Abstract
Acquired amegakaryocytic thrombocytopenia (AATP) is a rare disease characterized by thrombocytopenia and the disappearance of marrow megakaryocytes. A 43-year-old man was admitted because of thrombocytopenia of 1.0×109/L. Bone marrow aspirate demonstrated normal hematopoiesis lacking megakaryocytes, and AATP was diagnosed. The serum concentration of thrombopoietin (TPO) was high (7.72 fmol/mL). Prednisolone (PSL) at 60 mg/day was started and the platelet count recovered to 1,335×109/L; however, excessive megakaryocytopoiesis and subsequent decline in platelet count were noted 14 days later. At the peak platelet count, the TPO remained at 3.79 fmol/mL and returned to a normal level of 0.40 fmol/mL during the period of normal platelet count after PSL tapering. The marked thrombocytosis in response to prednisolone may have been caused by the high TPO after the resolution of suppressed megakaryopoiesis. Marked rebound thrombocytosis beyond 1,000×109/L after successful PSL treatment for AATP has not been previously reported.
Keywords: : acquired amegakaryocytic thrombocytopenia, glucocorticoid, thrombopoietin, rebound thrombocytosis
INTRODUCTION
Acquired amegakaryocytic thrombocytopenia (AATP) is a rare disease characterized by severe thrombocytopenia and the disappearance of megakaryocytes in the bone marrow, which consists of hematopoietic cells without dysplastic features.1 Immune mechanisms are considered to be involved in the thrombocytopenia and amegakaryocytic marrow.1 However, this disease is usually resistant to steroid therapy and many therapies have been employed with some success.1-26 We treated a patient with AATP who exhibited marked rebound thrombocytosis to more than 1,300×109/L in response to glucocorticoid therapy. Such marked rebound thrombocytosis has not been previously reported, and a high serum concentration of thrombopoietin (TPO) may have played a role in the high platelet count.
CASE REPORT
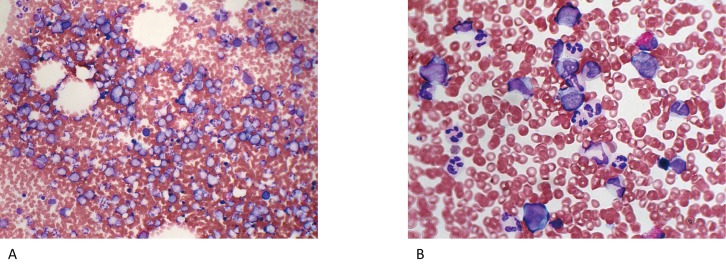
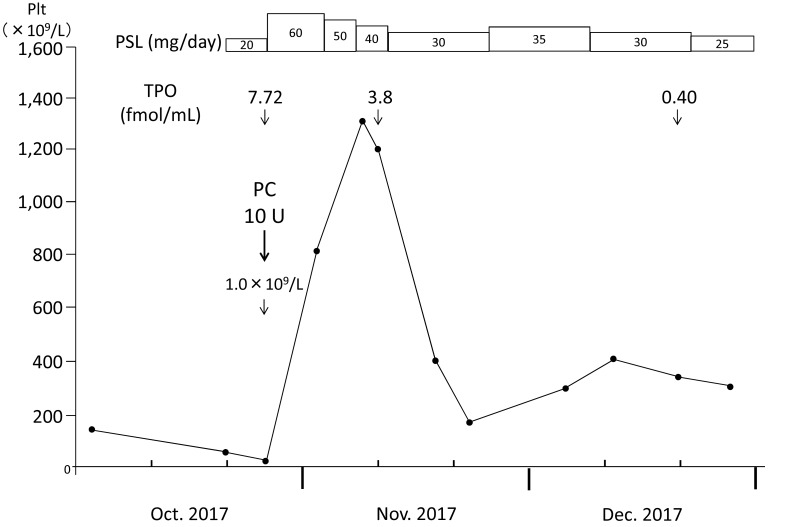
A 43-year-old man was referred to our hospital because of thrombocytopenia at the end of August 2017. At the beginning of the month, he had developed a sore throat without fever. He did not have any specific past or family history. Physically, neither purpura/petechiae of the extremities nor bleeding in the oral cavity was observed. Superficial lymph node swelling and hepatosplenomegaly were not noted. Laboratory examinations (Table 1) revealed a platelet count of 60×109/L, a white blood cell count (WBC) of 9.2×109/L with 36.0% neutrophils, 12.0% monocytes, and 48.5% lymphocytes, and a hemoglobin concentration of 15.6 g/dL. An increased number of large granular lymphocytes was not noted. Monoclonal rearrangements of T-cell receptor-γ and immunoglobulin heavy chain genes in circulating lymphocytes were not present, as examined by PCR (performed in July 2018) (data not shown). The serum concentration of platelet-associated IgG (PA-IgG) was elevated to 64 ng/107cells (normally below 46 ng/107cells). Hemostatic tests, including PT, APTT, and fibrinogen, yielded normal results. Antibodies against Helicobacter pylori were not detected in the urine. Antinuclear factor was negative, and serum levels of complement (C3 and C4) were within normal limits. Based on these findings, a tentative diagnosis of immune thrombocytopenia (ITP) was made. One month later, the patient visited again with petechiae on the extremities. The platelet count had decreased to 4.0×109/L; therefore, treatment with oral prednisolone (PSL: 20 mg/day) was started. Three days later, his platelet count had further decreased to 1.0×109/L, and he was admitted to our department. Bone marrow tap was performed after platelet transfusion. The marrow aspirate exhibited normocellular marrow lacking megakaryocytes (0/μL) (Figure 1A). No dysplastic features or an increase in the number of blasts was observed (Figure 1B). The aspirate was successfully obtained and we carefully observed the entire smear. Megakaryocytes were not observed in two smears and one particle smear preparation regardless of the reasonable numbers of granulocytes and erythrocyte precursors (Figure 1A). From these results, a diagnosis of AATP was made. Soon after the platelet transfusion, the platelet count increased to 33×109/L, and then decreased to 29×109/L and 24×109/L on days 1 and 3 after the transfusion, respectively. The dosage of PSL was increased to 60 mg/day on day 1 after admission. In response to the increased dosage of PSL, the platelet count became elevated to 95×109/L and 1,335×109/L on days 7 and 14, respectively. Therefore, the dosage of PSL was tapered with a gradual decrease in the platelet count to 858×109/L on day 26, and the patient was discharged on the same day (Figure 2).
Table 1. Laboratory findings on admission (August 2017).
| Hematology | Chemistry | Serology | |||
|---|---|---|---|---|---|
| WBC | 11.2×109/L | TP | 6.4g/dL | ANA | - |
| Neu | 70.5% | Alb | 4.2g/dL | IgA | 168mg/dL |
| Eos | 0.0% | AST | 19IU/L | IgG | 1,471mg/dL |
| Bas | 0.0% | ALT | 27IU/L | IgM | 42.0mg/dL |
| Mon | 9.0% | T-Bil | 0.9mg/dL | C3 | 78.0mg/dL |
| Lym | 20.0% | D-Bil | 0.1mg/dL | C4 | 25.8mg/dL |
| Aty-Lym | 0.5% | ALP | 147IU/L | ||
| RBC | 453×1010/L | LDH | 285IU/L | (August 25th) | |
| Hb | 14.0g/dL | γ-GTP | 37IU/L | PAIgG | 64ng/107cells |
| Ht | 38.2% | BUN | 14.6mg/dL | ||
| Plt | 1.0×109/L | Cr | 1.00mg/dL | (October 23rd) | |
| Coagulation | Na | 140mEq/L | PAIgG | 3,300ng/107cells | |
| PT-INR | 0.99 | K | 3.7mEq/L | ||
| APTT | 27.1S | Cl | 105mEq/L | ||
| Fib | 281mg/dL | Glu | 98mg/dL | ||
Abbreviations: ANA: anti-nucleolar antibody, atyp.lym: atypical lymphocytes, PAIgG: platelet-associated IgG.
