Abstract
The c-fms proto-oncogene is also known as macrophage colony stimulating factor receptor (M-CSFR) or colony-stimulating factor-1 receptor (CSF-1R), and is expressed on several types of malignant tumor cells and myeloid cells. In the present study, we found that overexpression of M-CSFR was present in adult T-cell leukemia/lymphoma (ATLL) cases. M-CSFR signaling was associated with lymphoma cell proliferation, and M-CSFR inhibition induced apoptosis in lymphoma cells. The ATLL cell line ATL-T expressed M-CSF/CSF-1 and interleukin (IL)-34, which are both M-CSFR ligands. M-CSF and IL-34 expression was seen in ATLL cases, and co-expression of these ligands was detected in 11 of 13 ATLL cases. M-CSFR inhibition suppressed programmed death-1 and -2 ligand in ATL-T cells and macrophages stimulated with conditioned medium from ATL-T cells. Thus, an M-CSFR inhibitor may be useful as additional therapy against ATLL due to direct and indirect mechanisms.
Keywords: M-CSFR, CSF-1R, ATLL, macrophage, PD-L1
INTRODUCTION
Adult T-cell leukemia/lymphoma (ATLL) is one of the most aggressive lymphomas arising in people infected with human T-cell leukemia virus type 1 (HTLV-1). The disease is classified into four categories: acute (60%); lymphomatous (20%); chronic (15%); and smoldering (5%).1,2 Acute and lymphomatous ATLLs are the most aggressive, with a reported median survival time of <1 year in patients who do not undergo bone marrow transplantation.1-3
The c-fms proto-oncogene is also known as macrophage colony stimulating factor receptor (M-CSFR) or colony-stimulating factor-1 receptor.4 Recent studies have shown that in addition to M-CSF/CSF-1, interleukin (IL)-34 also binds to M-CSFR.5,6 M-CSFR signaling is essential for macrophage differentiation, placental development, and lactogenic differentiation of the human breast.7
M-CSFR signaling is also essential for tumor cell growth, and increased expression of M-CSFR in tumor cells is linked to aggressive behavior and a worse clinical course in several types of malignant tumors including breast cancer and glioma. Overexpression of M-CSF in tumor cells has also been reported in many types of malignancies.8-10 M-CSFR is overexpressed in several types of hematological malignancies including acute myeloid leukemia and Hodgkin lymphoma.11,12 ATLL cells show high production of M-CSF,13 but no information about M-CSFR expression has been reported in ATLL.
Many companies have developed M-CSFR inhibitors,14,15 but a minimal effect of monotherapy against malignant tumors has been reported in clinical trials.16 Notably, a preclinical study showed that combination therapy using an M-CSFR inhibitor and cytotoxic therapy produces significant anti-tumor effects via induction of anti-tumor immune responses.17 Increased anti-tumor immune responses following treatment with an M-CSFR inhibitor were seen in other preclinical models,18,19 and depletion of immunosuppressive myeloid cells may contribute to this immune response. Many clinical trials with combination therapy using an M-CSFR inhibitor are currently underway (https://clinicaltrials.gov/).
In the present study, we found that M-CSFR was expressed in human ATLL cells and cell lines. M-CSFR signaling was associated with cell growth, and an M-CSFR inhibitor abrogated ATLL cell proliferation. Thus, M-CSFR inhibitors may be potentially useful as anti-ATLL therapy.
Materials and methods
Samples
Paraffin-embedded samples were prepared from specimens obtained from 13 patients diagnosed with lymphomatous ATLL between 2010 and 2017 at Kumamoto University Hospital. Written informed consent was obtained from all patients in accordance with protocols of the Kumamoto University Review Board, and study design was approved by the Kumamoto University Review Board (#1174).
Immunohistochemistry (IHC)
Single and double immunohistochemical staining was performed as described previously.20 In brief, deparaffinized sections were microwave treated in 1 mM EDTA (pH 8.0). Then, sections were reacted with anti-M-CSFR antibody (clone SP211, Abcam, Cambridge, UK), anti-M-CSF antibody (clone EP1179Y, Abcam), or isotype-matched control antibody (DAKO, Glostrup, Denmark). For IHC of macrophages, anti-CD68 antibody (clone PG-M1, DAKO) was used. IHC for IL-34 was performed as described previously.21 Horseradish peroxidase (HRP)-labeled anti-mouse or rabbit immunoglobulin antibody (Nichirei, Tokyo, Japan) was used as the secondary antibody. Diaminobenzidine (DAB, brown color) and HistoGreen (green color) substrate (#AYS-E109, Cosmo Bio, Tokyo, Japan) were used for visualization of positive signals.
Cell lines and cell culture
The human ATLL cell lines, ATL-T and ED, were previously established by Taylor and Matsuoka.22 The T-cell leukemia cell line MOLT-4 was purchased from the RIKEN Cell Bank (WAKO, Japan). Control TF-1 cells (TF-1CT) and TF-1 cells expressing M-CSFR (TF-1MCSFR) were previously established by Suzu.23 Cells were maintained in RPMI1640 supplemented with 10% fetal bovine serum. Mycoplasma testing was performed using a polymerase chain reaction (PCR) detection kit (Takara Bio Inc., Otsu, Japan). Conditioned medium (CM) from cell lines was obtained as described previously.24 Recombinant M-CSF was a gift from Morinaga Milk Industry (Kanagawa, Japan). IL-34 was purchased from R&D Systems (Minneapolis, MN, USA). PLX3397 (pexidartinib) was obtained from APExBio (Houston, TX, USA). