Abstract
Voltage-gated K+ (Kv) channels are major determinants of membrane potential in vascular smooth muscle cells (VSMCs), and regulate the diameter of small cerebral arteries and arterioles. However, the intracellular structures that govern the expression and function of vascular Kv channels are poorly understood. Scaffolding proteins including postsynaptic density 95 (PSD95) recently were identified in rat cerebral VSMCs. Primarily characterized in neurons, the PSD95 scaffold has more than 50 known binding partners and it can mediate macromolecular signaling between cell surface receptors and ion channels. In cerebral arteries, Shaker-type Kv1 channels appear to associate with the PSD95 molecular scaffold, and PSD95 is required for the normal expression and vasodilator influence of members of this K+ channel gene family. Furthermore, recent findings suggest that the β1-subtype adrenergic receptor is expressed in cerebral VSMCs and forms a functional vasodilator complex with Kv1 channels on the PSD95 scaffold. Activation of β1-subtype adrenergic receptors in VSMCs enables protein kinase A-dependent phosphorylation and opening of Kv1 channels in the PSD95 complex; the subsequent K+ efflux mediates membrane hyperpolarization and vasodilation of small cerebral arteries. Early evidence from other studies suggests that other families of Kv channels and scaffolding proteins are expressed in VSMCs. Future investigations into these macromolecular complexes that modulate the expression and function of Kv channels may reveal unknown signaling cascades that regulate VSMC excitability and provide novel targets for ion channel–based medications to optimize vascular tone.
Introduction
Cerebral arteries regulate blood flow to the brain by reducing their diameter in response to increased intraluminal pressure – an event also known as the myogenic response.1, 2 One important mechanism of vasoregulation is the activity of potassium (K+) channels in the cerebral vascular smooth muscle cells (cVSMCs).2–4 The closing of K+ channels causes membrane depolarization, opening of voltage-sensitive Ca2+ channels, and vasoconstriction.2, 3 Thus, voltage-gated K+ (Kv) channels represent a major K+ channel superfamily that contributes to the resting diameter of small cerebral arteries.5–14 There are twelve gene families (Kv1 to Kv12) in the Kv channel superfamily.15 At least three gene families, Kv1, Kv2, and Kv7 channels, are expressed in cVSMCs and regulate myogenic tone.8, 11, 14
The open-state probability of Kv channels increases in response to a depolarized membrane potential, and multiple phosphorylation sites also regulate channel activity.16–23 In this review, we will focus mainly on the Shaker-type Kv1 channels and expand our discussion to other Kv channel gene families when appropriate. The Kv1 channels consist of four pore-forming α-subunits and auxiliary β-subunits.8, 10 In the rat cerebral circulation, the α1.2 and α1.5 subunits minimally form the heterotetrameric pore structure.8 Seemingly contradicting reports describe how phosphorylation of the α-subunits regulates the function of Kv1 channels. For example, the adenylyl cyclase activator, forskolin, elicits protein kinase A (PKA)-dependent phosphorylation and increases delayed rectifier-type K+ current in smooth muscle cells from rabbit portal vein, suggesting that PKA can signal effectively to open Kv1 channels in native VSMCs.24 In contrast, several studies using forskolin or cAMP analogues to elicit PKA phosphorylation of recombinant Kv1.2 channels report an increase in Kv1.2 protein expression, but little change in Kv1 channel current.20, 23, 25, 26 Instead, the direct application of protein kinase A (PKA) catalytic subunits is required to activate cloned Kv1.2 channels.21 This apparent dichotomy infers that a signaling complex in native VSMCs closely associates PKA and Kv1 channels to enable PKA phosphorylation of Kv1 channel α-subunits; however, the close association of PKA and Kv1 channels is absent in transfected expression systems, necessitating the direct application of exogenous PKA for phosphorylation and activation of Kv1 channels. To date, the Kv1 channel signaling complexes in VSMCs are largely enigmatic. However, the topology and amino acid sequences of the Kv1 channel α-subunits provide important clues with regard to possible protein interaction domains. The Kv1 channel α-subunits have six transmembrane segments flanked by intracellular N- and C-termini. Of particular interest are the final four amino acids on the C-terminus, i.e., -LTDV for the α1.2 subunit and -ETDL for the α1.5 subunit. These sequences form so-called Class I “PDZ binding motifs”,27, 28 which selectively bind to PDZ domains on scaffolding proteins as explained in the next section.
Postsynaptic density 95 (PSD95) scaffolding of Kv channels
Postsynaptic density 95 (PSD95),29 also known as synapse associated protein 90 (SAP90),30 was first characterized as a 95-kilodalton protein found in the postsynaptic density fraction of rat brain.29 PSD95 belongs to a large family of scaffolding proteins known as MAGUKs (membrane-associated guanylate kinase homologues).31 As the name suggests, MAGUKs are not transmembrane proteins per se, but associate with membrane-bound proteins to form clustered multimeric structures near the plasma membrane.32, 33 Uniquely positioned to interact with ion channels and cell surface receptors, as well as cell adhesion molecules and cytoplasmic proteins, MAGUKs modulate the surface expression and function of ion channels and receptors.28, 34–37 Several structural characteristics of MAGUKs enable them to play such a unique role in integrating signaling and effector molecules in a cell-specific manner.31 The basic core of MAGUK proteins including PSD95 contains a Src homology 3 (SH3) domain,38 a guanylate kinase (GK) domain, and one or more PDZ domains.39 The PDZ domain refers to the ~90 amino acid residues that form a hydrophobic pocket commonly found in MAGUK proteins including PSD95,29, 30 Drosophila septate junction protein Discs-large,40 and epithelial tight junction protein ZO-1.41 The PSD95 protein has three PDZ domains followed by the SH3 and GK domains (Figure 1A). Although the PDZ domains were first identified in MAGUKs, they are present in a wide variety of proteins in diverse organisms including bacteria, yeast, and plants.42 In the human genome, the PDZ domain family ranks as the 19th most abundant of domain families, suggesting a diverse and intricate role in protein-protein interactions.43 The crystal structure of the pocket-like PDZ domain and the structure of the PDZ binding motif have been resolved for several proteins.44–46 The PDZ domain contains a series of GLGF (Gly-Leu-Gly-Phe) repeats,29 which can tightly bind to the hydrophobic PDZ binding motifs of its protein partners. Differences in the side chains on the pocket of the PDZ domains confer differential affinity for the various PDZ binding motifs found in receptors and ion channels.28, 47–49
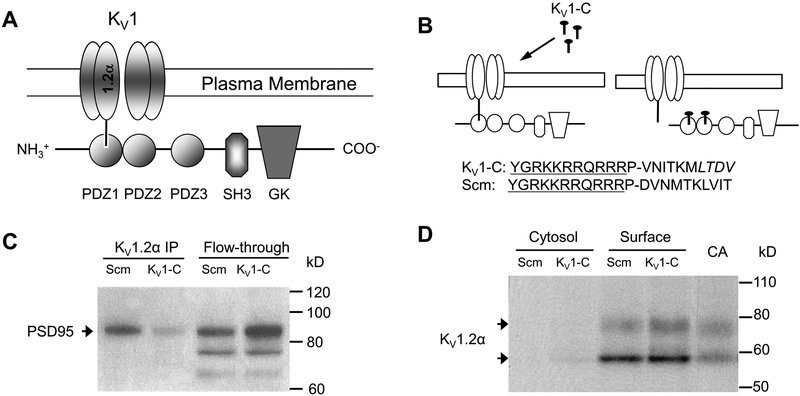
Figure 1. PSD95 interaction with the Kv1 channel and disruption by Kv1-C peptide.
A) Schematic of the association of the Kv1 channel α1.2 subunit with the PSD95 scaffold via the PDZ1 binding domain. PSD95 contains three PDZ binding domains (PDZ1–3), and Src-homology (SH3) and guanylate kinase (GK) domains. B) The Kv1-C dominant negative peptide was designed to compete for the PDZ binding domain on PSD95. The last 10 amino acids of the C-terminus of the α1.2 pore protein were conjugated to HIV-tat (YGRKKRRQRRR) to confer cell-permeability. P is a spacer. LTDV is a class-1 PDZ binding motif on the α1.2 subunit. A peptide with same amino acid composition in a scrambled order (Scm) was used as control. C) Immunoprecipitation using anti-Kvα1.2 of rat cerebral arterial lysate treated with Scm or Kv1-C peptide for 30 min. The Kvα1.2 immunoprecipitate and column flow-through (Flow-through) were probed for PSD95 on a Western blot. Depicted is a representative scan from three similar experiments showing that Kv1-C peptide disrupted PSD95 association with α1.2. D) Biotinylation of rat cerebral arteries treated with Scm or Kv1-C peptide for 30 min. Cytosolic and surface fractions were probed for the Kv1 channel α1.2 subunit. Control lysate from freshly isolated cerebral arteries (CA) was loaded for size comparison. Depicted is a representative blot from five similar experiments. Kv1-C did not alter the surface expression of Kv1 channel α1.2 subunits, which appear as a doublet band at ~ 58 kD and 80 kD; the upper band represents the glycosylated form. Figures and legend from reference 82.
The PDZ domains on the PSD95 scaffold bind to a subset of PDZ binding motifs recognized as X-S/T-X-V, where S/T is serine or threonine, V is valine, and X is any amino acid.50, 51 Notably, more than 50 proteins associate with PSD95 in neurons and other cells.28, 37 The best known binding partners of PSD95 include the N-methyl-D-aspartate receptors (NMDAR),50 amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (AMPA) receptors,52 and neuronal nitric oxide synthase (nNOS).53 Interestingly, some of the binding partners of PSD95 identified in neurons, also are expressed in rat cerebral arteries.54 This fact drew attention to PSD95 as a potentially unappreciated molecular scaffold in cVSMCs. For example, inwardly rectifying K+ (Kir) channels and TWIK-related acid-sensitive K+ channels are expressed in cerebral arteries and possess the PDZ binding motif for PSD95.37, 55 G-protein coupled receptors such as the serotonin receptor subunit 5-HT2C56, 57 and the β1-subtype adrenergic receptor (β1AR)58 also associate with PSD95 as potential regulators of vascular tone. Additionally, PSD95 can bind to A-kinase anchoring protein 150 (AKAP150) via the SH3 and GK domains to pair PKA59 and Kv1 channels on the PSD95 scaffold, and enable phosphorylation and opening of Kv1 channels.60 Interestingly, a previous report of AKAP150 in cVSMCs illustrates a punctate structure near the plasma membrane reminiscent of scaffolding clusters by PSD95.61 The authors indicate the AKAP150 scaffold facilitates protein kinase C (PKC)–elicited opening of voltage-gated calcium channels and vasoconstriction.61 Collectively, these data suggest the existence of an intricate latticework of intracellular scaffolds near the plasma membrane of cVSMCs, which dynamically aligns different ion channels with cell-surface receptors and signaling molecules to optimally regulate cVSMC excitability and vessel diameter.
