critical to biofilms.
Herein, we report our transcript profiling investigations of methicillin-resistant S. aureus (MRSA BAA-1707, or MW-2) biofilms treated with a halogenated phenazine (HP) biofilm-eradicating agent to define cellular targets and pathways critical for biofilm survival. HPs have demonstrated biofilm eradication (killing) activities against several pathogens, including MRSA, methicillin-resistant S. epidermidis (MRSE), and vancomycin-resistant Enterococcus faecium (VRE).[17–20] HPs operate through a unique, and not well understood, metal-dependent mechanism that enables high levels of bacterial targeting (minimal mammalian cytotoxicity or cell-membrane lysis).[18–20] During these investigations, we aimed to gain extensive mechanistic and biological information regarding HP-14 (minimum biofilm eradication concentration, MBEC = 6.25 μm, MRSA-1707)[17,18] and MRSA biofilm viability through transcript profiling using RNA-seq technology (Figure 1).
We began these investigations by treating 20 hour (established) MRSA-1707 biofilms with HP-14 at sub-MBEC concentrations (1/10 MBEC, 0.625 μm; 20 hour treatment) in 24-well plates. Following compound treatment, media and planktonic cells were removed, and total RNA was isolated from HP-14 treated and vehicle (DMSO) treated biofilms. Total RNA was subjected to RNA sequencing at the Interdisciplinary Center for Biotechnology Research (RNA Integrity Number, RIN ≥ 7 quality control cutoff; see the Supporting Information).
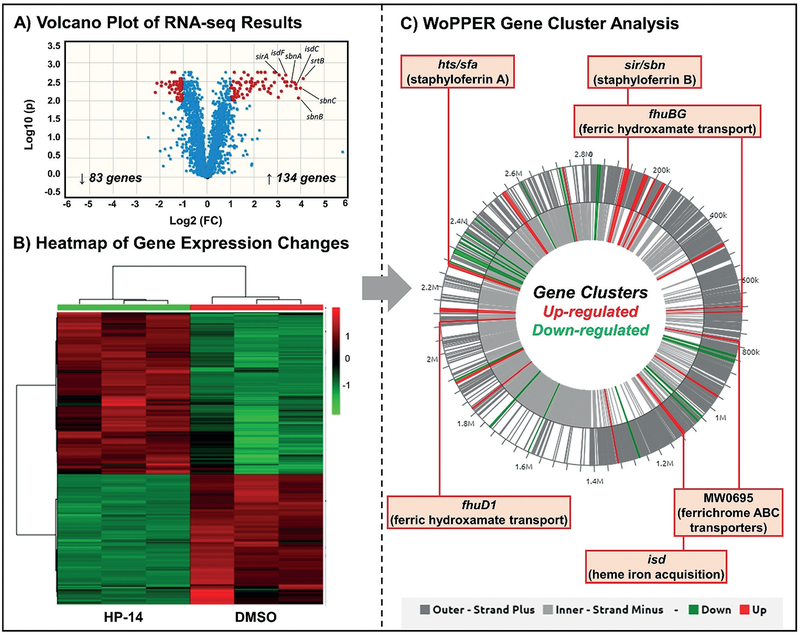
Results from these experiments revealed 217 of 2738 gene transcripts to be differentially expressed between HP-14-treated and untreated MRSA biofilm samples (≥ 2.0-fold change in gene expression). Volcano plot (Figure 2A) and heatmap analysis (Figure 2B) of our RNA-seq results show 83 genes down-regulated and 134 genes up-regulated in MRSA biofilms as a result of HP-14 treatment. We found the WoPPER analysis tool[21] to be the most useful approach to analyzing our RNA-seq data. The WoPPER tool enabled us to determine gene clusters up-regulated (“activated”) and down-regulated (“inhibited”) in response to HP-14 (Figure 2C).
Figure 2.
A) Volcano plot and B) heatmap analysis of RNA-seq profiles of MRSA biofilms treated with HP-14. C) WoPPER analysis reveals that HP-14 up-regulates MRSA biofilm gene clusters involved in iron acquisition.
WoPPER results showed alterations in gene expression profiles for 37 gene clusters, including several involved in iron acquisition (Figures 2C and 3A): hts/sfa (staphyloferrin A[22]), sir/sbn (staphyloferrin B[23]), isd (iron-regulated surface determinant; heme iron acquisition[24]), MW0695 (hypothetical protein, similar to ferrichrome ABC transporters), and fhu (ferric hydroxamate uptake[25]).[26,27] Other MRSA biofilm gene clusters with changes in expression profiles due to HP-14 include: spl (serine proteases;[28] activated), opp (oligopeptide transporters;[29] activated), gap (glycolysis;[30] inhibited), arc (arginine deiminase;[31] inhibited), ure (urease;[32] inhibited), hem (heme biosynthesis;[33] inhibited), and an uncharacterized gene cluster containing dnaC (DNA synthesis and repair;[34] inhibited; see Figures 1 and 2 in the Supporting Information). Several gene clusters with unknown functions or lower levels of activation/inhibition in response to HP-14 are presented in the Supporting Information (for WoPPER details, see Tables 6 and 7). One representative gene from each of the up- and down-regulated clusters was subjected to real-time qPCR assessment (RT-qPCR; Figure 3E) to confirm activation or inhibition results from our WoPPER analysis.
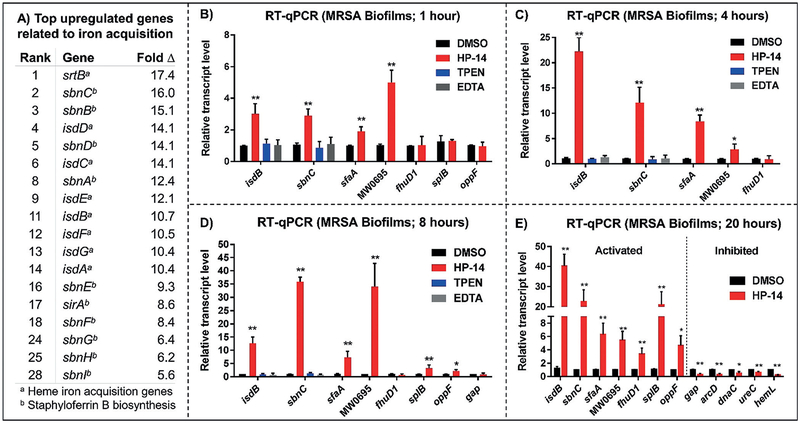
Figure 3.
A) Top iron-acquisition genes in MRSA biofilms affected by HP-14 (initial results). B–E) Time–course validation of genes selected from WoPPER analysis. RT-qPCR results of MRSA biofilms treated with HP-14 for 1 hour (B), 4 hours (C), 8 hours (D), and 20 hours (E). Note: *p value ≤0.05, **p value ≤0.01.
A time–course assessment using RT-qPCR of MRSA biofilms (Figure 3B–E) treated with low concentrations of HP-14 (1/10 MBEC = 0.625 μm) revealed that four iron-acquisition gene clusters (isd, sbn, sfa, MW0695) were activated in 1 hour. We found this rapid activation of iron-uptake genes to be profound, as bacterial biofilms are notorious for high levels of dormancy and persistence. We were unable to find other reports to suggest that biofilms rapidly respond to small molecules and conclude the rapid activation of iron-uptake systems to be the primary mechanism by which HP-14 operates through the iron starvation of MRSA biofilms. Our data demonstrate that, although dormant, bacterial biofilms are highly sensitive and possess the ability to rapidly respond to small-molecule threats (e.g., HP-14) to enable survival.
The RNA-seq data align with previous results demonstrating that HPs bind metal(II) cations and cotreatment of iron(II) in antibacterial assays against S. aureus results in reduced potencies.[18,19] We believe that the loss in antibacterial activity is most likely due to free iron(II) co-administered in the MIC assay being directly bound to the HP test compound (inactivation of the HP occurs through iron(II) chelation between the hydroxy oxygen and adjacent nitrogen atoms to form a five-membered iron(II) chelate, a 2:1 HP:iron(II) complex according to UV/Vis spectroscopy[18,19]).
Iron-acquisition genes isdB, sbnC, sfaA, MW0695 demonstrated increased levels of activation in MRSA biofilms subjected to HP-14 for 4 hours (isdB: 22.3-fold up-regulation; Figure 3C) and 8 hours (sbnC: 36.0-fold up-regulation; Figure 3D) through RT-qPCR. Known metal-chelating agents N,N,N’,N’-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; 5 μm) and ethylenediaminetetraacetic acid (EDTA; 5 μm) were unable to activate isdB and sbnC upon treatment of MRSA biofilms for 1, 4, and 8 hours (Figure 3B–D), thus demonstrating a unique mechanism of action for HP-14. These results align with previous findings that TPEN and EDTA are unable to eradicate MRSA biofilms at high concentrations (MBEC > 2000 μm) when tested alongside HP-14 (MBEC = 6.25 μm).[18]
Time–course studies provided insight into the timing of gene activation and inhibition, thus enabling us to differentiate primary biofilm activities (early time points, RT-qPCR) from secondary changes in gene expression triggered by HP-14. Interestingly, fhuD1 (ferric hydroxamate xenosiderophore, iron acquisition) was not activated upon HP-14 treatment of MRSA biofilms at 1, 4, or 8 hours, despite being activated at 20 hours. This result suggests the fhu gene cluster does not play as critical a role in iron acquisition as the isd, sir/sbn, hts/sfa, and MW0695 (ferrichrome ABC transporters) gene clusters, which demonstrated rapid activation in MRSA biofilms upon HP-14 treatment.
Gene transcripts unrelated to iron uptake, such as splB (serine protease) and oppF (oligopeptide transporter), were not activated in MRSA biofilms upon HP-14 treatment for 1 hour; however, both genes were up-regulated in MRSA biofilms treated with HP-14 after 8 hours. This result suggests that serine proteases and oligopeptide transporters are involved in secondary responses to HP-14 in addition to the possibility of interplay with iron-acquisition genes, or the iron-starvation response in MRSA biofilms. Additionally, gap (glycolysis) expression was investigated in MRSA biofilms treated with HP-14 after 8 hours; however, no change in gap expression was observed at this earlier time point, thus suggesting the down-regulation of gap is a downstream response to HP-14 treatment (gap gene expression reduced after 20 hour treatment, aligns with KEGG assessment, as described below).
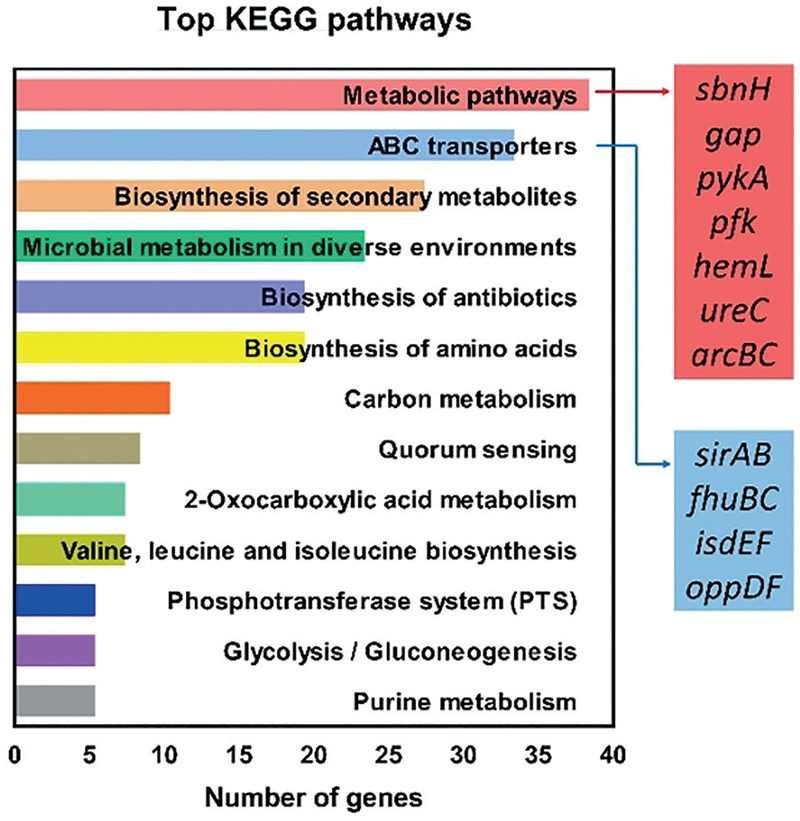
We analyzed the RNA-seq data using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway assessment[35] to gain insight into the impact HP-14 has on functional pathways critical to MRSA biofilms. By assessing differentially expressed genes from our RNA-seq findings, genes can be assigned to functional bacterial pathways. The top 13 pathways are presented in Figure 4, with the most frequently assigned functional pathway alterations being metabolic pathways (38 genes affected by HP-14 treatment). Metabolic-pathway genes identified by KEGG analysis align with our WoPPER findings, including: sbnH (6.2-fold Δ; staphyloferrin B), gap (−3.7-fold Δ; glycolysis), pykA (−2.8-fold Δ; glycolysis), pfk (−2.4-fold Δ; glycolysis), hemL (−2.6-fold Δ; heme biosynthesis), ureC (−2.2-fold Δ; urease alpha subunit), arcB (−2.1-fold Δ; arginine deiminase), and arcC (−2.9-fold Δ; arginine deiminase). These findings indicate that metabolic pathways are important to the response of MRSA biofilm cells to HP-14. We found this result to be intriguing, as biofilm cells are considered metabolically dormant; however, under the threat of iron starvation, it appears that metabolic pathways in MRSA biofilms are greatly impacted.
Figure 4.
KEGG analysis to characterize functional bacterial pathways in MRSA biofilms affected by HP-14.
The second most altered KEGG functional pathway by HP-14 was ABC transporters (33 genes affected). ABC transporters are crucial transport systems for nutrient uptake by bacteria,[36] including iron.[27] Altered genes expressed in the ABC transporter category include sirAB, fhuBC, isdEF, and oppDF. Overall, this KEGG analysis leads us to conclude that metabolic pathways and ABC transporters are important for MRSA biofilm survival, as HP-14 significantly impacts genes involved in these functions.
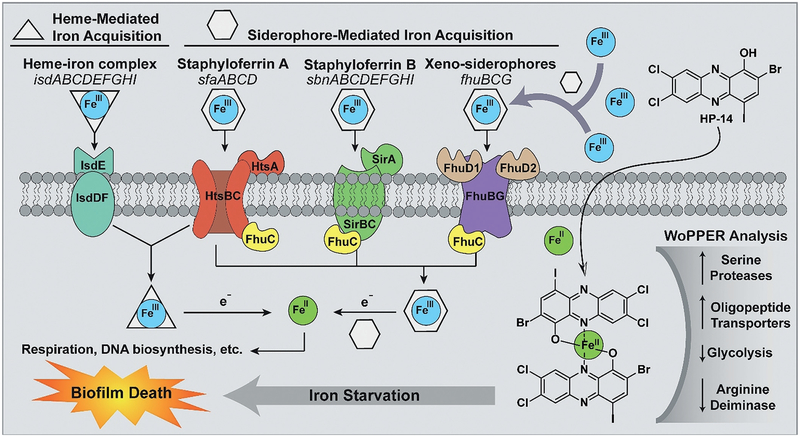
From our findings, it is clear that MRSA biofilms are highly sensitive to HP-14-induced iron starvation. It is well-established that iron is essential for bacteria to thrive;[26,27] however, iron(III) is unable to diffuse through bacterial membranes. Bacteria utilize siderophore molecules with high binding affinities to acquire iron(III). Siderophores are synthesized by bacteria and secreted into their surrounding environment to sequester iron(III) and deliver the iron(III)–siderophore complex into bacteria through protein transporters that recognize the complex. Once the iron(III)–siderophore complex has entered the bacterial cell, iron(III) is reduced to iron(II) by the reductive cytoplasm of the bacterium. Upon reduction to iron(II), the siderophore loses affinity and releases iron(II) for the bacterium to utilize (for respiration, DNA biosynthesis, metalloproteins, etc.).[27]
HP-14 is a potent biofilm-eradicating agent that directly binds iron(II); however, other metal chelators (EDTA, TPEN) are unable to eradicate biofilms.[18] During these studies, EDTA and TPEN were unable to activate iron-acquisition genes when tested alongside HP-14. On the basis of these findings and considering the lipophilic properties of HP-14 (cLogP = 6.25), we propose that this HP rapidly diffuses through the cellular membrane of biofilm cells to bind iron(II), which has been released from a siderophore for utilization by the bacterium (Figure 5). Once HP-14 binds iron(II), intracellular concentrations of free iron(II) decreases. Insufficient iron(II) is sensed by the MRSA biofilm cell, which elicits rapid activation of iron-acquisition gene clusters to counteract iron starvation (isd, heme iron acquisition; sbn, staphyloferrin B, siderophore; sfa, staphyloferrin A, siderophore; MW0695, ferrichrome ABC transporter).
Figure 5.
Model for the mechanism of MRSA biofilm eradication by HP-14. The primary mode of action is iron starvation, with additional MRSA biofilm gene clusters affected based on WoPPER analysis.
In conclusion, transcript profiling of MRSA biofilms treated with the biofilm-eradicating agent HP-14 has been performed to define cellular targets and pathways critical to biofilm survival. RNA-seq was utilized in conjunction with WoPPER analysis to identify MRSA biofilm gene clusters differentially expressed in response to HP-14. We found that HP-14 rapidly up-regulated iron-acquisition gene clusters after 1 hour. We found this rapid activation of iron-acquisition gene clusters to be profound, as bacterial biofilms are notorious for their metabolic dormancy. KEGG analysis revealed that HP-14 significantly impacts 1) metabolic pathways and 2) ABC transporters in MRSA biofilms. Combined, our data demonstrate that HP-14 induces rapid iron starvation in MRSA biofilms while impacting other genes (e.g., spl, opp, gap, ure) and functional pathways critical for the viability of the surface-attached bacterial community. We aim to extend this platform to other biofilm-eradicating agents that operate through alternative modes of action to identify new targets and pathways that can be exploited to eradicate persistent biofilm infections in the clinic.
Supplementary Material
Acknowledgements
We acknowledge the University of Florida (start-up funds) and National Institute of General Medical Sciences of the National Institutes of Health for supporting this research (R35GM128621 to R.W.H.). We thank Professor Hendrik Luesch and Professor Yousong Ding for helpful discussions during these studies.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201809785.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Yasmeen Abouelhassan, Department of Medicinal Chemistry, Center for Natural Products, Drug Discovery and Development (CNPD3), College of Pharmacy, University of Florida, 1345 Center Drive, Gainesville, FL (USA).
Yanping Zhang, Interdisciplinary Center for Biotechnology Research (ICBR), Gene Expression and Genotyping, University of Florida (USA).
Shouguang Jin, Department of Molecular Genetics and Microbiology College of Medicine, University of Florida (USA).
Robert W. Huigens, III, Department of Medicinal Chemistry, Center for Natural Products, Drug Discovery and Development (CNPD3), College of Pharmacy, University of Florida, 1345 Center Drive, Gainesville, FL (USA).
References
- [1].Garrison AT, Huigens RW, Curr. Top. Med. Chem 2017, 17, 1954–1964. [Google Scholar]
- [2].Hall-Stoodley L, Costerton JW, Stoodley P, Nat. Rev. Micro-biol 2004, 2, 95–108. [DOI] [PubMed] [Google Scholar]
- [3].Waters CM, Bassler BL, Annu. Rev. Cell Dev. Biol 2005, 21, 319–346. [DOI] [PubMed] [Google Scholar]
- [4].Lewis K, Annu. Rev. Microbiol 2010, 64, 357–372. [DOI] [PubMed] [Google Scholar]
- [5].Wood TK, Biotechnol. Bioeng 2016, 113, 476–483. [DOI] [PubMed] [Google Scholar]
- [6].Conlon BP, BioEssays 2014, 36, 991–996. [DOI] [PubMed] [Google Scholar]
- [7].Musk DJ, Hergenrother PJ, Curr. Med. Chem 2006, 13, 2163–2177. [DOI] [PubMed] [Google Scholar]
- [8].Wolcott R, Dowd S, Plast. Reconstr. Surg 2011, 127, 28S–35S. [DOI] [PubMed] [Google Scholar]
- [9].Resch A, Rosenstein R, Nerz C, Gçtz F, Appl. Environ. Microbiol 2005, 71, 2663–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PM, Kielian T, Infect. Immun 2013, 81, 4363–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marguerat S, Bähler J, Cell. Mol. Life Sci 2010, 67, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fisher RA, Gollan B, Helaine S, Nat. Rev. Microbiol 2017, 15, 453–464. [DOI] [PubMed] [Google Scholar]
- [13].Castro J, Franca A, Bradwell KR, Serrano MG, Jefferson KK, Cerca N, NPJ Biofilms Microbiomes 2017, 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amador CI, Sternberg C, Jelsbak L, Methods Mol. Biol 2018, 1734, 131–158. [DOI] [PubMed] [Google Scholar]
- [15].Qin N, Tan X, Jiao Y, Liu L, Zhao W, Yang S, Jia A, Sci. Rep 2014, 4, 5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tan X, Qin N, Wu C, Sheng J, Yang R, Zheng B, Ma Z, Liu L, Peng X, Jia A, Sci. Rep 2015, 5, 11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garrison AT, Abouelhassan Y, Kallifidas D, Bai F, Ukhanova M, Mai V, Jin S, Luesch H, Huigens RW, Angew. Chem. Int. Ed 2015, 54, 14819–14823; Angew. Chem. 2015, 127, 15032–15036. [DOI] [PubMed] [Google Scholar]
- [18].Garrison AT, Abouelhassan Y, Norwood IV VM, Kallifidas D, Bai F, Thu Nguyen M, Rolfe M, Burch BM, Jin S, Luesch H, Huigens RW, J. Med. Chem 2016, 59, 3808–3825. [DOI] [PubMed] [Google Scholar]
- [19].Yang H, Abouelhassan Y, Burch GM, Kallifidas D, Huang G,Yousaf H, Jin S, Luesch H, Huigens RW, Sci. Rep 2017, 7, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Garrison AT, Abouelhassan Y, Kallifidas D, Tan H, Kim YS, Jin S, Luesch H, Huigens RW, J. Med. Chem 2018, 61, 3962–3983. [DOI] [PubMed] [Google Scholar]
- [21].Puccio S, Grillo G, Licciulli F, Severgnini M, Liuni S, Bicciato S, De Bellis G, Ferrari F, Peano C, Nucleic Acids Res. 2017, 45, W109–W115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakaminami H, Chen C, Truong-Bolduc QC, Kim ES, Wang Y, Hooper DC, Infect. Immun 2017, 85, e00358–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Laakso HA, Marolda CL, Pinter TB, Stillman MJ, Heinrichs DE, J. Biol. Chem 2016, 291, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP, Bacteriol J. 2006, 188,8421–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sebulsky MT, Speziali CD, Shilton BH, Edgell DR, Heinrichs DE, J. Biol. Chem 2004, 279, 53152–53159. [DOI] [PubMed] [Google Scholar]
- [26].Hammer ND, Skaar EP, Annu. Rev. Microbiol 2011, 65, 129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bilitewski U, Blodgett JAV, Duhme-Klair A-K, Dallavalle S, Laschat S, Routledge A, Schobert R, Angew. Chem. Int. Ed 2017, 56, 14360–14382; Angew. Chem. 2017, 129, 14552–14575. [DOI] [PubMed] [Google Scholar]
- [28].Pusteiny K, Zdzalik M, Stach N, Stec-Niemczyk J, Cichon P,Czarna A, Popowicz G, Mak P, Drag M, Salvesen GS, Wladyka B, Potempa J, Dubin A, Dubin G, J. Biol. Chem 2014, 289, 15544–15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hiron A, Borezée-Durant E, Piard J-C, Juillard V, Bacteriol J. 2007, 189, 5119–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yugueros J, Temprano A, Berzal B, Sánchez M, Hernanz C,Luengo JM, Naharro G, J. Clin. Microbiol 2000, 38, 4351–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Makhlin J, Kofman T, Borovok I, Kohler C, Engelmann S, Cohen G, Aharonowitz Y, J. Bacteriol 2007, 189, 5976–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Konieczna I, Żarnowiec P, Kwinkowski M, Kolesińska B, Frączyk J, Kamiński Z, Kaca W, Curr. Protein Pept. Sci 2012, 13, 789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, Warren MJ, Microbiol. Mol. Biol. Rev 2017, 81, e00048–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaito C, Kurokawa K, Hossain MS, Akimitsu N, Sekimizu K, FEMS Microbiol. Lett 2002, 210, 157–164. [DOI] [PubMed] [Google Scholar]
- [35].Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M, Nucleic Acids Res. 2010, 38, D355–D360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wilkens S, F1000Prime Rep. 2015, 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.