Abstract
We report a 40-year-old woman who presented with multiple osteolytic bone lesions and hypercalcemia, which are rarely caused by chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Although receiving intensive chemotherapy and allogeneic transplantation, the patient had a poor outcome with an overall survival of 2 years. To our knowledge, this presentation is extremely rare for B-chronic lymphocytic leukemia, and new treatment strategies may be needed for long-term control of the disease.
Keywords: chronic lymphocytic leukemia, small lymphocytic lymphoma, hypercalcemia, osteolysis
INTRODUCTION
CLL/SLL is the most common form of leukemia in Western countries, accounting for approximately 30% of all leukemia cases.1 However, its incidence in Japan is approximately 10-fold less than in Western countries.1 The clinical course of CLL/SLL is highly variable. Some patients survive for decades, whereas others develop aggressive disease and die within 2 to 3 years of diagnosis. In particular, transformation of CLL/SLL to Richter’s syndrome leads to a poor prognosis with a median survival of less than 1 year.2 Here, we report a patient with refractory CLL/SLL complicated by osteolytic bone lesions and hypercalcemia with no evidence of Richter’s syndrome, who presented with an aggressive clinical course and was resistant to intensive chemotherapy.
CASE REPORT
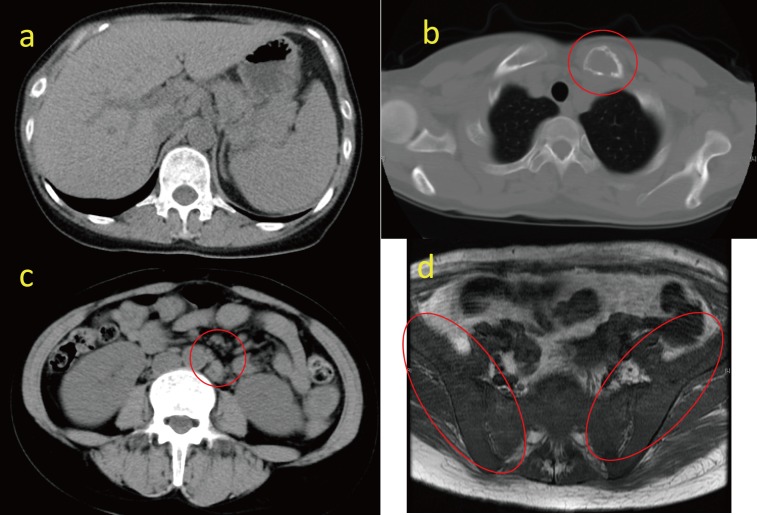
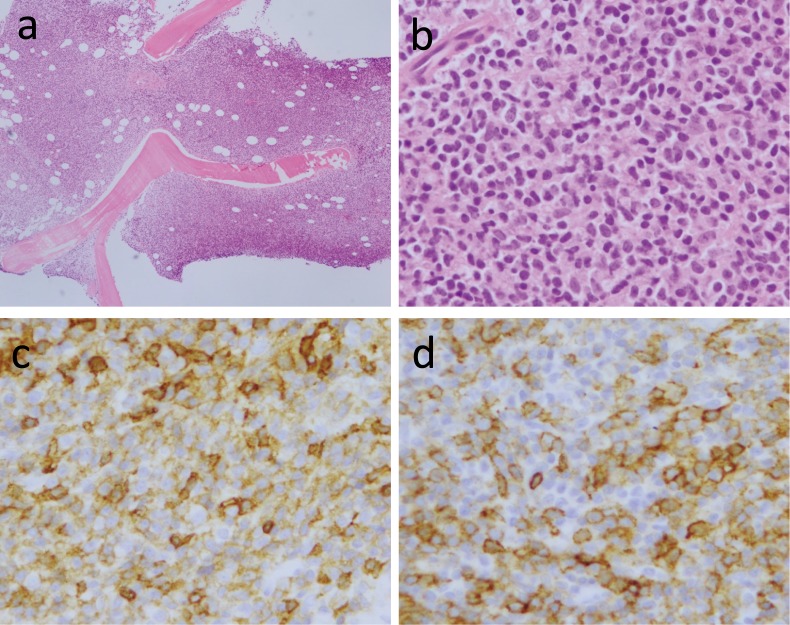
A 40-year-old woman was admitted to our hospital in April, 2014 for anorexia, vomiting and pain in the left clavicle area. Computed tomographic scanning revealed hepatosplenomegaly and slight lymphadenopathy in the paraaortic region, and a lytic bone lesion on the left clavicle (Figure 1a-c). The T1 weighted magnetic resonance imaging revealed diffuse low-signal regions on bilateral iliac bones, which suggested tumor infiltration in these regions (Figure 1d). Laboratory evaluation disclosed a white blood cell of 6.5×109/L with 65% lymphocytes, a hemoglobin level of 70 g/L and a platelet count of 124×109/L. A peripheral blood smear demonstrated lymphocytes with narrow cytoplasmic borders, and dense nuclei that were positive for CD5, CD23, CD19, CD22 and κ light chain immunoglobulin, dim for CD20, and negative for CD103 based on flow cytometric analysis (data not shown). The bone marrow examination was a ‘dry tap’ on aspiration, but biopsy revealed diffuse infiltration of abnormal lymphocytes (Figure 2a-b) with a similar morphology to the peripheral lymphocytes. These lymphocytes were strongly positive for CD5 and CD23 on immunohistochemistry (Figure 2c-d), were dim for CD20, and were negative for CD3, CD10, Cyclin D1 and SOX11 (data not shown). The serum total protein level was 77 g/L, the albumin level was 44 g/L, the creatinine level was 1.63 