Among the different α-subunits of the Kv1 channel family, only the C-terminus of the Kv1 channel pore-forming α1.2 subunit (-LTDV) exactly matches the definition of the PDZ binding motif for PSD95, whereas its partner in pore formation, α1.5, has a slightly different C-terminus (-ETDL) that may confer less affinity for PSD95. Other Kv1 channel α-subunits (α1.3 and α1.4) abundant in neurons also bind to the first two PDZ domains of PSD95.27, 51, 62–67 By means of this PDZ binding, PSD95 can regulate Kv1 channel clustering and surface expression. For example, postsynaptic clustering of Kv1 channels is abolished by mutation of the PSD95-homologue in Drosophila.68 Additionally, PSD95 slows the internalization of Kv1 channels in heterologous expression systems.65 Similarly, the surface expression of Kv1.2 channels increases when co-expressed with PSD95.69 Since its first discovery in 1992,29 PSD95 has been extensively studied as evidenced by more than 1,000 original research articles, but primarily it was regarded as a marker for postsynaptic density in neurons and rarely studied in non-neuronal tissues. However, we observed that PSD95 is abundantly expressed near the plasma membrane of rat cVSMCs to anchor Kv1 channels. Although mice with gene deletion of PSD95104 or PSD95 mutants lacking the first two PDZ domains105 have been designed, chronic global knockdown or mutation of PSD95 that is densely expressed in brain may trigger changes in the cerebral circulation unrelated to the loss of protein in the vasculature. For that reason, in our initial study we used an antisense-mediated knockdown of PSD95 in rat cerebral arteries, which caused a concomitant loss of Kv1 channel protein and its tonic vasodilator influence, leading to cVSMC depolarization and abnormal vasoconstriction.54
Notably, we also reported that a similar scaffolding protein, SAP97, is expressed abundantly in rat cerebral arteries, but it does not co-immunoprecipitate with Kv1 channels and is mainly found in the intracellular, juxtanuclear structures of cVSMCs under normal conditions.54 SAP97 shares a structural similarity with PSD95,70 but may have a different set of binding partners. For example, SAP97 in cardiac myocytes interacts with Kv471 and Kir72 channels. Considering the robust mRNA and protein expression of SAP97 in rat cerebral arteries,54 it would be interesting to investigate whether SAP97 is involved in the regulation of these additional K+ channel types in cVSMCs, or potentially alters their expression and function under pathological conditions, possibly by binding with other PDZ motif-containing proteins. It is also possible that different scaffolding proteins are expressed in non-cerebral vascular beds, where they may play unrecognized roles in regulating ion channel expression and function. For example, in the mesenteric circulation of hypertensive mice, the Kir2 and Kir4 channel proteins are down-regulated as part of the extensive electrical remodeling of arterial K+ channels.73 In diabetic rats, an exaggerated impact of oxidative stress signaling on Kir1.1 and Kir2.1 channel function may contribute to the loss of renal afferent arteriolar tone that predisposes to kidney damage.74
Dynamic and reversible interaction of Kv1 channels and PSD95
The PDZ interaction between the pore-forming α1.2 subunit of Kv1 channels and PSD95 appears to be dynamic and reversible.27, 51, 67, 69 In particular, the C-terminus of Kv1 channels is intrinsically disordered and can act as a molecular “fishing rod” to bind to PSD95 scaffolds.67, 75 This property has been compared to the ball-and-chain model of channel inhibition, whereby the N-terminus of the Kv channel swings around to block the channel pore.76 Interestingly, analysis of the unfoldability profiles of the C-terminal tails using a dataset of 70 Kv channel sequences revealed that 80% of all Kv channels contain intrinsically disordered C-terminal segments immediately adjacent to the terminal PDZ-binding motif.75 Thus, the intrinsic instability of the C-terminus that promotes PDZ binding to Kv1 channels also is a feature of other Kv channel families.
This dynamic and reversible interaction between Kv1 channels and PSD95 can be taken advantage of to elucidate the physiological outcome of the interaction.54 Because the PDZ domains of MAGUKs form unique interactions with their binding partners, “interfering peptides” that disrupt PDZ binding to its intended protein partners have been used as tools to selectively target unique scaffolding interactions without affecting the rest of the protein complex.50, 77 Using this approach, a pharmacological dose of short dominant negative peptide (~10 amino acids) containing the C-terminus PDZ binding motif of the protein is introduced into the cell to bind to PDZ domains, and thereby disrupt the PDZ-mediated interaction between PSD95 and its biological binding partner. In neurons, overloading cells with peptide sequences that mimic the C-terminus PDZ binding motif of NMDAR will serve as a PSD95 decoy, effectively uncoupling PSD95 from biological NMDAR to limit its access to nNOS. The end result is reduced excitotoxicity triggered by the NMDAR-nNOS signaling pathway.50, 77 A similar strategy was extended to rodents and non-human primates in vivo as a therapeutic intervention to reduce cortical damage after experimental stroke.78–81 In these studies, a cell-permeable HIV-tat sequence was coupled to the dominant negative peptide to ensure penetration into the cells of interest for disruption of NMDAR-nNOS signaling.
Separately, we devised a related strategy to elucidate whether a dynamic interaction with PSD95 is required for the vasodilator function of Kv1 channels in rat cerebral arteries.82 Here, we used a cell-permeable, dominant-negative peptide (“Kv1-C peptide”) that couples the HIV-tat sequence to the C-terminus PDZ binding motif of the Kv1.2 subunit to specifically disrupt the scaffolding interaction of PSD95 and native Kv1 channels (Figure 1B, 1C).82 Compared to our earlier work using antisense-based knockdown of PSD95, which resulted in the concurrent loss of both PSD95 and Kv1 channel proteins,54 this new strategy allowed us to evaluate the impact of disrupting the PSD95 - Kv1 channel interaction in rat cVSMCs without a loss of cell surface expression of Kv1 channels (Figure 1D).82 Surprisingly, a profound vasoconstriction was elicited within 5 minutes of the application of the Kv1-C peptide to rat cerebral arteries ex vivo and in vivo. The magnitude of the vasoconstriction was equivalent to that caused by a Kv1 channel-selective blocker, Psora-4 (100 nmol/L).54, 83, 84 A secondary binding site in the unique side pocket of Kv1 channels confers selectivity of submicromolar concentrations of Psora-4 for Kv1 channels compared to other Kv channel families.83, 85 Finally, the constrictor effects of the Kv1-C peptide and Psora-4 were not additive when administered together, suggesting a shared mechanism of action.82 The rapid constriction of rat cerebral arteries by Kv1-C peptide, presumed to be caused by loss of PKA phosphorylation of Kv1 channels, was blunted by phosphatase inhibitors okadaic acid and calyculin-A.82 Collectively, these results indicate that the “dynamic” PSD95 scaffolding of Kv1 channels is a critical requirement for Kv1 channel α-subunit phosphorylation and opening, hyperpolarization, and subsequent dilation of rat cerebral arteries.82 Because PKA does not directly bind to PSD95, these results raise the possibility that PKA indirectly associates with PSD95 through AKAP-PSD95 interaction to provide a functional macromolecular scaffolding complex in cVSMCs. In neurons, AKAP-PSD95 interaction is required for the PKA-dependent phosphorylation of AMPA receptors,59 and it appears a similar AKAP-PSD95 complex also may be required for PKA phosphorylation of vascular Kv1 channels. Potassium channels in the Kv superfamily other than Kv1 and Kv4 are not known to interact directly with PDZ binding domains. However, these Kv channels may indirectly associate with multi-protein complexes that include the PSD95 scaffold. As one possibility, Kv7 channels encoded by the KCNQ1 gene associate with an AKAP9 protein, Yotiao, and are phosphorylated by PKA.86 Similar associations that form molecular networks of interacting receptors, ion channels and signaling molecules may exist in VSMCs and underlie the complex interaction between Kv channels and other proteins involved in the regulation of vascular tone.