Normal range: C3 65-135 mg/dL, C4 13-35 mg/dL, PAIgG < 46 ng/107cells.
Fig. 1.
Bone marrow images with particle and smear preparations at the diagnosis of AATP (October 24, 2017). A: A particle smear preparation of the marrow aspirate (Wright-Giemsa staining, ×200). The nucleated cell count was normal (66.3×109/L) with a normal myeloid erythroid cell ratio (M/E ratio) and absence of megakaryocytes. B: A smear preparation of the same aspirate (Wright-Giemsa staining, ×400). No dysplastic features or an increase in the number of blasts was observed.
Fig. 2.
Clinical course of the present patient. The platelet count markedly increased, with a peak count of 1,335×109/L, after the initiation of glucocorticoid therapy, and gradually decreased as prednisolone was tapered. PSL: prednisolone, TPO: thrombopoietin.
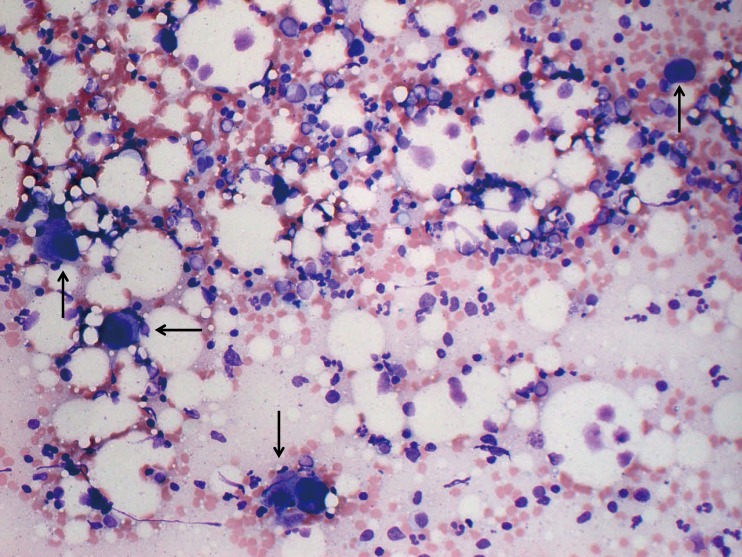
On day 10, bone marrow re-examination was performed, revealing a marked increase in megakaryocytes (312/μL) (Figure 3). Of note, these megakaryocytes were mature but small to medium in size without dysplastic features or other lineage hematopoietic cells. The serum concentration of TPO on day 1 after admission was as high as 7.72 fmol/mL and slightly decreased to 3.79 fmol/mL on day 9 when the platelet count was 1,328×109/L. Furthermore, the TPO concentration decreased to below 0.4 fmol/mL, which was the normal level, at the outpatient department when the platelet count was 361×109/L.
Fig. 3.
A particle smear preparation of the bone marrow (×200) at the peak platelet count after glucocorticoid therapy (November 9, 2017). The nucleated cell count was normal (161.9×109/L), with a normal myeloid erythroid cell ratio (M/E ratio) and increased number of mature megakaryocytes (312/μL). Megakaryocytes are mature, but most are medium in size (arrows).
At the outpatient department, we tapered the dosage of PSL cautiously to prevent AATP relapse and secondary refractoriness of PSL to this disease because AATP is generally resistant to glucocorticoid therapy. During the tapering of PSL, the platelet count rapidly decreased from 418×109/L to 186×109/L with 30 mg of PSL; therefore, the dosage of PSL was increased to 35 mg/day with the recovery of platelet count at approximately 350×109/L (Figure 2). After this episode, PSL was tapered, and the platelet count has remained around 220 ×109/L with 7 mg of PSL as of July 2018.
