SUMMARY
We have previously reported that co-transplantation of the kidney with vascularized donor thymus from α−1,3-galactosyltransferase gene knockout pigs with an anti-CD154 with rituximab-based regimen led to improved xenograft survival in baboons with donor-specific unresponsiveness. However, nephrotic syndrome emerged as a complication in which the glomeruli showed mild mesangial expansion with similarities to minimal change disease (MCD) in humans. Since MCD is associated with CD80 expression in glomeruli and elevated urinary excretion, we evaluated a potential role for CD80 in xenograft nephropathy. Study 1 confirmed high urinary CD80 excretion in nephrotic animals with renal xenografts showing CD80 expression in glomeruli. In Study 2, baboons receiving xenografts received CTLA4-Ig once a week from the second postoperative week or no CTLA4-Ig. The non-CTLA4-Ig group developed severe proteinuria with modest mesangial expansion with high urinary excretion of CD80 and documented CD80 expression in glomerular podocytes. All of the recipients in non-CTLA4-Ig groups had to be euthanized before POD 60. In contrast, CTLA4-Ig group showed a marked reduction in proteinuria and survived significantly longer, up to 193 days. These results demonstrate that anti-CD80 targeted therapy represents a promising strategy for reduction of proteinuria following renal xeno-transplantation with improved survival.
Keywords: CD80, kidney transplantation, large animal model, proteinuria, xenograft
Introduction
The recent use of α−1,3-galactosyltransferase knockout (GalT-KO) pigs as donors for xeno-transplantation has led to a significant reduction in hyperacute rejection and has provided a promising solution to the problem of organ shortage [1–4]. Beyond hyperacute rejection, a major challenge has been a robust T cell-mediated immune response against the xenograft. We have previously reported that a strong direct pathway of porcine antigen recognition by human T cells is present [5], suggesting a requirement for higher immunosuppression to inhibit pig-to-primate xeno-transplantation. In order to inhibit anti-pig T cell responses specifically, we developed a strategy of co-transplantation of porcine thymus as a composite vascularized graft with the donor kidney, a so called thymokidney (TK) graft [6]. Our initial attempt at co-transplantation of vascularized thymus and kidney from GalT-KO pigs to baboons resulted in stable serum creatinine for up to 83 days with no evidence of sensitization in vitro assays [3].
Although adequate control of xenogeneic cellular and humoral mediated rejection was achieved, a majority of the recipients developed nephrotic-range proteinuria as early as postoperative day (POD) 2, despite demonstrating minimal histologic changes in the glomeruli (i.e., primarily mild-to-moderate mesangial expansion) and evidence of normal renal function [7,8]. Indeed, the nephrotic syndrome in the baboons clinically and histologically resembled minimal change disease (MCD) [9,10], including the increased risk for bacterial infections and thrombosis [11]. Indeed, thrombosis and infection are frequent complications observed in xenotransplantation, and while multiple causes are likely, this also provides an additional rationale to identify the etiologic mechanism(s) driving the nephrotic response.
We have recently reported that rituximab, when administered in the perioperative period, protects sphingomyelin phosphodiesterase acid-like 3B (SMPDL-3b)/sphingomyelinase activity on porcine podocytes, which in turn delays the development of proteinuria [8]. However, since this effect lasts only 2–3 weeks, additional strategies are required to ensure a long-lasting resolution.
As mentioned, the nephrotic syndrome in xenografts resembles MCD, and a similar type of nephrotic syndrome can also occur following allogeneic transplantation of bone marrow, stem cells, or kidneys [12,13]. In many patients, it is associated with chronic graft-versus-host disease [13,14]. Recently, we reported that urinary levels of CD80 were extremely high in a patient who developed a minimal change-like nephrotic syndrome following allogeneic stem cell transplantation [14]. While CD80 is normally expressed by antigen-presenting cells, it has also been reported to be expressed by activated podocytes that may also have the capability of being antigen-presenting cells [15,16]. Moreover, we have found high levels of CD80 in the urine of children with MCD, and we also reported that CD80 is expressed in glomerular podocytes in renal biopsies [17,18]. These results led us to hypothesize that the nephrotic syndrome occurring in pig-to-baboon transplantation may involve a similar mechanism. Furthermore, CTLA4 is known to bind CD80 and inhibit activation of the CD80-expressing cell [19]. Thus, we also tested whether the administration of CTLA4-Ig might help reduce proteinuria in baboons with xenograft nephropathy.
Materials and methods
Study groups
Experiments were divided into two study groups. A total of ten baboon recipients of porcine GalT-KO kidneys were enrolled in these two studies (Table 1), of which nine received TK [3,7] and one received a kidney transplant with combined bone marrow transplantation. Donors were MGH-Gal-TKO pigs without additional gene modification [1,3].
Table 1.
Description of Study Groups and Treatment Regimens.
| Study group | Baboon ID | Xeno-organs | Regimen |
|---|---|---|---|
| Study 1: Pilot study using available saved urine and kidney biopsy samples from three recipients of GalT-KO kidney | |||
| Pilot study | B289 | Thymokidney | Anti-CD154 mAb-based regimen |
| B314 | Thymokidney | Anti-CD154 mAb/rituximab-based regimen | |
| B324 | Kidney + BM | ||
| Study 2: Determine the effects of multiple doses of CTLA4-Ig treatment on the development of proteinuria following GalT-KO thymokidney transplantation in current (2013–2016) cases | |||
| CTLA-4Ig group (2015–2016) | B393 | Thymokidney | Weekly doses of CTLA-4Ig + anti-CD154 mAb/rituximab-based |
| B394 | regimen | ||
| 14P5 | |||
| Comp: non-CTLA4-Ig group (2013–2014) | B341 | Thymokidney | Anti-CD154 mAb/rituximab-based regimen |
| B358 | |||
| B363 | |||
| B366 | |||
Study 1
In order to test our hypothesis that xenograft nephropathy is similar to MCD, we first determined whether CD80 excretion increased in the urine after GalT-KO kidney transplantation in baboons. We used saved urine and kidney biopsy samples from three recipients of GalT-KO kidneys that had been transplanted previously and had died within 30 days because of either ischemic heart failure or baboon CMV (bCMV) (details in Results). All had low total protein in their serum (<4.5 g/dl) despite albumin supplementation intravenously, because of massive proteinuria. The three animals (B289, B314, and B324) received slightly different regimens, with B289 and B314 receiving composite TKs [3,7] and B324 receiving a kidney with combined bone marrow transplantation [20]. All received treatments that included anti-thymocyte globulin (ATG, 20 mg/kg on day −2 and −10 mg/kg on day 1) and rituximab preoperatively in order to deplete T and B cells transiently during the induction period, followed by anti-CD154 mAb (20 mg/kg on day 2, 5, and 7, then 15 mg/kg, 3 times/week) and MMF (continuous infusion of 110 mg/kg/day) (anti-CD154 Ab regimen) [7]. In addition, B314 and B324 received rituximab (10 mg/kg) at day 0 in order to prevent development of proteinuria (anti-CD154 mAb/rituximab-based regimen) [8].