Interestingly, the PDZ-mediated interaction of PSD95 with its binding partners appears to be dynamic and reversible as shown in similar studies in which application of a competing peptide disrupts NMDAR-PSD95 binding in cultured neurons77 and in neurons in vivo.79–81 In Canada and the United States, clinical trials using cell-permeable interfering peptides targeting NMDAR-PSD95 interaction (dubbed “PSD95 inhibitors”) are underway to explore PSD95 inhibitors as medications for ischemic brain damage.87 Considering that the Kv1 channel α1.2 subunit and NMDAR share the ability to bind to the PDZ1 and PDZ2 domains of PSD95,79 therapeutic peptides that bind to the PDZ domains of PSD95 to prevent NMDAR-PSD95 interaction, also would make PSD95 unavailable to its other biological binding partners, including Kv1 channels in cVSMCs. The loss of functional Kv1 channels caused by “unscaffolding” from PSD95 would favor cerebral vasoconstriction. Thus, it may be important to consider the broader pharmacological implications of the PSD95 inhibitors79, 80 and constriction of cerebral arteries could be a potential off-target effect.
Activation of Kv1 channels by the β1-subtype adrenergic receptor
Adrenergic receptors (ARs) mediate sympathetic responses to norepinephrine (NE) released locally by nerve endings, or to circulating epinephrine and NE released by the adrenal medulla. The typical response of most systemic arterioles and arteries to sympathetic activation is vasoconstriction mediated by α1-subtype adrenergic receptors (α1AR), although vasodilation of skeletal muscle arteries can result from activation of β2-subtype adrenergic receptors (β2AR).88 Traditionally, the final response of an artery to sympathetic activation was thought to depend on the relative expression levels of α1AR and β2AR in the VSMCs, and the effectiveness of their signaling pathways. However, newer studies using subtype-specific β1- and β2- adrenergic receptor knockout mice have revealed a mixed distribution of functional β1AR and β2AR in many arteries, challenging initial dogma of predominant β2AR expression in VSMCs.89 For example, in pulmonary and femoral arteries, β1ARs instead of β2ARs mediate the vasodilator response to isoproterenol, a nonselective β-AR agonist.89
The precise contribution of sympathetic influences to the regulation of cerebral blood flow still is debated. The cerebral arteries of several species including human90, 91 and rat92 express both α-AR and β-AR. However, some early studies in cat, dog and monkey failed to detect direct diameter responses to cervical nerve stimulation93 despite heavy sympathetic innervation of cerebral arteries.94 Regardless, later studies in cerebral arteries from several species including rat and cat suggested that β-AR activation induces cerebral vasodilation.94–98 Interestingly, studies dating back to the 1980’s report β1AR-mediated dilation in cerebral arteries of rats and other mammals, instead of prevalent dilation by β2AR.99, 100 Realizing that β1AR has a C-terminus PDZ binding motif (-ESKV) that binds to the PDZ3 domain of PSD95,54, 82, 101 this receptor has surfaced as a prime candidate to be a member of the PSD95-Kv1 vasodilator complex in cVSMCs (Figure 2).54, 82, 101 The predominance of β1AR in cVSMCs has important relevance for the regulation of cerebral blood flow, because: 1) β1AR but not β2AR contains the C-terminus PDZ binding motif required for binding to PSD95;58, 102, 103 2) NE as an endogenous mixed adrenergic agonist has high affinity for α-AR and β1AR, but not for β2AR; and 3) the most widely used β-AR blocking drug, metoprolol, selectively blocks β1AR over β2AR.104
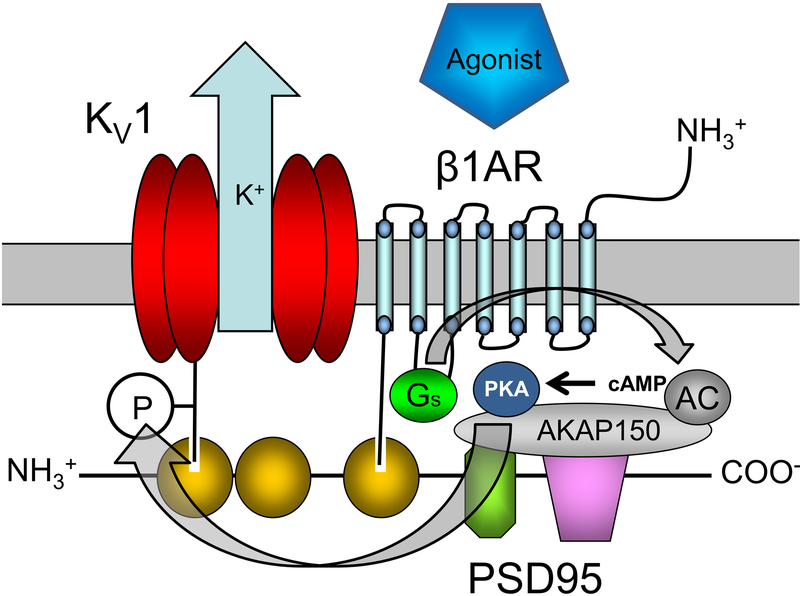
Figure 2. Proposed association of the β1-subtype adrenergic receptor (β1AR) and Kv1 channel on a PSD95 scaffold to form a “vasodilator signalosome” in cVSMCs.
The carboxyl termini of the β1AR and α1.2 pore protein of the Kv1 channel bind to PDZ domains on the PSD95 scaffold to create a receptor-effector signaling complex or “signalosome”. Agonist binding to β1AR initiates the GS-protein, adenylyl cyclase (AC), protein kinase A (PKA) cascade. This second-messenger cascade in cVSMCs culminates in PKA-phosphorylation (P) and activation of Kv1 channels, which promotes vasodilation of cerebral arteries. PDZ domains (yellow), SH3 domain (green octagon), guanylate kinase domain (purple), A-kinase anchoring protein (AKAP150), cyclic adenosine monophosphate (cAMP). Involvement of gray-colored proteins has not been tested directly in cVSMCs, but is speculated based on reported associations in other cell types. For simplicity, PSD95 is depicted linearly to illustrate proposed association of the receptor, signaling proteins and Kv1 channel. This two-dimensional schematic may not represent the actual relative positions of components in three-dimensional space. Figures and legend from reference 101.
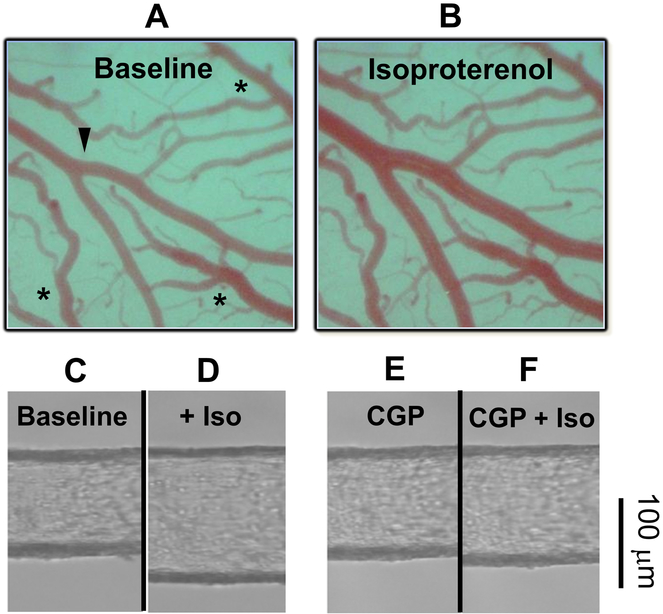
Considering these facts, we recently proposed that a β1AR-PSD95-Kv1 complex in cVSMCs enables β1AR to signaling through PKA-mediated phosphorylation of Kv1 channels, which mediates NE-induced dilation of rat cerebral arteries.101 Notably, the expression of β1AR and its association with PSD95 in rat cerebral arteries were confirmed by real-time RT-PCR, Western blots, co-immunoprecipitation and confocal microscopy of cVSMCs.101 Indeed, NE and isoproterenol (Iso) dilated rat cerebral arteries in vivo (Figure 3A–B), and similarly dilated and hyperpolarized rat cerebral arteries in vitro (Figure 3C–D). The vasodilator responses were inhibited by either the β1AR-selective blocker CGP20712 (Figure 3E–F)105, 106 or the Kv1 channel blocker Psora-4, but not the β2AR-selective blocker, ICI18551. Additionally, isoproterenol-induced dilation was inhibited by the Kv1-C peptide that disrupts PSD95-Kv1 channel association and by a PKA inhibitor.88 Collectively, these results suggest that a β1AR-PSD95-Kv1 signalosome (Figure 2) mediates the vasodilator response of cerebral arteries and arterioles to endogenous catecholamines or exogenous β-AR agonists.101
Figure 3. Isoproterenol-induced dilation of cerebral arteries in vivo.
A-B) Representative images of a rat middle cerebral artery branch (arrowhead) in a cranial window at (A) baseline and (B) in response to 10 μmol/L isoproterenol. Veins (asterisks) are unresponsive to isoproterenol. Figures and legend from reference 101. C-F) Image of a rat superior cerebellar artery cannulated and pressurized to 80 mmHg as performed in reference 101. After 1 hour of equilibration (C, Baseline), isoproterenol (Iso) was added to the bath in half-log concentrations between 1 nM and 10 μmol/L. The diameter response to the highest Iso concentration of 10 μmol/L is shown (D, Iso). After washout, the β1AR blocker CGP20712 was applied for 10 minutes (C, CGP) and then Iso was added cumulatively up to 10 μmol/L (D, CGP+Iso). Scale bar, 100 μm.
It is interesting then to consider whether β-AR blocking drugs (i.e., beta blockers); which are commonly administered to block cardiac β1ARs and thereby lower heart rate, slow atrioventricular conduction, and reduce blood pressure; could potentially compromise cerebral blood flow by disabling the β1AR-PSD95-Kv1 signalosome in cVSMCs. In this regard, a meta-analysis comparing β-AR blockers to other antihypertensive drugs showed that the relative risk of stroke was 16% to 26% higher in patients taking β-AR blockers compared to other antihypertensive drugs with similar blood pressure reduction.107 Also, post-surgical patients with atherosclerotic risk administered a β-AR blocker for 30 days had a significantly higher incidence of stroke compared to a placebo group.108 Furthermore, the new guidelines by the Eighth Joint National Committee (JNC 8) removed β-AR blockers as a first-line antihypertensive drug option,109 reversing a previous recommendation by JNC 7.110 This decision was based on evidence of increased stroke incidence after β-AR blocker use.111 Alarmingly, β-AR blockers represent one of the most frequently prescribed drug classes. For example, metoprolol, a β1AR-selective blocker, is dispensed in more than 80 million prescriptions annually. It ranked 4th for medicines prescribed in the United States in 2013.104 With growing recognition of the vasodilator function of β1AR in the cerebral circulation, and its association with other signaling molecules including Kv1 channels via PSD95, the impact of β-AR blockers and other medications that modify macromolecular signaling in cVSMCs will have to be considered when conducting risk:benefit assessment.
Future directions
The β1AR-PSD95-Kv1 signalosome described in this chapter represents only one of many potential receptor-ion channel interactions mediated by PSD95 and other MAGUK scaffolds in the cerebral vasculature. Considered alone, PSD95 has more than 50 known binding partners, most of which have not been explored as components of the PSD95 signalosome in cVSMCs. Future studies are needed to identify the upstream signals that regulate signaling molecules in cVSMCs and the downstream effectors including ion channels that impact vascular tone. Additionally, the function of other MAGUK proteins in the cerebral circulation, for example, SAP97, is completely unexplored. SAP97 is known to interact with Kir channels72 that contribute to neurovascular coupling, and impaired function of Kir channels has been implicated in a poor blood flow response to transient global cerebral ischemia.112 The initial reports detailing scaffolding proteins in the rat cerebral circulation, including AKAP150 scaffolding of voltage-gated Ca2+ channels,61 and PSD95-mediated association of β1AR and Kv1 channels,101 may provide impetus to explore MAGUK-scaffolded signalosomes in other vascular beds. A clear definition of these vascular macromolecular complexes may represent an immediate opportunity to better decipher the complex networks that regulate vascular tone and affect pharmacological responses to medications, thereby impacting the lives of millions of patients with cardiovascular disorders.