DISCUSSION
AATP is a rare disease, and only 64 cases have been reported in the past 2 decades.1,5,6,12-16,21-23 In the present case, bone marrow examination should have performed when we made a tentative diagnosis of ITP because thrombocytopenic conditions include ITP, AATP, and other thrombocytopenic disorders. An autoimmune mechanism has been considered as the cause of AATP such as an antibody directed against TPO1 or c-Mpl,21,22,24 or cytotoxic immune cells against megakaryocyte precursors or progenitors.23,25,26 In support of an autoimmune mechanism, associations with other autoimmune diseases, such as systemic lupus erythematosus,21,24,25 rheumatoid arthritis,12 Sjögren’s syndrome,18 and adult-onset Still’s disease,7 have been reported. As another cause, the involvement of estrogen has been suggested.5 In the present patient, the presence of an antibody against c-Mpl or cytotoxic mechanism was suggested because the blood level of TPO was high. In AATP caused by a c-Mpl antibody, the response to glucocorticoid, rituximab, or bolus intravenous immunoglobulin (IVIg) therapy was delayed and insufficient in 3 reported cases.21,22,24 On the other hand, in AATP caused by a cytotoxic mechanism, the platelet recovery after glucocorticoid therapy was relatively rapid.23,25,26 Therefore, in the present patient, some cytotoxic factor against megakaryocytes may have played a role. The upper respiratory symptom that preceded the onset of thrombocytopenia may have triggered the cytotoxic mechanism.
Regarding the mechanism of marked rebound thrombocytosis in the present patient, high blood TPO levels may have played an important role. Previously, high (13.7±11.2 fmol/mL), mild (1.25±0.39 fmol/mL), and normal (0.55±0.20 fmol/mL) blood TPO levels have been reported in patients with AATP or ITP, and in healthy persons, respectively.27 The reason for the high TPO level in AATP has been explained by the sponge theory, proposing that megakaryocytes absorb blood TPO through c-Mpl, which they abundantly express.28 In accordance with this theory, the serum concentration of TPO was as high as 7.72 fmol/mL during the amegakaryocytic period in the present patient, and remained at 3.79 fmol/mL even in the recovery phase of thrombocytopenia. The marked rebound thrombocytosis may have been caused by the remaining TPO even after resolution of the immune-mediated suppression of megakaryopoiesis by glucocorticoid therapy. As for the remaining blood TPO during marked thrombocytosis, it may have taken time to sufficiently absorb blood TPO regardless of the increased number of marrow megakaryocytes. To the best of our knowledge, this role of TPO in the recovery phase of AATP and a high platelet count of more than 1,000×109/L after glucocorticoid therapy have not been previously reported.
As therapeutic strategies for AATP, glucocorticoid,1,3,4,7,8,10-12,22,23,25,26 high-dose IVIg,1,7,8,22,23 anti-thymocyte globulin,4,9 cyclosporine A,7,9-12,23 rituximab,10,11 splenectomy,8 allogeneic hematopoietic stem cell transplantation,8 and agonists for c-Mpl,10 androgens,5 and vincristine7,8 have been employed. Of these, glucocorticoid therapy, which is widely used and effective for ITP, is less effective for AATP. However, in the present patient, a rapid response to PSL was observed at 60 mg/day, presumably due to a cytotoxic factor against megakaryocytes as stated above. Although low-dose PSL (7 mg/day) should be sufficient to maintain remission, it may be challenging to maintain remission without glucocorticoids in the present patient.
In conclusion, we treated an AATP patient who exhibited marked rebound thrombocytosis after successful glucocorticoid therapy. High blood TPO levels may have led to the thrombocytosis.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest in this study.
REFERENCES
- 1.Agarwal N, Spahr JE, Werner TL, Newton DL, Rodgers GM. Acquired amegakaryocytic thrombocytopenic purpura. Am J Hematol. 2006; 81: 132-135. 10.1002/ajh.20510 [DOI] [PubMed] [Google Scholar]
- 2.Tristano AG. Acquired amegakaryocytic thrombocytopenic purpura: review of a not very well-defined disorder. Eur J Intern Med. 2005; 16: 477-481. 10.1016/j.ejim.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T, Kono I, Kabashima T, et al. Amegakaryocytic thrombocytopenia associated with systemic lupus erythematosus successfully treated by a high-dose prednisolone therapy. Jpn J Med. 1984; 23: 135-138. 10.2169/internalmedicine1962.23.135 [DOI] [PubMed] [Google Scholar]
- 4.Manoharan A, Williams NT, Sparrow R. Acquired amegakaryocytic thrombocytopenia: report of a case and review of literature. Q J Med. 1989; 70: 243-252. [PubMed] [Google Scholar]
- 5.Kashyap R, Choudhry VP, Pati HP. Danazol therapy in cyclic acquired amegakaryocytic thrombocytopenic purpura: A case report. Am J Hematol. 1999; 60: 225-228. [DOI] [PubMed] [Google Scholar]
- 6.El Omri H, Skouri H, Kraiem I, et al. [Acquired amegakaryocytic thrombocytopenic purpura treated with intravenous immunoglobulins]. Ann Med Interne (Paris). 2000; 151: 223-226 [in French with English abstract]. [PubMed] [Google Scholar]
- 7.Her MY, Kim TH, Chang HK, Lee WS, Yoo DH. Successful treatment of acquired amegakaryocytic thrombocytopenia with cyclosporine in adult onset Still’s disease. Rheumatol Int. 2006; 27: 295-298. 10.1007/s00296-006-0202-8 [DOI] [PubMed] [Google Scholar]
- 8.Lonial S, Bilodeau PA, Langston AA, et al. Acquired amegakaryocytic thrombocytopenia treated with allogeneic BMT: a case report and review of the literature. Bone Marrow Transplant. 1999; 24: 1337-1341. 10.1038/sj.bmt.1702063 [DOI] [PubMed] [Google Scholar]
- 9.Brown GE, Babiker HM, Cantu CL, Yeager AM, Krishnadasan R. “Almost bleeding to death”: the conundrum of acquired amegakaryocytic thrombocytopenia. Case Rep Hematol. 2014; 2014: 1-5. 10.1155/2014/806541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cela I, Miller IJ, Katz RS, Rizman A, Shammo JM. Successful treatment of amegakaryocytic thrombocytopenia with eltrombopag in a patient with systemic lupus erythematosus (SLE). Clin Adv Hematol Oncol. 2010; 8: 806-809. [PubMed] [Google Scholar]
- 11.Fukushima T, Dong L, Sakai T, et al. Successful treatment of amegakaryocytic thrombocytopenia with anti-CD20 antibody (rituximab) in a patient with systemic lupus erythematosus. Lupus. 2008; 17: 210-214. 10.1177/0961203307086032 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto A, Kanisawa Y, Fujimi A, et al. Thrombocytopenia and anemia with anti-c-Mpl antibodies effectively treated with cyclosporine in a patient with rheumatoid arthritis and chronic renal failure. Intern Med. 2016; 55: 683-687. 10.2169/internalmedicine.55.5190 [DOI] [PubMed] [Google Scholar]
- 13.Ai DL, Li BT, Peng XM, et al. Acquired amegakaryocytic thrombocytopenic purpura induced by percutaneous ethanol injection during treatment of hepatocellular carcinoma: A case report. Oncol Lett. 2016; 11: 798-800. 10.3892/ol.2015.3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddou H, Zinebi A, Khalloufi A, et al. [Acquired amegacaryocytic thrombocytopenic purpura hiding acute myeloid leukemia]. Pan Afr Med J. 2017; 26: 32 [in French with English abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto A, Fujimi A, Kanisawa Y, et al. [Successful rituximab treatment for acquired amegakaryocytic thrombocytopenic purpura complicated with Coombs-negative autoimmune hemolytic anemia]. Rinsho Ketsueki. 2013; 54: 568-573 [in Japanese with English abstract]. [PubMed] [Google Scholar]