Study 2
Since results in Study 1 suggested upregulation of CD80 on podocytes in the short-term follow-up, we next studied the effects of multiple doses of CTLA4-Ig treatment on the development of proteinuria in the porcine TK Tx model. Seven animals received TKs in this study. Four baboon recipients of TKs (B341, B358, B363, B366) received the CD154 mAb/rituximab-based regimen [8] (non-CTLA4-Ig group). Three baboons (B393, B394, 14P5) received a weekly belatacept therapy at 10 mg/kg of recipient body weight that was started 7–9 days after TK Tx, in addition to the CD154 mAb/rituximab-based regimen (CTLA4-Ig group). Additionally, since recent reports suggest rapamycin may have anti-viral effects [21], we converted MMF into rapamycin in the maintenance period to avoid bCMV complications. We tapered MMF with reduction of the dose by 10 mg/kg every week and started rapamycin at 0.1 mg/kg from the fifth postoperative week (B393) or the fourth postoperative week (B394 and 14P5). The target levels of rapamycin were 10 ng/ml.
Pig-to-baboon renal transplantation
MGH GalT-KO pigs [1] were used as kidney donors, and baboons (Papio hamadryas, Manheim Foundation, Homestead, FL, USA) were used as recipients. Ten xenogeneic kidney transplantations were performed. Both native baboon kidneys were removed at the time of xenogeneic kidney transplantation. Graft function was assessed by serum creatinine levels. Animals were divided into two studies (see above). All procedures were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital and Columbia University.
Analysis of CD80
Serum sample preparation and storage for CD80 analysis
Baboon serum and urine samples were stored at −80 °C until analysis. Samples were first thawed to 37 °C in a water bath, vortexed, and then centrifuged at room temperature at 1000 g for 2 min prior to use.
Urine analysis
Urine samples were analyzed using an autoanalyzer (Alfa Wassermann, West Caldwell, NJ, USA) in which urinary creatinine was measured using the alkaline picrate (Jaffe Reaction) method (reportable range of 0.2–25.0 mg/dl) and albumin was analyzed by the dye-binding BCG method (Alfa Wasserman, range of 0.1–7.0 g/dl). For samples in which albumin concentration was below 0.1 g/dl, urine was measured using a more sensitive microalbumin kit (Alfa Wasserman, range 0.1–30 mg/dl). For Study 2, we used a urine dipstick (Multistix, Siemens AG, Munich, Germany) that qualitatively measures urinary albumin concentration with the following range (1+; 30 mg/dl, 2+; 100 mg/dl, 3+; 500 mg/dl, 4+; >500 mg/dl).
Western blot analysis for CD80
The appropriate volume of each urine sample was added to the 5× loading buffer based on a total creatinine value of 720 μg/lane. Of note, we loaded lanes based on urine creatinine to account for urine volume changes as it would not be reasonable to load based on urine protein as the proteinuria is variable among samples, and we were interested in the amount of CD80 that is being shed into the urine. Samples were placed in boiling water for 5 min to thermally denature the sample and loaded in a 4–20% polyacrylamide gel (Bio-rad, Criterion, 18-well, #345–0028). Electrophoresis was carried out in a Tris-glycine-SDS buffer system. Gels were electroblotted to a PVDF transfer membrane (Millipore Immobilon-Psq, ISEQ00010) for 2 h at 250 mA, blocked in 5% milk (Biorad, Hercules, CA, USA, #170–6404) in TTBS, and incubated with primary antibody overnight at 4 °C. Primary antibody was a mouse monoclonal anti-CD80 antibody from Lifespan Biosciences with known reactivity with porcine CD80 (Seattle, WA, USA), with an anti-mouse HRP-linked secondary antibody (Cell Signaling, Danvers, MA, USA). After washing, the membrane was developed using Immun-Star HRP reagent (Bio-rad). Normal human kidney lysate (Biochain, Newark, CA, USA, 50 μg/lane) was loaded in each Western blot for normalizing CD80 images and densitometry across gels. We also included standard MW ladder to assess the molecular weight of CD80. Images were scanned and analyzed using an Image Station 440CF with 1D IMAGE Software (Kodak Digital Science, Rochester, NY, USA).
Immunohistochemistry
Snap frozen fresh renal tissues in liquid nitrogen were embedded in OCT Compound and stored at −80 °C. Immunostaining of CD80 in glomeruli was performed using antihuman monoclonal CD80 mouse antibody (2A2; Life Science Bio, Seattle, WA, USA) that is predicted to react with porcine CD80 or with goat antihuman polyclonal CD80 antibody (AF-140; R&D Systems, Minneapolis, MN, USA) with appropriate secondary antibodies. For double immunostaining to evaluate the podocyte, we used goat antihuman polyclonal CD80 antibody with a mouse anti-pig synaptopodin mouse antibody (G1D4; Gene Tex, Irvine, CA, USA), whereas for double staining of CD80 and CD31, because of cross-reactivity, we used antihuman monoclonal CD80 mouse antibody (2A2; Life Science Bio) with anti-pig polyclonal CD31 rabbit antibody (Abcam, Cambridge, MA, USA). Sections were incubated in normal donkey serum blocking solution and then incubated for 1 h at room temperature using both primary antibodies and then rinsed twice with PBS-Tween 20 for 3 min each time. Antigen–antibody complexes were visualized with donkey anti-goat IgG conjugated with red fluorescent 594 dye secondary antibodies (Alexa Fluor, #A-11058; Invitrogen, Carlsbad, CA, USA) or anti-mouse IgG conjugated with green secondary antibodies (fluorescein, FI-2001; Vector, Burlingame, CA, USA). Sections were incubated with the secondary antibody for 30 min at room temperature and rinsed with PBS-Tween three times for 2 min. A coverslip with anti-fade fluorescent mounting medium containing DAPI was placed and sealed with nail polish. Slides were examined by immunofluorescence microscopy using a Nikon Eclipse TE2000-S with a 20X Pan Fluor objective. All images were acquired at 500 ms exposure and 410–1800 image scaling. Frozen tissue sections were stained by direct immunofluorescence using fluorescein isothiocyanate (FITC)-conjugated rabbit polyclonal antibody to human IgG and IgM (Dako, Carpinteria, CA, USA). All figures have been enhanced by increasing the brightness and contrast by 40%. Intensity of CD80 expression was quantified using IMAGEJ software [22]. Differences between two groups were analyzed using t test.
Upregulation of CD80 expression and effect of CTLA4Ig on CD80 expression on porcine PBMC and podocytes
Peripheral blood mononuclear cells (PBMC) from MGH-Gal KO pigs were isolated by the Ficoll method. Pig podocyte cell lines were established utilizing previously reported methods [8]. 1 × 105 cells were cultured with or without LPS (5 μg/ml). For the analysis of the effect of CTLA4-Ig, PBMCs or podocytes were cultured with CTLA4-Ig for 3 h. After incubation with CTLA4-Ig, LPS was added in culture medium. CD80 expression was evaluated 24 h after incubation by flow cytometry. Anti-mouse CD80 antibody or IgG isotype controls (BioLegend, San Diego, CA, USA) were used.