SOURCES OF FUNDING
National Institutes of Health grant R01-HL097107 (SWR), American Heart Association grant 17GRNT33670970 (SWR), and a Fund-to-Cure-Stroke grant from the University of Arkansas for Medical Sciences (SWR).
References
- 1.Faraci FM, Baumbach GL, Heistad DD. Myogenic mechanisms in the cerebral circulation. J Hypertens Suppl 1989; 7: S61–4; discussion S65. [PubMed] [Google Scholar]
- 2.Knot HJ and Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol 1995; 269: H348–55. [DOI] [PubMed] [Google Scholar]
- 3.Robertson BE and Nelson MT. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol 1994; 267: C1589–97. [DOI] [PubMed] [Google Scholar]
- 4.Kitazono T, Faraci FM, Taguchi H, Heistad DD. Role of potassium channels in cerebral blood vessels. Stroke 1995; 26: 1713–23. [DOI] [PubMed] [Google Scholar]
- 5.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [KVα1] subunits in terminal arterioles of rabbit. J Physiol 2001; 534: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheong A, Dedman AM, Xu SZ, Beech DJ. KVα1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol 2001; 281: H1057–65. [DOI] [PubMed] [Google Scholar]
- 7.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res 2001; 89: 1030–7. [DOI] [PubMed] [Google Scholar]
- 8.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol 2003; 551: 751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole WC, Chen TT, Clement-Chomienne O. Myogenic regulation of arterial diameter: role of potassium channels with a focus on delayed rectifier potassium current. Can J Physiol Pharmacol 2005; 83: 755–65. [DOI] [PubMed] [Google Scholar]
- 10.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric KV1 channels contribute to myogenic control of arterial diameter. Circ Res 2005; 96: 216–24. [DOI] [PubMed] [Google Scholar]
- 11.Amberg GC and Santana LF. KV2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol 2006; 291: C348–56. [DOI] [PubMed] [Google Scholar]
- 12.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of KV1 channels in vasoregulation. Circ Res 2006; 99: 53–60. [DOI] [PubMed] [Google Scholar]
- 13.Joseph BK, Rhee SW, Hirenallur DK, Rusch NJ, Potassium channels in vascular smooth muscle: Structure, function and experimental intervention, in New Frontiers in Smooth Muscle Biology and Physiology, Savineau J-P, Editor. 2007. p. 173–194. [Google Scholar]
- 14.Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol 2010; 588: 3277–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutman GA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 2005; 57: 473–508. [DOI] [PubMed] [Google Scholar]
- 16.Baumann A, Grupe A, Ackermann A, Pongs O. Structure of the voltage-dependent potassium channel is highly conserved from Drosophila to vertebrate central nervous systems. Embo J 1988; 7: 2457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch AE, Varnum MD, North RA, Adelman JP. An amino acid mutation in a potassium channel that prevents inhibition by protein kinase C. Science 1992; 255: 1705–7. [DOI] [PubMed] [Google Scholar]
- 18.Drain P, Dubin AE, Aldrich RW. Regulation of Shaker K+ channel inactivation gating by the cAMP-dependent protein kinase. Neuron 1994; 12: 1097–109. [DOI] [PubMed] [Google Scholar]
- 19.Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol 1996; 74: 439–47. [DOI] [PubMed] [Google Scholar]
- 20.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A 2007; 104: 20055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang XY, Morielli AD, Peralta EG. Molecular basis of cardiac potassium channel stimulation by protein kinase A. Proc Natl Acad Sci U S A 1994; 91: 624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winklhofer M, Matthias K, Seifert G, Stocker M, Sewing S, Herget T, Steinhauser C, Saaler-Reinhardt S. Analysis of phosphorylation-dependent modulation of Kv1.1 potassium channels. Neuropharmacology 2003; 44: 829–42. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP, Cole WC. Identification and Functional Characterization of Protein Kinase A-catalyzed Phosphorylation of Potassium Channel KV1.2 at Serine 449. J Biol Chem 2009; 284: 16562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol 1995; 268: H926–34. [DOI] [PubMed] [Google Scholar]
- 25.Connors EC, Ballif BA, Morielli AD. Homeostatic regulation of KV1.2 potassium channel trafficking by cyclic AMP. J Biol Chem 2008; 283: 3445–53. [DOI] [PubMed] [Google Scholar]
- 26.Cerda O and Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett 2010; 486: 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature 1995; 378: 85–8. [DOI] [PubMed] [Google Scholar]
- 28.Sheng M and Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 2001; 24: 1–29. [DOI] [PubMed] [Google Scholar]
- 29.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 1992; 9: 929–42. [DOI] [PubMed] [Google Scholar]
- 30.Kistner U, Wenzel BM, Veh RW, Cases-Langhoff C, Garner AM, Appeltauer U, Voss B, Gundelfinger ED, Garner CC. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J Biol Chem 1993; 268: 4580–3. [PubMed] [Google Scholar]
- 31.Anderson JM. Cell signalling: MAGUK magic. Curr Biol 1996; 6: 382–4. [DOI] [PubMed] [Google Scholar]
- 32.Hsueh YP and Sheng M. Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel KV1.4. J Biol Chem 1999; 274: 532–6. [DOI] [PubMed] [Google Scholar]
- 33.Christopherson KS, Sweeney NT, Craven SE, Kang R, El-Husseini Ael D, Bredt DS. Lipid- and protein-mediated multimerization of PSD-95: implications for receptor clustering and assembly of synaptic protein networks. J Cell Sci 2003; 116: 3213–9. [DOI] [PubMed] [Google Scholar]
- 34.Sheng M and Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science 2002; 298: 776–80. [DOI] [PubMed] [Google Scholar]
- 35.McGee AW and Bredt DS. Assembly and plasticity of the glutamatergic postsynaptic specialization. Curr Opin Neurobiol 2003; 13: 111–8. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery JM, Zamorano PL, Garner CC. MAGUKs in synapse assembly and function: an emerging view. Cell Mol Life Sci 2004; 61: 911–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim E and Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci 2004; 5: 771–81. [DOI] [PubMed] [Google Scholar]
- 38.Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M. Crystal structure of a Src-homology 3 (SH3) domain. Nature 1992; 359: 851–5. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy MB. Origin of PDZ (DHR, GLGF) domains. Trends Biochem Sci 1995; 20: 350. [DOI] [PubMed] [Google Scholar]
- 40.Woods DF and Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 1991; 66: 451–64. [DOI] [PubMed] [Google Scholar]
- 41.Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 1993; 121: 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponting CP. Evidence for PDZ domains in bacteria, yeast, and plants. Protein Sci 1997; 6: 464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lander ES, et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860–921. [DOI] [PubMed] [Google Scholar]
- 44.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell 1996; 85: 1067–76. [DOI] [PubMed] [Google Scholar]