mg/dL, the blood urea nitrogen was 38 mg/dL and the serum calcium level was 15.9 mg/dL. The parathyroid hormone-related protein level (PTHrP; less than 1.0 pmol/L), intact prohormone concentration (PTH; 10 pg/mL) and calcitonin level (34 pg/mL) were within the normal range. IgG, IgA and IgM levels were also normal. The soluble interleukin 2 receptor concentration was 3140 U/mL (normal <512). The free light chain κ concentration was 41.1 mg/L (<19.4) and λ concentration was 20.1 mg/L (<26.3). Alkaline phosphatase and uric acid levels increased to 649 U/L (< 376) and 14.4 mg/dL (<7), respectively. The lactate dehydrogenase level was normal. Based on these data, the patient was diagnosed with CLL/SLL (stage Rai 3) complicated by hypercalcemia and osteolytic bone lesions.
Fig. 1.
Computed tomography showing hepatosplenomegaly (1a), a lytic bone lesion on the left clavicle (1b) and slight lymphadenopathy in the paraaortic region (1c).T1-weighted magnetic resonance image showing diffuse low-signal regions on bilateral iliac bones (1d).
Fig. 2.
Bone marrow biopsy examination revealed diffuse lymphocyte infiltration (2a and 2b), as stained by hematoxylin-eosin with original magnification at 4x and 40x, respectively. These lymphocytes were strongly positive for CD5 (2c) and CD23 (2d) on immunocytochemistry (original magnification 40x)
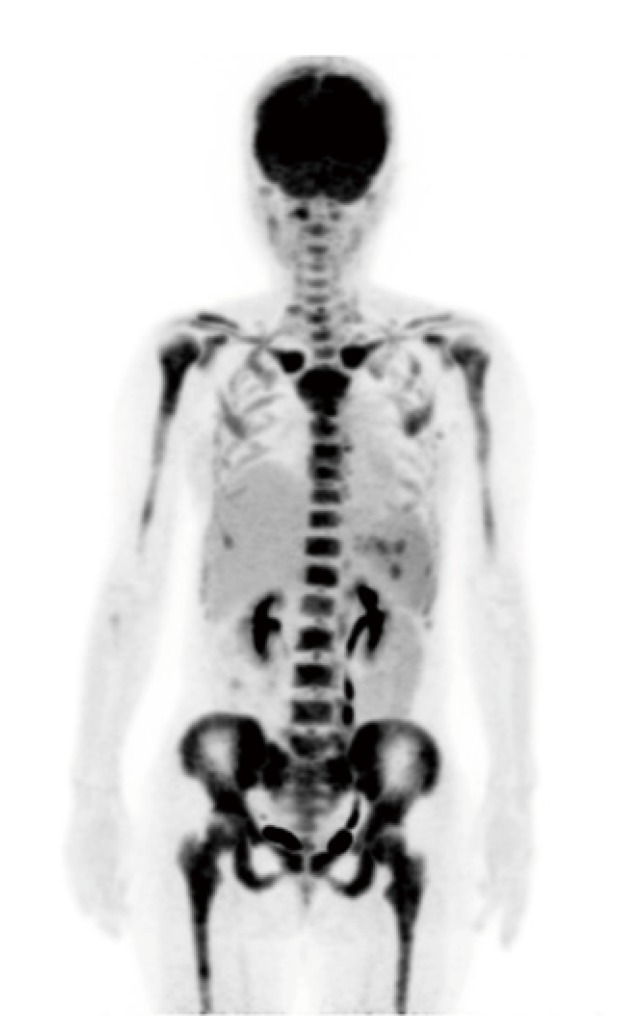
After treatment with calcitonin and hydration with normal saline to resolve hypercalcemia, the patient was started on a standard FCR (fludarabine, cyclophosphamide, rituximab) regimen. After two cycles of FCR, positron emission tomography-computed tomography (PET-CT) was performed, and the median maximum standardized uptake value was 8.48 in multiple bone lesions, which indicated no remission (Figure 3). Therefore, examination of bone marrow aspirate was repeated, demonstrating marked infiltration of lymphocytes (nucleated cells of 85%) with 65% co-positive CD5 and CD23 on flow cytometric immunostaining. G-banding analysis revealed complex chromosome abnormalities, including t(2;14)(p13;q32), del(9)(q?), (9) and (11)(q?), and add (12)(p11.2) and (14)(q11.2). In order to exclude the possibility of transformation to high-grade non-Hodgkin lymphoma, bone biopsy was performed, revealing bone infiltration by CLL/SLL without Richter transformation. The patient was readmitted for full-body skeletal pain and loss of appetite. Laboratory examinations demonstrated the recurrence of hypercalcemia, suggesting disease progression. The patient underwent salvage treatment with bendamustine and rituximab for three 28-day cycles. Partial remission was achieved based on the results of PET-CT and bone marrow examinations (data not shown). However, one month later, the patient developed difficulty walking due to hip pain, and the recurrence of CLL/SLL complicated by hypercalcemia was confirmed. The patient was transferred to a second hospital for allogeneic transplantation where she underwent 1 cycle of clofarabine, which was approved by the institutional review board of the hospital for treatment of CLL/SLL, plus cytarabine, followed by 1 cycle of vincristine, asparaginase and dexamethasone. Although the patient underwent these intensive therapies, she only achieved partial remission. After full-match allogeneic transplantation was performed, complete remission lasted for one year. The patient died of recurrence of the disease even though umbilical cord blood transplant was performed.
Fig. 3.
PET-CT showing multiple bone lesions with a median SUVmax value of 8.48
DISCUSSION
Hypercalcemia is a well-known complication of many neoplastic diseases, such as lung cancer, myeloma, Burkitt lymphoma and adult T cell lymphoma, but it rarely occurs in CLL/SLL.3 The most common form of hypercalcemia is mediated by PTHrP, often presenting in patients with bone metastases of solid tumors such as breast cancer and non-small cell lung cancer.4 Another important type is local osteolytic hypercalcemia, majorly exhibited in multiple myeloma; however, this type is extremely rare in B-CLL, with only 9 cases reported in English from 1980.5-12 In our case, there was no evidence of elevated serum PTH or PTHrP, corresponding to this type of osteolytic hypercalcemia. Unlike the type with elevated PTH or PTHrP, the mechanisms for hypercalcemia remain unclear, although several speculations have been made. In general, the predominant cause of this hypercalcemia is suggested to be bone resorption by activated osteoclasts in the vicinity of tumor cells metastatic to bone. Although not well-characterized, numerous cytokines and local regulatory factors have been implicated in this type of hypercalcemia.12 Koutroumpakis et al. suggested that TNF-α and IL-6 can increase bone resorption.12 Bone marrow infiltration by CLL/SLL can be divided into four common patterns: interstitial, nodular, mixed interstitial and nodular, and diffuse. The diffuse pattern was reported to be associated with a median survival of 28 months,2 similar to our case. Hypercalcemia is considered to be a negative prognostic factor in several hematological malignancies.12 We reviewed the previously reported cases of CLL/SLL complicated by osteolytic bone lesions and/or hypercalcemia, excluding those with elevated PTH or PTHrP levels. As shown in table 1, the patients in four of eight cases, including ours, died within 2 years, consistent with previous reports, demonstrating that hypercalcemia with osteolytic bone lesions is associated with a poorer outcome in CLL/SLL.7,9
Table 1. Overview of previously reported cases of CLL compromised by osteolytic bone lesions and/or hypercalcemia.
| Ref. | Age/Sex | Stage | WBC (109/L)/Lym | Hb (g/dL) |
Plt (109/L) |
M protein | Osteolytic bone lesions | Hypercalcemia | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 65/M | NA | 68.3/90% | 11 | 22 | Kappa | + | + | NA | NA |
| 6 | 73/F | NA | 2.8/78% | 9.3 | NA | NA | + | + | Chlor | 3 W Alive |
| 7 | 72/M | NA | 14.8/61% | 9.1 | 14.2 | NA | + | + | Chlor+PSL | 3 W Dead |
| 7 | 70/F | Binet C | NA/NA | NA | NA | NA | + | + | NA | 10 M Dead |
| 8 | 81/M | Binet A | NA/NA | NA | NA | Kappa | + | - | NA | NA |
| 9 | 60/M | NA | 3.52/57% | 12.7 | 6.2 | lambda | + | - | CHOP | 2 M Dead |
| 10 | 83/M | NA | NA/40X109/L | 10.1 | 21.3 | NA | + | + | CHOP | 6 M CR |
| 11 | 59/F | NA | NA/NA | NA | NA | NA | + | NA | FCR | Alive |
| 12 | 70/M | Rai III | 10.9/93.6% | 9.1 | 18.9 | Kappa | - | + | BR | Alive |
| Present | 40/F | Rai III | 6.5/65% | 7 | 12.4 | Kappa | + | + | FCR/BR | 2 Y Dead |
WBC, white blood cell; Lym, lymphocyte; Hb, hemoglobin; Plt, platelet; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; Chlor, chlorambucil; FCR, fludarabine, cyclophosphamide, rituximab; BR, bendamustine, rituximab; PSL, prednisolone; CR, complete remission; NA; not available
In CLL/SLL, t(2;14)(p13~16;q32) is a rare chromosomal aberration.13,14 Molecular genetic studies demonstrated that this translocation juxtaposed the BCL11A gene at 2p13~16 with the IgH gene at 14q32.13,14 CLL/SLL with t(2;14)(p13~16;q32) and BCL11A/IgH rearrangement was characterized by non-mutated IgVH genes, and atypical morphological features, such as a heterogeneous mixture of a small and large cells, plasmacytoid differentiation, increased prolymophocytes, or conspicuous nuclear indentations, suggesting an unfavorable prognosis for CLL/SLL with t(2;14)(p13~16;q32).13,14 In our case, a complex chromosomal aberration including t(2;14)(p13;q32) may have been associated with a distinct clinicopathological entity, different from CLL/SLL without these abnormal chromosomal phenomena. However, we were unable to confirm the relationship between the chromosome aberration of t(2;14)(p13~16;q32) and the hypercalcemia and osteolytic bone lesions in our patient.
In conclusion, we report a very rare case of CLL/SLL presenting with hypercalcemia and osteolytic bone lesions. Although the patient received intensive treatment and allogeneic transplantation, the patient had a poor outcome with an overall survival of 2 years. Further studies are needed to clarify the characteristics and pathogenic mechanisms underlying CLL/SLL-related osteolysis and hypercalcemia.
Footnotes
CONFLICT OF INTEREST: None of the authors have any conflicts of interests to declare.