Assessments of anti-non-Gal IgM and IgG antibodies
Anti-non-Gal IgM and IgG serum antibodies were evaluated using GalT-KO PBMCs and detected by indirect FACS using Becton-Dickinson FACScan (Sunnyvale, CA, USA) and FITC-conjugated goat antihuman IgM or IgG antibody (Invitrogen). Data were expressed as median fluorescence intensity.
Baboon CMV in sera
qPCR was run using the ViiA7 by Applied Biosytems (Thermo Fisher Scientific Inc, Waltham, MA, USA). Standards ranging from 100 to 106 were used for absolute quantification with a quantitative detection limit of 4.5 copies per reaction. Data were analyzed using Quant Studio TM Real-Time PCR software v 1.3 (Thermo Fisher Scientific Inc.). Results are the average of three experiments.
Statistics
All blots were run at least three times. Linear regression was used to determine correlation of urinary albumin with CD80 densitometry, with a P < 0.05 considered to be significant.
Results
Study 1: urinary excretion of CD80 increases with development of proteinuria following pig-to-baboon xeno-kidney transplantation in baboons
As a pilot study, we first determined whether excretion of CD80 in the urine increased following pig-to-baboon xeno-kidney transplantation using saved urine and kidney biopsy samples from three recipients of GalT-KO kidneys (Table 1). These animals died from either ischemic heart failure (B289) on postoperative day (POD) 21, or bCMV (B314 and B324) on POD27 (B314) and POD19 (B324), respectively. All three animals had low total protein in their serum (<4.5 g/dl) even with intravenous albumin supplementation subsequent to the development of massive proteinuria. Using samples of urine saved during this period and available biopsies, we examined excretion of CD80 in the urine retrospectively following pig-to-baboon xeno-kidney transplantation as well as characteristics of CD80 excretion.
Excretion of CD80 in the urine following pig-to-baboon xeno-kidney transplantation
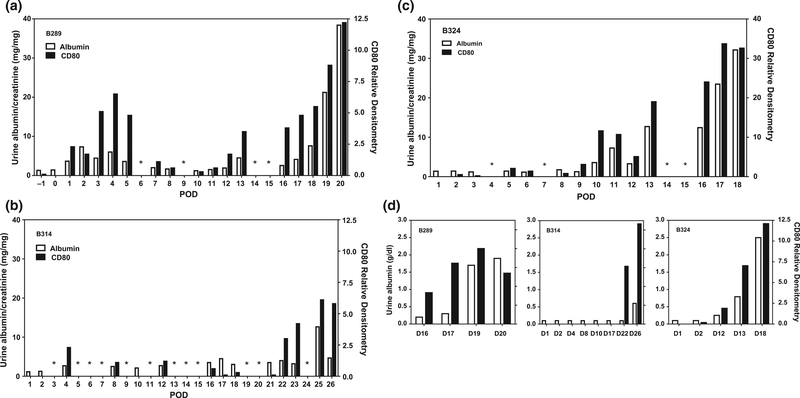
B289 received a TK with the basic regimen and died 21 days after transplant with pleural effusions and ascites. At necropsy, there were ischemic changes in the heart (left and right ventricle). The TK graft appeared grossly normal, and the serum creatinine was 0.8 mg/dl at the time of sacrifice. Urinary CD80 increased transiently around day 3, peaking at day 4. A second rise began around day 12 in association with a stepwise increase in proteinuria (Fig. 1a). Urinary albumin excretion began to rise markedly on POD19.
Figure 1.
Time course of urinary CD80 excretion relative to albuminuria in three baboons with porcine xenografts. Shown is the time course in postoperative days for baboon 289 (a), baboon 314 (b), and baboon 324 (c). A narrowed time course in these baboons shows urinary CD80 expression tended to increase in serial samples prior to increases in urine albumin excretion (d). Dark bars represent CD80 excretion (y bar on right) while urine albumin excretion is shown as open bars (y bar for albuminuria on left). *No sample was available.
Both B314 and B324 baboons received anti-CD154 mAb/rituximab-based regimens [8] and exhibited delayed development of proteinuria. B314 received a TK and survived 27 days, with a serum creatinine at death of 0.9 mg/dl. The baboon died of hemorrhagic pneumonia associated with bCMV infection. Urinary albumin and CD80 excretion remained nearly absent until POD22, which was attributed to day 0 rituximab effect [8]. However, CD80 excretion began to rise at POD22, followed by an increase in urinary albumin excretion at POD25 (Fig. 1b). B324 was euthanized on POD19 with a serum creatinine of 1.0 mg/dl because of bCMV. Urinary CD80 excretion began to increase on POD 10, followed by a slight reduction on POD12, only to increase markedly on POD13 (Fig. 1c). Albuminuria followed with the first substantial rise on POD13. Proteinuria increased severely after POD16.
Characteristics of CD80 excretion
Detailed analyses show that urinary CD80 excretion rose 1–3 days prior to the rise in urinary albumin excretion (Fig. 1d), with the exception being the first-week posttransplant in B289 (Fig. 1a). In these baboons, there was a direct correlation between urinary CD80 excretion and the level of albuminuria (r2 = 0.62, P < 0.01, data from 31 samples). The CD80 in the urine had a molecular weight of 53 KD, consistent with the cell membrane form.
Study 2: multiple doses of CTLA4-Ig markedly inhibited development of proteinuria during the maintenance period
The observation in Study 1 that urinary CD80 excretion was increased alludes to the possible protective effects of administering CTLA-4 Ig, since CTLA-4 can bind CD80 and reduce activation of CD80-expressing cells [19]. Therefore, we next studied the effects of multiple doses of CTLA4-Ig treatment on the development of proteinuria in the porcine TK Tx model with the anti-CD154 mAb/rituximab-based regimen [8] (Table 1). Three baboon recipients of TKs received weekly belatacept therapy starting 7–9 days after TK Tx in addition to the CD154 mAb/rituximab-based regimen [8] (CTLA4-Ig group). We compared results of the CTLA4-Ig-treated group with four baboon recipients of TKs with the CD154 mAb-/rituximabbased regimen that survived >30 days (non-CTLA4-Ig group) without the postoperative complications observed in the earlier animals (i.e., those reported in Study 1, above).
Clinical course of baboon recipients of porcine TK
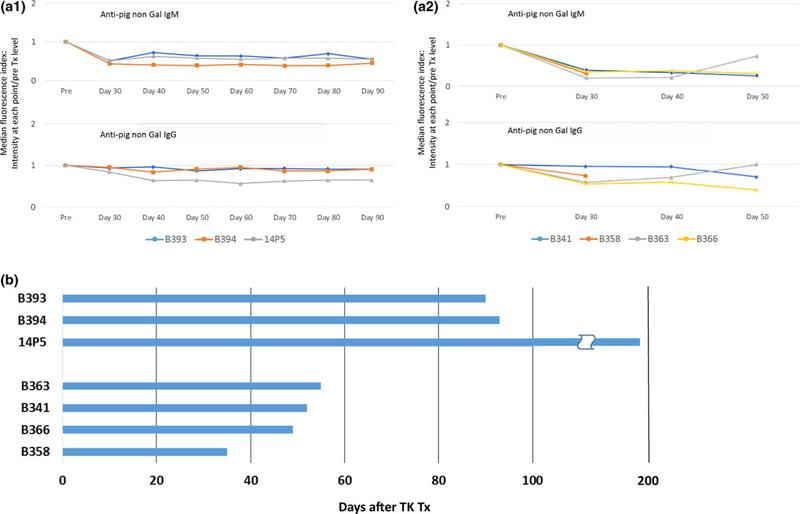
We previously reported that co-transplantation of vascularized thymus as a part of kidney grafts from the donors (TKs) induces transplant tolerance in allogeneic kidneys [6] with an in vitro donor-specific unresponsiveness in GalTKO pig-to-baboon models when an anti-CD154 mAb-based regimen was used [7]. Similar to these previous studies, none of the animals in the CTLA4-Ig group and non-CTLA4-Ig groups developed anti-non-Gal elicited antibodies following TK Tx (Fig. 2a-1 and a-2).
Figure 2.
(a) Anti-pig non-Gal antibodies assessed by FCM. Line graphs show median fluorescence index that was calculated by intensity at each point postoperative day (30, 40, 50, 60, 70, 80, and 90)/pre-Tx level. All baboons had preformed anti-non-Gal Nabs, IgM, and IgG, prior to thymokidney (TK) Tx that were adsorbed by TKs after revascularization. Anti-pig antibody levels never exceeded above pre-Tx levels (index less than 1), indicating no elicited anti-pig antibodies, IgM and IgG, developed after TK Tx in both CTLA4-Ig-treated (a-1) and CTLA4-Ig-nontreated (a-2) groups. (b) Baboon recipient survival days after porcine TK Tx in CTLA4-Ig group (B393, B394, 14P5) and non-CTLA4-IG group (B363, B341, B366, B358).
Although TK grafts did not show signs of immunological rejection, these animals were euthanized for either catheter trouble or graft compartment syndrome [23] in the CTLA4-Ig group, and for pleural effusion or bCMV in the non-CTLA4-Ig group. All three animals with CTLA4-Ig treatment survived for longer than 3 months after TK Tx (B393: 90 days; B394: 93 days; 14P5: 193 days. Mean survival days = 125) (Fig 2b; Table 2). No animals developed lethal bCMV infection. Briefly, B394 had stable creatinine (<1.5 mg/dl) except for one episode in which a transient rise in serum creatinine occurred (peaking at 4 mg/dl) because of ureteral stent occlusion which was surgically repaired at POD 51. At POD 93, however, the animal was euthanized because of extensive thrombus in the left common femoral artery and vein, which extended down the leg and was associated with the intravenous and arterial catheters, possibly because of anti-CD154 mAb thrombotic side effects. The other two animals lost graft function at later time points because of graft compartment syndrome secondary to growth of porcine grafts in small baboons [23]. In contrast, the four animals in the non-CTLA4-Ig group did not develop early postoperative complications, but were euthanized before POD60 for other reasons as follows. Baboon survival of non-CTLA4-Ig group was 55 days (B363), 52 days (B341), 49 days (B366), and 35 days (B358), respectively (Table 2). B363 and B341 were euthanized because of pleural effusion with low total protein and albumin levels in the serum despite albumin supplementation. B358 and B366 also had low total protein in the serum and massive proteinuria and were eventually euthanized because of pneumonia with bCMV+ in lung specimens (Table 2). Mean survival of these non-CTLA4-Ig-treated animals was markedly shorter than that observed in the CTLA4-Ig group (average SE: 125.3 ± 33.8 with CTLA4-Ig vs. 47.75 ± 4.42 without CTLA4-Ig. P = 0.0436).
Table 2.
Comparison of CTLA4-lg Treated Group and Control Group.
| Group | Baboon ID | Xeno-organs | Regimen | Survival (days) |
Complication/cause of euthanasia |
|---|---|---|---|---|---|
| CTLA4-lg group (2015–2016) | B393 | Thymokidney | Weekly doses of CTLA-4lg from POD 7–9 + anti-CD154mAb/rituximab-based regimen | 90 | Graft compartment syndrome |
| B394 | 93 | Extensive thrombosis | |||
| 14P5 | 193 | Graft compartment syndrome | |||
| Non-CTLA4-lg group (2013–2014) | B341 | Thymokidney | AnthCD154mAb/rituximab-based regimen | 52 | Pleural effusion |
| B358 | 35 | Pneumonia, baboon CMV+ in lung (27 576 copies per 300 ng input DNA) | |||
| B363 | 55 | Pleural effusion | |||
| B366 | 49 | Pneumonia, baboon CMV+ in lung (1255 copies per 300 ng input DNA) |
Proteinuria was markedly lower in CTLA4-treated animals
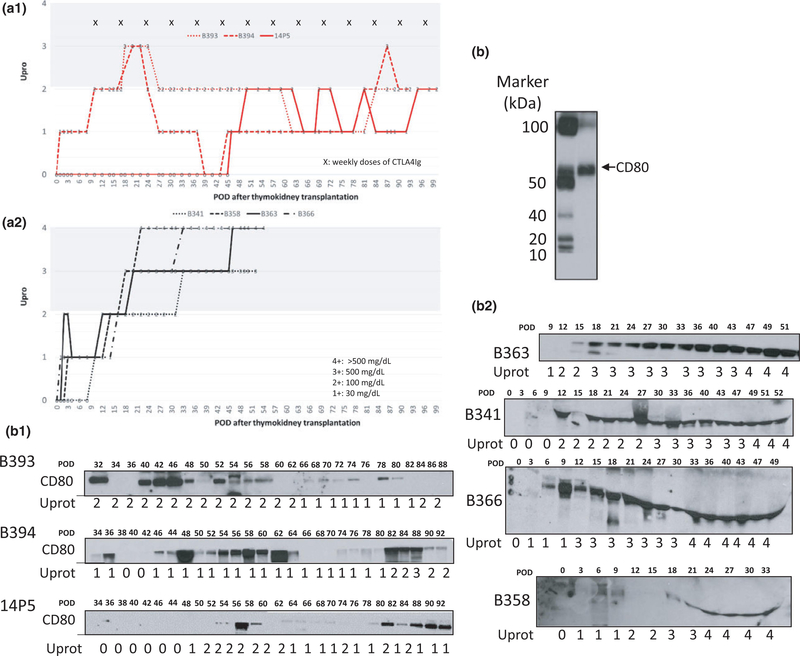
Proteinuria was lower in the CTLA4-Ig group compared to non-CTLA4-Ig group (Fig. 3a-1 vs. a-2). Figure 3a shows individual levels of proteinuria assessed by dipstick during the clinical course after transplantation. Urine proteinuria was assessed three times a week. Proteinuria is described based on the following scale: 1+; 30 mg/dl, 2+; 100 mg/dl, 3+; 500 mg/dl, 4+; >500 mg/dl. Two of the three baboons in the CTLA4 group developed proteinuria 3+ in the short term in the second or third postoperative week (POW). However, after two to three doses of CTLA administration, all three baboons in the CTLA4-Ig group had less than 2+ proteinuria except at one time point in B394 (Fig. 3a). 14P5 maintained TK function up to POD193 with minimal proteinuria (always <2+) that persisted after POD 100. In contrast, baboons in the non-CTLA4-Ig group had levels of proteinuria exceeding 2+ from the third POW and all of the animals developed proteinuria 3+ or greater, which remained persistent after the fourth POW and caused significant pleural effusion and infection (Fig. 3a-2, Table 2).
Figure 3.
(a) Proteinuria assessed by dipstick in CTLA4-Ig group (a-1) and non-CTLA4-Ig group (a-2). Urine protein excretion was assessed three times a week. Proteinuria is described based on the following scale: 1+; 30 mg/dl, 2+; 100 mg/dl, 3+; 500 mg/dl, 4+; >500 mg/dl. (b) Western blot analysis of CD80 with molecular weight markers reveals CD80 to be 53 KDa. Also shown is the urinary excretion of CD80 in three recipients of TK that were treated with multiple doses of CTLA4-Ig (b-1: B394, B393, 14P5, b-1) and four recipients of TK without CTLA4-Ig therapy (b-2: B363, B341, B366, B358, b-2). Non-CTLA4-Ig-treated recipients consistently showed high urinary CD80 excretion following transplantation and greater proteinuria (b-2). In contrast, long-term survivors of TK with CTLA4Ig had many days with absent or minimal CD80 secretion and less proteinuria (b-1) although transient CD80 excretion was seen when nonimmunological complications such as ureteral obstruction or catheter complications developed.
Characteristics of CD80 excretion
We next analyzed urine samples for CD80 excretion after POD 30 from those seven recipients. Figure 3b shows Western blot analysis of CD80 with a molecular weight of slightly more than 50 KDa based on molecular weight markers (Fig. 3b) with urinary excretion of CD80 in three recipients of TK that were treated with multiple doses of CTLA4-Ig (B-1: B394, B393, 14P5, Fig. 3b-1) and four recipients of TK without CTLA4-Ig therapy (B-2: B363, B341, B366, B358, Fig. 3b-2). Non-CTLA4-Ig-treated recipients consistently showed high urinary CD80 excretion following transplantation in association with greater proteinuria (Fig. 3b-2). In contrast, long-term survivors of TK with CTLA4Ig had many days with absent or minimal CD80 excretion that correlated with lower proteinuria (Fig. 3b-1) although transient CD80 excretion was seen when nonimmunological complications such as ureteral obstruction or catheter complications developed.
Upregulation of CD80 expression in podocytes of porcine xenografts
Five kidney grafts were stained for CD80 (three nephrotic animals B358 from the second study, and B314 and B324 from the first pilot study) and two nonnephrotic animals that had received belatacept (CTLA4-Ig)). CD80 expression was observed in glomeruli that were nephrotic but in neither nonnephrotic kidney samples (Fig. 4a–c).
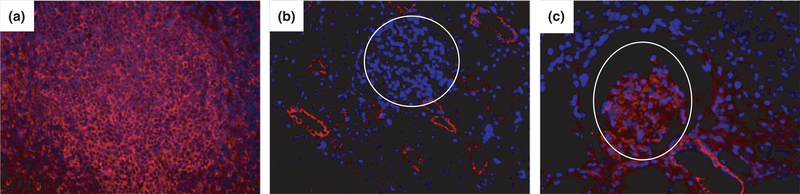
Figure 4.
CD80 Expression in a Nephrotic baboon. Baboon 314 developed severe nephrotic syndrome. CD80 was present in the glomeruli (c) but was negative in a normal porcine glomerulus (negative control, b), whereas it was positive in porcine spleen (a) [400×, CD80 in red, DAPI counterstain shows nuclei in blue].
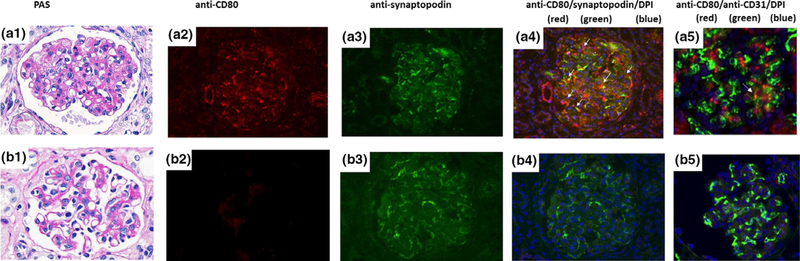
To identify the cell type expressing CD80, we performed double immunofluorescence staining of the biopsy of B358 in which the thymokidney graft was removed at POD 35 (serum creatinine 0.8 mg/dl). This animal had severe nephrotic syndrome with moderate mesangial expansion in glomeruli (Fig. 5a-1). CD80 expression was observed in glomeruli as well as the vascular pole (red in Fig. 5a-2). The intensity of CD80 expression in glomeruli was quantified using IMAGEJ software [22]. The expression of CD80 in non-CTLA4-Ig group was significantly higher than that of CTLA4-Ig group (22.3 × 1.9 vs. 90.3 × 3.1, P < 0.001). By utilizing an anti-pig synaptopodin antibody, which marks synaptopodin in podocytes (green in Fig. 5a-3), many of the CD80-expressing cells could be documented as podocytes (Fig. 5a1–4. Double-positive cells are indicated with white arrows). In contrast, the glomerulus appeared normal (Fig. 5b-1) with no upregulation of CD80 in glomerular cells in two baboons which did not develop nephrotic syndrome (Fig. 5b2–5. Figure 5b showed the PAS and immunofluorescence findings of the baboon 14P5, which had a serum creatinine of 0.8 mg/dl at the time of biopsy on POD 121).
Figure 5.
CD80 expression in glomeruli of Thymokidney Grafts. The excised thymokidney graft at POD35 (B358, serum creatinine 0.8 mg/dl) appeared histologically normal (PAS. a-1) but CD80 upregulation was observed (a-2. red). (a3) Showed an image with anti-pig synaptopodin Ab alone (a-3. green). Double staining with anti-pig synaptopodin Ab (green) showed many CD80-positive cells which were double stained for synaptopodin (white arrows in a-4). Double staining for CD80 and CD31 was also performed. Rare CD31+ capillary endothelial cells (green) express CD80 (red) (double-positive cells were indicated by white arrow in a-5) of the thymokidney. Blue staining was DAPI. In contrast, a biopsy performed at POD 121 from baboon 14P5 which was not nephrotic with normal renal function (serum creatinine 0.8 mg/dl) showed a normal-appearing glomerulus (PAS. b-1), with no upregulation of CD80 by both single immunofluorescence staining (b-2) or double immunofluorescence staining (anti-CD80/anti-pig synaptopodin Ab, Fig. 4b4; anti-CD80/anti-CD31 ab, b-5) [4009, CD80, red; synaptopodin or CD31, green; DAPI, blue].