- 45.Morais Cabral JH, Petosa C, Sutcliffe MJ, Raza S, Byron O, Poy F, Marfatia SM, Chishti AH, Liddington RC. Crystal structure of a PDZ domain. Nature 1996; 382: 649–52. [DOI] [PubMed] [Google Scholar]
- 46.Tochio H, Hung F, Li M, Bredt DS, Zhang M. Solution structure and backbone dynamics of the second PDZ domain of postsynaptic density-95. J Mol Biol 2000; 295: 225–37. [DOI] [PubMed] [Google Scholar]
- 47.Hall RA and Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 2002; 91: 672–80. [DOI] [PubMed] [Google Scholar]
- 48.Piserchio A, Pellegrini M, Mehta S, Blackman SM, Garcia EP, Marshall J, Mierke DF. The PDZ1 domain of SAP90. Characterization of structure and binding. J Biol Chem 2002; 277: 6967–73. [DOI] [PubMed] [Google Scholar]
- 49.Piserchio A, Spaller M, Mierke DF. Targeting the PDZ domains of molecular scaffolds of transmembrane ion channels. AAPS J 2006; 8: E396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 1995; 269: 1737–40. [DOI] [PubMed] [Google Scholar]
- 51.Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J Gen Physiol 1999; 113: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A 2006; 103: 19535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenman JE, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996; 84: 757–67. [DOI] [PubMed] [Google Scholar]
- 54.Joseph BK, Thakali KM, Pathan AR, Kang E, Rusch NJ, Rhee SW. Postsynaptic density-95 scaffolding of Shaker-type K(+) channels in smooth muscle cells regulates the diameter of cerebral arteries. J Physiol 2011; 589: 5143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, Fujita A, Horio Y, Kurachi Y. Cloning and functional expression of a novel cardiac two-pore background K+ channel (cTBAK-1). Circ Res 1998; 82: 513–8. [DOI] [PubMed] [Google Scholar]
- 56.Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem 2004; 279: 20257–66. [DOI] [PubMed] [Google Scholar]
- 57.Gavarini S, Becamel C, Altier C, Lory P, Poncet J, Wijnholds J, Bockaert J, Marin P. Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell 2006; 17: 4619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA. Beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem 2000; 275: 38659–66. [DOI] [PubMed] [Google Scholar]
- 59.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 2000; 27: 107–19. [DOI] [PubMed] [Google Scholar]
- 60.Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol 1998; 275: H448–59. [DOI] [PubMed] [Google Scholar]
- 61.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 2008; 102: e1–e11. [DOI] [PubMed] [Google Scholar]
- 62.Sheng M and Wyszynski M. Ion channel targeting in neurons. Bioessays 1997; 19: 847–53. [DOI] [PubMed] [Google Scholar]
- 63.Arnold DB and Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron 1999; 23: 149–57. [DOI] [PubMed] [Google Scholar]
- 64.Cayabyab FS, Khanna R, Jones OT, Schlichter LC. Suppression of the rat microglia KV1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur J Neurosci 2000; 12: 1949–60. [DOI] [PubMed] [Google Scholar]
- 65.Jugloff DG, Khanna R, Schlichter LC, Jones OT. Internalization of the KV1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem 2000; 275: 1357–64. [DOI] [PubMed] [Google Scholar]
- 66.Imamura F, Maeda S, Doi T, Fujiyoshi Y. Ligand binding of the second PDZ domain regulates clustering of PSD-95 with the KV1.4 potassium channel. J Biol Chem 2002; 277: 3640–6. [DOI] [PubMed] [Google Scholar]
- 67.Magidovich E, Orr I, Fass D, Abdu U, Yifrach O. Intrinsic disorder in the C-terminal domain of the Shaker voltage-activated K+ channel modulates its interaction with scaffold proteins. Proc Natl Acad Sci U S A 2007; 104: 13022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gramates LS and Budnik V. Assembly and maturation of the Drosophila larval neuromuscular junction. Int Rev Neurobiol 1999; 43: 93–117. [DOI] [PubMed] [Google Scholar]
- 69.Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K+ channel surface expression and clustering. J Cell Biol 2000; 148: 147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujita A and Kurachi Y. SAP family proteins. Biochem Biophys Res Commun 2000; 269: 1–6. [DOI] [PubMed] [Google Scholar]
- 71.El-Haou S, Balse E, Neyroud N, Dilanian G, Gavillet B, Abriel H, Coulombe A, Jeromin A, Hatem SN. Kv4 potassium channels form a tripartite complex with the anchoring protein SAP97 and CaMKII in cardiac myocytes. Circ Res 2009; 104: 758–69. [DOI] [PubMed] [Google Scholar]
- 72.Vaidyanathan R, Taffet SM, Vikstrom KL, Anumonwo JM. Regulation of cardiac inward rectifier potassium current (I(K1)) by synapse-associated protein-97. J Biol Chem 2010; 285: 28000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tajada S, Cidad P, Moreno-Dominguez A, Perez-Garcia MT, Lopez-Lopez JR. High blood pressure associates with the remodelling of inward rectifier K+ channels in mice mesenteric vascular smooth muscle cells. J Physiol 2012; 590: 6075–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Troncoso Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K(+) channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension 2012; 59: 657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magidovich E, Fleishman SJ, Yifrach O. Intrinsically disordered C-terminal segments of voltage-activated potassium channels: a possible fishing rod-like mechanism for channel binding to scaffold proteins. Bioinformatics 2006; 22: 1546–50. [DOI] [PubMed] [Google Scholar]
- 76.Zandany N, et al. Alternative splicing modulates Kv channel clustering through a molecular ball and chain mechanism. Nat Commun 2015; 6: 6488. [DOI] [PubMed] [Google Scholar]
- 77.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999; 284: 1845–8. [DOI] [PubMed] [Google Scholar]
- 78.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 2002; 298: 846–50. [DOI] [PubMed] [Google Scholar]
- 79.Bach A, et al. A high-affinity, dimeric inhibitor of PSD-95 bivalently interacts with PDZ1–2 and protects against ischemic brain damage. Proc Natl Acad Sci U S A 2012; 109: 3317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 2012; 483: 213–7. [DOI] [PubMed] [Google Scholar]
- 81.Sun HS, et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke 2008; 39: 2544–53. [DOI] [PubMed] [Google Scholar]
- 82.Moore CL, Nelson PL, Parelkar NK, Rusch NJ, Rhee SW. Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries. Circ Res 2014; 114: 1258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rusch NJ, Rhee SW. Loss of cerebrovascular Shaker-type K(+) channels: a shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol 2009; 297: H293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marzian S, et al. Side pockets provide the basis for a new mechanism of Kv channel-specific inhibition. Nat Chem Biol 2013; 9: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hansel W, Chandy KG. Kv1.3-blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol Pharmacol 2004; 65: 1364–74. [DOI] [PubMed] [Google Scholar]
- 86.Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc Natl Acad Sci U S A 2004; 101: 16374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.https://clinicaltrials.gov/ct2/show/NCT02930018. Accessed August 11, 2017.