- 16.Niparuck P, Atichartakarn V, Chuncharunee S. Successful treatment of acquired amegakaryocytic thrombocytopenic purpura refractory to corticosteroids and intravenous immunoglobulin with antithymocyte globulin and cyclosporin. Int J Hematol. 2008; 88: 223-226. 10.1007/s12185-008-0125-0 [DOI] [PubMed] [Google Scholar]
- 17.Zafar T, Yasin F, Anwar M, Saleem M. Acquired amegakaryocytic thrombocytopenic purpura (AATP): a hospital based study. J Pak Med Assoc. 1999; 49: 114-117. [PubMed] [Google Scholar]
- 18.Ergas D, Tsimanis A, Shtalrid M, Duskin C, Berrebi A. T-γ large granular lymphocyte leukemia associated with amegakaryocytic thrombocytopenic purpura, Sjögren’s syndrome, and polyglandular autoimmune syndrome type II, with subsequent development of pure red cell aplasia. Am J Hematol. 2002; 69: 132-134. 10.1002/ajh.10024 [DOI] [PubMed] [Google Scholar]
- 19.Maslovsky I, Gefel D, Uriev L, Ben Dor D, Lugassy G. Malignant thymoma complicated by amegakaryocytic thrombocytopenic purpura. Eur J Intern Med. 2005; 16: 523-524. 10.1016/j.ejim.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Chen Y, Ding R. [Study on the pathogenesis of acquired pure amegakaryocytic thrombocytopenic purpura]. Zhonghua Xue Ye Xue Za Zhi. 1999; 20: 124-126 [in Chinese with English abstract]. [PubMed] [Google Scholar]
- 21.Kuwana M, Okazaki Y, Kajihara M, et al. Autoantibody to c-Mpl (thrombopoietin receptor) in systemic lupus erythematosus: relationship to thrombocytopenia with megakaryocytic hypoplasia. Arthritis Rheum. 2002; 46: 2148-2159. 10.1002/art.10420 [DOI] [PubMed] [Google Scholar]
- 22.Katsumata Y, Suzuki T, Kuwana M, et al. Anti-c-Mpl (thrombopoietin receptor) autoantibody-induced amegakaryocytic thrombocytopenia in a patient with systemic sclerosis. Arthritis Rheum. 2003; 48: 1647-1651. 10.1002/art.10965 [DOI] [PubMed] [Google Scholar]
- 23.Lai DW, Loughran TP, Jr, Maciejewski JP, et al. Acquired amegakaryocytic thrombocytopenia and pure red cell aplasia associated with an occult large granular lymphocyte leukemia. Leuk Res. 2008; 32: 823-827. 10.1016/j.leukres.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 24.Kuwana M, Kaburaki J, Okazaki Y, Miyazaki H, Ikeda Y. Two types of autoantibody-mediated thrombocytopenia in patients with systemic lupus erythematosus. Rheumatology. 2006; 45: 851-854. 10.1093/rheumatology/kel010 [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa T, Sakurai T, Kashiwagi H, Abe T. Cell-mediated amegakaryocytic thrombocytopenia associated with systemic lupus erythematosus. Blood. 1986; 67: 479-483. [PubMed] [Google Scholar]
- 26.Gewirtz AM, Sacchetti MK, Bien R, Barry WE. Cell-mediated suppression of megakaryocytopoiesis in acquired amegakaryocytic thrombocytopenic purpura. Blood. 1986; 68: 619-626. [PubMed] [Google Scholar]
- 27.Mukai H, Kojima H, Todokoro K, et al. Serum thrombopoietin (TPO) levels in patients with amegakaryocytic thrombocytopenia are much higher than those with immune thrombocytopenic purpura. Thromb Haemost. 1996; 76: 675-678. 10.1055/s-0038-1650641 [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa T, Hasegawa Y, Shimizu S, et al. Serum thrombopoietin level is mainly regulated by megakaryocyte mass rather than platelet mass in human subjects. Br J Haematol. 1998; 101: 242-244. 10.1046/j.1365-2141.1998.00683.x [DOI] [PubMed] [Google Scholar]