Since not all CD80-positive cells were podocytes, we also performed double immunofluorescence staining of CD80 with CD31, which is an endothelial cell marker. Occasional CD80-positive cells were CD31 positive (Fig. 5a-5. CD31+ cells are shown in green and CD80+ cells are shown in red; double-positive cells are indicated with a white arrow).
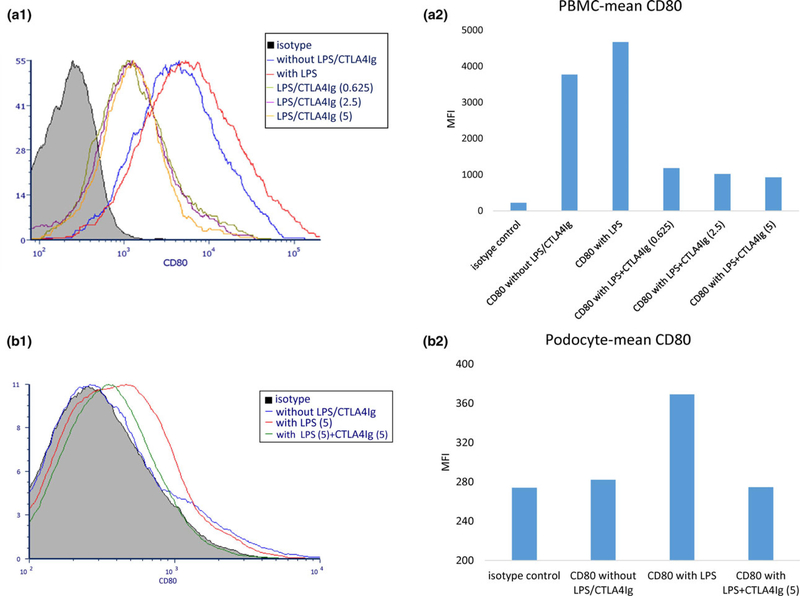
CTLA4-Ig-suppressed CD80 expression on porcine podocytes in vitro
Our in vivo study demonstrated CD80 expression was upregulated in glomerular cells, predominantly podocytes, and this was inhibited by CTLA4-Ig therapy. In order to further strengthen the role of CD80, we next assessed whether activated porcine podocytes upregulate CD80 expression in vitro and whether CTLA4 Ig inhibits this upregulation in vitro. We first titrated CTLA4 Ig to identify a concentration that inhibited CD80 expression in porcine PBMC activated by lipopolysaccharide (LPS). LPS has been previously shown to upregulate CD80 in cultured podocytes [16]. Figure 6a showed CD80 expression in PBMC. A histogram demonstrates resting porcine PBMC express CD80 on CD3-negative lymphoid cells as well as macrophages (Fig. 6a-1, blue line). This expression was upregulated by LPS (Fig. 6a-1, red line and Fig. 6a-2). Moreover, this effect was inhibited in a dose-dependent manner when CTLA4-Ig was added to the podocyte culture medium before the LPS stimulation (Fig. 6a-1,2). Based upon this data of PBMC and the number of cultured podocytes, we chose 5 μg/ml as the concentration of CTLA4-Ig for the podocyte assay. CD80 expression was not observed in resting podocytes (Fig. 6b-1, blue line and Fig. 6b-2). However, it was induced by LPS stimulation (Fig. 6b-1, red line and Fig. 6b-2) and this upregulation was completely inhibited by CTLA4-Ig (Fig. 6b-1, green line and Fig. 6b-2).
Figure 6.
CD80 expression on porcine peripheral blood mononuclear cell (PBMC) and podocytes. (a) Pig PBMCs were stimulated with lipopolysaccharide (LPS) (5 μg/ml) for 24 h. CTLA4-Ig (at 0.25–5 μg/ml) was added and incubated for 3 h before LPS stimulation. CD80 expression was evaluated with flow cytometry (a-1). The mean MFI value in each of the groups was shown in the bar graph (a-2). Pig PBMC expressed CD80 (red line in the histogram, and third bar from left in the bar graph). CTLA4 Ig markedly inhibited CD80 expression. (b) Although resting pig podocytes did not express CD80 (blue line in the histogram and the second bar in the bar graph), LPS (5 μg/ml) stimulation induced CD80 upregulation (b-1, 2, red in histogram, and third bar from left in the bar graph). Pretreatment of CTLA4-Ig completely suppressed CD80 expression (green line in the histogram, and the fourth bar in the bar graph).
Baboon CMV replication is not an initiator to develop posttransplant proteinuria
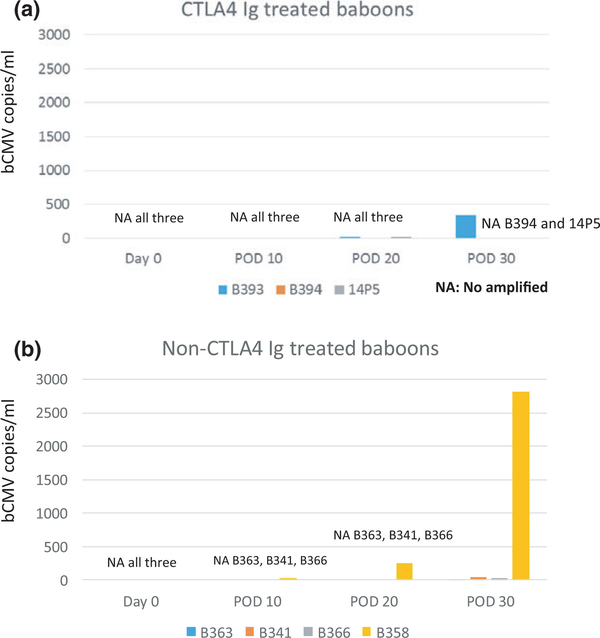
Since two of the non-CTLA4-Ig-treated baboons died with high copies of bCMV in lungs, and development of bCMV may influence the development of proteinuria [24,25], we tested the sera for evidence of bCMV infection in the early postoperative period in recipients of Study 2. Despite all animals developing proteinuria in the first 20 days in the non-CTLA4 Ig-treated group in Study 2, no bCMV replication was observed in three of four baboons by POD 20 and the remaining baboon had only 19.4 copies/ml of serum at POD 10 and 242.95 copies/ml of serum at POD 20 (Fig. 7b). These data suggest that the proteinuria preceded bCMV infection and therefore bCMV is unlikely an initiator of posttransplant proteinuria in this model.
Figure 7.
Replication of baboon CMV in sera of recipients in Study 2. (a) No bCMV replication was observed in all three baboons that were treated with CTLA4 Ig. (b) Three of four baboons in non-CTLA4-Ig-treated group did not develop any replication of bCMV by POD 20, and the remaining animal had only 19.4 copies/ml of serum at POD 10 and 242.95 copies/ml of serum at POD 20.