- 88.Hall JE. Guyton and Hall Textbook of Medical Physiology Philadelphia, PA: Saunders; 2015. [Google Scholar]
- 89.Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of β-adrenergic receptor subtypes in blood vessels of knockout mice lacking β1- or β2-adrenergic receptors. Mol Pharmacol 2001; 60: 955–62. [DOI] [PubMed] [Google Scholar]
- 90.Alexander E 3rd, and Friedman AH. The identification of adrenergic receptors in human pial membranes. Neurosurgery 1990; 27: 52–9. [DOI] [PubMed] [Google Scholar]
- 91.Tsukahara T, Taniguchi T, Shimohama S, Fujiwara M, Handa H. Characterization of beta adrenergic receptors in human cerebral arteries and alteration of the receptors after subarachnoid hemorrhage. Stroke 1986; 17: 202–7. [DOI] [PubMed] [Google Scholar]
- 92.Mayhan WG. Responses of cerebral arterioles to activation of beta-adrenergic receptors during diabetes mellitus. Stroke 1994; 25: 141–6. [DOI] [PubMed] [Google Scholar]
- 93.Heistad DD and Marcus ML. Evidence that neural mechanisms do not have important effects on cerebral blood flow. Circ Res 1978; 42: 295–302. [DOI] [PubMed] [Google Scholar]
- 94.Lincoln J Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 1995; 68: 473–501. [DOI] [PubMed] [Google Scholar]
- 95.Muravchick S and Bergofsky EH. Adrenergic receptors and vascular resistance in cerebral circulation of the cat. J Appl Physiol 1976; 40: 797–804. [DOI] [PubMed] [Google Scholar]
- 96.Dora E and Kovach AG. Effect of the adrenergic beta receptor blocker propranolol on the dilatation of cerebrocortical vessels evoked by arterial hypoxia. Acta Physiol Hung 1984; 63: 35–41. [PubMed] [Google Scholar]
- 97.Kitazono T, Faraci FM, Heistad DD. Effect of norepinephrine on rat basilar artery in vivo. Am J Physiol 1993; 264: H178–82. [DOI] [PubMed] [Google Scholar]
- 98.Inoue S, Kawaguchi M, Kurehara K, Sakamoto T, Kitaguchi K, Furuya H. Effect of mild hypothermia on inodilator-induced vasodilation of pial arterioles in cats. J Trauma 2002; 53: 646–53. [DOI] [PubMed] [Google Scholar]
- 99.Winquist RJ and Bohr DF. Characterization of the rat basilar artery in vitro. Experientia 1982; 38: 1187–8. [DOI] [PubMed] [Google Scholar]
- 100.Winquist RJ, Webb RC, Bohr DF. Relaxation to transmural nerve stimulation and exogenously added norepinephrine in porcine cerebral vessels. A study utilizing cerebrovascular intrinsic tone. Circ Res 1982; 51: 769–76. [DOI] [PubMed] [Google Scholar]
- 101.Moore CL, McClenahan SJ, Hanvey HM, Jang DS, Nelson PL, Joseph BK, Rhee SW. Beta1-adrenergic receptor-mediated dilation of rat cerebral artery requires Shaker-type Kv1 channels on PSD95 scaffold. J Cereb Blood Flow Metab 2015; 35: 1537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall RA. Beta-adrenergic receptors and their interacting proteins. Semin Cell Dev Biol 2004; 15: 281–8. [DOI] [PubMed] [Google Scholar]
- 103.He J, Bellini M, Inuzuka H, Xu J, Xiong Y, Yang X, Castleberry AM, Hall RA. Proteomic analysis of beta1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem 2006; 281: 2820–7. [DOI] [PubMed] [Google Scholar]
- 104.Aitken M, Kleinrock M, Lyle J, Caskey L, Medicine use and shifting costs of healthcare. 2014, IMS Institute for Healthcare Informatics: Parsippany, NJ. [Google Scholar]
- 105.Dooley DJ, Bittiger H, Reymann NC. CGP 20712 A: a useful tool for quantitating beta 1- and beta 2-adrenoceptors. Eur J Pharmacol 1986; 130: 137–9. [DOI] [PubMed] [Google Scholar]
- 106.Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144: 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366: 1545–53. [DOI] [PubMed] [Google Scholar]
- 108.Devereaux PJ, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371: 1839–47. [DOI] [PubMed] [Google Scholar]
- 109.James PA, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–20. [DOI] [PubMed] [Google Scholar]
- 110.Chobanian AV, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–72. [DOI] [PubMed] [Google Scholar]
- 111.Dahlof B, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003. [DOI] [PubMed] [Google Scholar]
- 112.Povlsen GK, Longden TA, Bonev AD, Hill-Eubanks DC, Nelson MT. Uncoupling of neurovascular communication after transient global cerebral ischemia is caused by impaired parenchymal smooth muscle Kir channel function. J Cereb Blood Flow Metab 2016; 36: 1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]