REFERENCES
- 1.Ruchlemer R, Polliack A. Geography, ethnicity and “roots” in chronic lymphocytic leukemia. Leuk Lymphoma. 2013; 54: 1142-1150. 10.3109/10428194.2012.740670 [DOI] [PubMed] [Google Scholar]
- 2.Sagatys EM, Zhang L. Clinical and laboratory prognostic indicators in chronic lymphocytic leukemia. Cancer Contr. 2012; 19: 18-25. 10.1177/107327481201900103 [DOI] [PubMed] [Google Scholar]
- 3.Vaturi M, Prokocimer M, Sidi Y. Hypercalcemia in chronic lymphatic leukemia patients. Am J Hematol. 1996; 53: 245-247. [DOI] [PubMed] [Google Scholar]
- 4.Danks JA, Ebeling PR, Hayman J, et al. Parathyroid hormone-related protein: immunohistochemical localization in cancers and in normal skin. J Bone Miner Res. 1989; 4: 273-278. 10.1002/jbmr.5650040221 [DOI] [PubMed] [Google Scholar]
- 5.Redmond J, III, Stites DP, Beckstead JH, et al. Chronic lymphocytic leukemia with osteolytic bone lesions, hypercalcemia, and monoclonal protein. Am J Clin Pathol. 1983; 79: 616-620. 10.1093/ajcp/79.5.616 [DOI] [PubMed] [Google Scholar]
- 6.McMillan P, Mundy G, Mayer P. Hypercalcaemia and osteolytic bone lesions in chronic lymphocytic leukaemia. BMJ. 1980; 281: 1107. 10.1136/bmj.281.6248.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littlewood TJ, Lydon AP, Barton CJ. Hypercalcemia and osteolytic lesions associated with chronic lymphatic leukemia (CLL). J Clin Pathol. 1990; 43: 877. 10.1136/jcp.43.10.877-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield HM, Hunt R, Lee LK, Jowitt SN. B-cell chronic lymphocytic leukaemia with extensive lytic lesions. Eur J Haematol. 2006; 76: 356-357. 10.1111/j.0902-4441.0000.t01-1-EJH2515.x [DOI] [PubMed] [Google Scholar]
- 9.Mian M, Cerú S, Billio A, Rosanelli C, Cortelazzo S. Osteolytic bone lesions as a rare sign of progression of chronic lymphocytic leukemia without evidence of Richter syndrome. Leuk Lymphoma. 2012; 53: 993-995. 10.3109/10428194.2011.634044 [DOI] [PubMed] [Google Scholar]
- 10.Narayan H, Bandyopadhyay D, Schmidt K, et al. Successful treatment of a patient with chronic lymphocytic leukaemia (CLL) presenting with bony metastases with aggressive antibody and chemotherapy. Clin Lab Haematol. 2005; 27: 405-408. 10.1111/j.1365-2257.2005.00733.x [DOI] [PubMed] [Google Scholar]
- 11.Langenberg JCM, Bosman WM, Bremer J, Ritchie ED. Pathological fractures in a patient with chronic lymphatic leucaemia without disease progression. Case Reports. 2015; 2015: bcr2014208118. 10.1136/bcr-2014-208118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koutroumpakis E, Lobe M, McCarthy L, Mehdi S. Symptomatic Hypercalcemia in a patient with B-cell chronic lymphocytic leukemia - A case report and review of the literature. In Vivo. 2016; 30: 691-694. [PubMed] [Google Scholar]
- 13.Dyer MJS, Oscier DG. The configuration of the immunoglobulin genes in B cell chronic lymphocytic leukemia. Leukemia. 2002; 16: 973-984. 10.1038/sj.leu.2402528 [DOI] [PubMed] [Google Scholar]
- 14.Yin CC, Lin KIC, Ketterling RP, et al. Chronic lymphocytic leukemia With t(2;14)(p16;q32) involves the BCL11A and IgH genes and is associated with atypical morphologic features and unmutated IgVH genes. Am J Clin Pathol. 2009; 131: 663-670. 10.1309/AJCPXLY46UPFLISC [DOI] [PubMed] [Google Scholar]