Discussion
Massive albuminuria with edema can lead to increased risk of infection and thrombosis, with subsequent increased morbidity and mortality [11]. Although co-transplantation of a vascularized thymus and kidney (TK) from GalT-KO pigs to baboons has resulted in stable serum creatinine and renal function for 3 months, the majority of these animals developed nephrotic syndrome [7]. We have demonstrated that baboon recipients of TKs developed proteinuria (2+) as early as POD 2 without signs of either cellular or humoral sensitization [8]. In this study, although neither CTLA4-Ig-treated animals nor non-CTLA-4-Ig-treated animals elicited anti-pig antibodies (Fig. 2a-1 and a-2), non-CTLA-4-Ig-treated animals developed massive proteinuria following TK Tx. Therefore, development of proteinuria in this TK model is not because of elicited anti-pig antibody-mediated rejection.
Development of proteinuria has been commonly reported following GalTKO pig-to-baboon xeno-trans-plantation, as well as GalT-KO/hCD39/hCD55/hCD59 and α−1,2-fucosyltransferase pig-to-cynomolgus xenotransplantation [7,8,26,27]. Recently, the Emory group has published data demonstrating >6 months survival with minimal proteinuria, no accompanying hypoalbuminemia, and low preformed natural antibodies in rhesus monkeys that received GalT-KO/hDAF kidneys although data on multi-time points were not presented [28]. In addition, Iwase et al. [29] reported that baboons which received multi-Tg (hCD46/hCD55/ EPCR/TFPI/hCD47) GalTKO kidneys treated with anti-IL6 receptor, anti-TNF-α, and anti-CD 40 mAb survived >8 months. However, Pintore et al. [26] reported that proteinuria does consistently occur in the hDAF transgenic Tg or multi-Tg GalTKO (including human decay accelerating factor, hDAF) pig-to-cynomolgus model. Our group has also observed the development of proteinuria following transplantation of hDAF/GalT-KO-to-cynomolgus monkey kidney xenografts using an anti-CD40L-based regimen without a vascularized thymic graft (Yamada et al., manuscript in preparation). Given these varying results, it is not yet conclusive if GalT-KO/hDAF donor kidneys are protected from developing proteinuria. In addition, recently published data in the pig-to-baboon model [29] have not yet addressed mechanistic studies to determine whether factors related to pharmacologic drugs or gene(s) lead to the development of proteinuria. Therefore, proteinuria remains a major issue and studies to determine the mechanism responsible for this phenomenon as well as the development of preventive strategies are crucial to the success of future xeno-transplantation studies.
Podocytes are the primary cells within the glomerulus that are responsible for preventing or developing proteinuria. We have previously studied the mechanism of postxeno-Tx proteinuria by developing a technique for the culture of pig podocytes and found that loss of sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b)/sphingomyelinase expression on porcine podocytes played an essential role in initiating proteinuria in vitro [8]. In addition, we demonstrated reduced SMPDL-3b expression in podocytes in porcine TK grafts in baboons with nephrotic syndrome and found that rituximab binding to SMPDL-3b on podocytes delayed development of proteinuria [8]. However, since this effect was limited, we expected that other mechanisms might also be involved in the induction of massive proteinuria and nephrotic syndrome. Glomeruli of TK xenografts appeared normal by light microscopy or showed mesangial expansion (Fig. 5) and thus have histologic similarities similar to MCD in humans [10]. Of potential relevance, our group has recently reported that treatment with CTLA4-Ig reduced proteinuria markedly in a child with frequently relapsing, steroid-dependent MCD, although the suppression of proteinuria was short-lived [30]. We hypothesized that if xenograft nephropathy also involves a disorder of CD80, then it may respond to CTLA4-Ig, which is known to bind to CD80, block T cell activation, and regulate CD80 on dendritic cells [19].
In MCD, there are reports of de novo expression of CD80 by podocytes in glomeruli, with increased excretion of CD80 in the urine [17,18,31,32]. We therefore evaluated this hypothesis in three baboons with porcine TKs and massive proteinuria. All baboons were found to excrete high amounts of CD80 in their urine, which increased immediately preceding the development of proteinuria. The CD80 had a molecular weight (MW) of 53 KD, consistent with the cell membrane form of CD80 as opposed to the circulating soluble CD80 (MW 23 KD). In a second set of studies, we showed that sequential administration of belatacept (CTLA4-Ig), which is known to bind CD80, was able to lower the proteinuria and urinary CD80 excretion in all three subsequent baboons that survived 90, 93, and 193 days respectively. Notably, we found that CD80 was expressed in the glomeruli of nephrotic but not non-nephrotic baboons, and by double immunostaining, we could show that many of the CD80-expressing cells were podocytes (as noted by double staining with the podocyte marker, synaptopodin). We also showed in cell culture studies that porcine podocytes could be induced to express CD80 by LPS and that treatment with CTLA4 Ig could suppress the CD80 expression.
Our regimen in the CTLA4-Ig group in Study 2 included rapamycin administered from the fourth or fifth postoperative week (POW) with a tapering of MMF 10 mg/kg every week to prevent development of bCMV. This modification appeared to be effective with bCMV disease being un-detectable in the CTL4-Ig group while two of four baboons in the non-CTLA4-Ig group developed bCMV disease. Importantly, the development of bCMV appears to have occurred after the proteinuria developed, consistent with CMV not being the initiator of the proteinuric response. These results also suggest that animals in the CTLA4-Ig group were not over-immunosuppressed by adding weekly CTLA4-Ig and rapamycin with tapering of MMF. In addition, animals receiving CTLA4-Ig had less proteinuria than the non-CTLA4-Ig group during the period in which rapamycin was not given. Clinical reports on administration of CTLA-4 Ig to subjects with focal segmental glomerulosclerosis (FSGS) following transplantation have indicated some successful responses [33], but also some negative results [30,34]. Recent reports have demonstrated that rapamycin can worsen proteinuria in clinical allotransplant cases and in a rodent model [35,36]. Taken together with our previous studies described above [17,18,37,38], the inhibitory effects on proteinuria is likely because of CTLA4-Ig therapy rather than rapamycin.
While our data suggest that the benefit of CTLA4-Ig therapy is likely because of suppression of the CD80 response in podocytes, it is also possible that the beneficial effects of CTLA4-Ig could involve effects on CD80 expression by other cell types, such as the glomerular endothelial cell or tubular cell, or possibly even general effects of CTLA4-Ig to suppress activation of T cells and local inflammation. However, the observation of a direct effect of CTLA4-Ig on CD80 expression in cultured podocytes would support a beneficial role of CTLA4-Ig to block podocyte activation. It should also be noted that while our data suggest a benefit of CTLA4-Ig on nephrotic syndrome in this xenograft model, the evidence that CTLA4-Ig is beneficial in FSGS in humans is mixed. However, this may relate to the amount of CD80 expressed in that condition, which appears to be less than that observed in MCD [30,33].
Although combination therapy with day 0 administration of rituximab and multiple doses of belatacept reduced proteinuria, complete prevention of proteinuria was not achieved. Thus, it is likely that other mechanisms are involved in the nephrotic response following xenogeneic kidney transplantation. In this regard, there are recent reports of incompatibility between porcine CD47 and its potential ligand, the baboon signal regulatory protein a (SIRP-a), which induces activation of macrophages and phagocytosis in some cases of xenogeneic transplantation [39,40]. Theoretically, immune activation of the porcine podocyte, which leads to expression of CD80, may potentially downregulate SIRP-a and SMPDL-3b. We are currently investigating the role of this SIRP-α-CD47 pathway and its interaction with CD80 and SMPDL-3b as a potential additional factor in xenograft proteinuria, using our hCD47 Tg GalTKO pigs [41] and a recently established technique for isolation of porcine podocytes [8].
In summary, we propose that the nephrotic syndrome following xenograft transplantation in baboons appears to involve CD80 upregulation in glomerular podocytes with increased urinary CD80 excretion and responds to CD80-targeted therapy (belatacept). The lesion has some similarities to minimal change disease in humans, although there are more mesangial expression and also some nonpodocyte expression of CD80 in glomeruli (which may represent endothelial cells and possibly mesangial cells and/or macrophages). Further studies on the role of CD80 in xenograft nephropathy appear warranted.
Acknowledgements
The authors gratefully acknowledge the generous gifts of thymoglobulin from Genzyme, of rituximab from Genentech, and of belatacept from Bristol-Myers Squibb. The authors also thank Dr. Hisashi Sahara for his critical review of the manuscript. We also appreciate Dr. Robert A. Wilkinson, MGH, for technical guidance in baboon CMV assays and Arsenoi Asfour for editorial assistance.
Funding
Supported by NIH 6PO1AI45897 and 2P01AI45897–11A1.
Footnotes
Conflicts of interest
The authors have declared no conflicts of interest.
REFERENCES
- 1.Kolber-Simonds D, Lai L, Watt SR, et al. Production ofalpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations.Proc Natl Acad of Sci USA 2004; 101: 7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferasedeficient pigs. Science 2003; 299: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 2005; 11: 32. [DOI] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005; 11: 29. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol 1995; 155: 5249. [PubMed] [Google Scholar]
- 6.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol 2000; 164: 3079. [DOI] [PubMed] [Google Scholar]
- 7.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant 2009; 9: 2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pigto-baboon xeno-kidney transplantation. J Am Soc Nephrol 2014; 25: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB. Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol 2012; 23: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 1981; 20: 765. [DOI] [PubMed] [Google Scholar]
- 11.Harris RC, Ismail N. Extrarenal complications of the nephrotic syndrome. Am J Kidney Dis 1994; 23: 477. [DOI] [PubMed] [Google Scholar]
- 12.Zafarmand AA, Baranowska-Daca E, Ly PD, et al. De novo minimal change disease associated with reversible posttransplant nephrotic syndrome. A report of five cases and review of literature. Clin Transplant 2002; 16: 350. [DOI] [PubMed] [Google Scholar]
- 13.Fofi C, Barberi S, Stoppacciaro A, Punzo G, Mene P. Focal segmental glomerulosclerosis as a complication of graft-versus-host disease. Nat Rev Nephrol 2009; 5: 236. [DOI] [PubMed] [Google Scholar]
- 14.Huskey J, Rivard C, Myint H, et al. Minimal change disease in graft versus host disease: a podocyte response to the graft? Clinl Nephrol 2013; 80: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwich A, Burkard M, Olke M, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol 2013; 24: 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest 2004; 113: 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garin EH, Diaz LN, Mu W, et al. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol 2009; 20: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin EH, Mu W, Arthur JM, et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int 2010; 78: 296. [DOI] [PubMed] [Google Scholar]
- 19.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322: 271. [DOI] [PubMed] [Google Scholar]
- 20.Tasaki M, Villani V, Shimizu A, et al. Role of bone marrow maturity, insulin-like growth factor 1 receptor, and forkhead box protein N1 in thymic involution and rejuvenation. Am J Transplant 2016; 16: 2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nashan B, Gaston R, Emery V, et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation 2012; 93: 1075. [DOI] [PubMed] [Google Scholar]
- 22.Rasband WS. ImageJ. Bethesda, MD: U. S. National Institutes of Health, 1996. [Google Scholar]
- 23.Tanabe T, Watanabe H, Shah JA, et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant 2017; 17: 1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgaki-Angelaki H, Lycopoulou L, Stergiou N, et al. Membranous nephritis associated with acquired cytomegalovirus infection in a 19-month-old baby. Pediatr Nephrol 2009; 24: 203. [DOI] [PubMed] [Google Scholar]
- 25.Hogan J, Fila M, Baudouin V, Peuchmaur M, Deschenes G, Niel O. Cytomegalovirus infection can mimic genetic nephrotic syndrome: a case report. BMC Nephrol 2015; 16: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pintore L, Paltrinieri S, Vadori M, et al. Clinicopathological findings in nonhuman primate recipients of porcine renal xenografts: quantitative and qualitative evaluation of proteinuria. Xenotransplantation 2013; 20: 449. [DOI] [PubMed] [Google Scholar]
- 27.Soin B, Smith KG, Zaidi A, et al. Physiological aspects of pig-to-primate renal xenotransplantation. Kidney Int 2001; 60: 1592. [DOI] [PubMed] [Google Scholar]
- 28.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015; 22: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 2017; 24: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garin EH, Reiser J, Cara-Fuentes G, et al. Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol 2014; 30: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling C, Liu X, Shen Y, et al. Urinary CD80 levels as a diagnostic biomarker of minimal change disease. Pediatr Nephrol 2015; 30: 309. [DOI] [PubMed] [Google Scholar]
- 32.Mishra OP, Kumar R, Narayan G, et al. Toll-like receptor 3 (TLR-3), TLR-4 and CD80 expression in peripheral blood mononuclear cells and urinary CD80 levels in children with idiopathic nephrotic syndrome. Pediatr Nephrol 2017; 32: 1355. [DOI] [PubMed] [Google Scholar]
- 33.Yu CC, Fornoni A, Weins A, et al. Abatacept in B7–1-positive proteinuric kidney disease. N Engl J Med 2013; 369: 2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alachkar N, Carter-Monroe N, Reiser J. Abatacept in B7–1-positive proteinuric kidney disease. N Engl J Med 2014; 370: 1263. [DOI] [PubMed] [Google Scholar]
- 35.Izzedine H, Brocheriou I, Frances C. Post-transplantation proteinuria and sirolimus. N Engl J Med 2005; 353: 2088. [DOI] [PubMed] [Google Scholar]
- 36.Ko HT, Yin JL, Wyburn K, et al. Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol Dial Transplant 2013; 28: 327. [DOI] [PubMed] [Google Scholar]
- 37.Shimada M, Ishimoto T, Lee PY, et al. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-kappaB-dependent pathway. Nephrol Dial Transplant 2012; 27: 81. [DOI] [PubMed] [Google Scholar]
- 38.Ishimoto T, Shimada M, Gabriela G, et al. Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice. Nephrol Dial Transplant 2013; 28: 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA 2007; 104: 5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, VerHalen J, Madariaga ML, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood 2007; 109: 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tena AA, Sachs DH, Mallard C, et al. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 2017; 101: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]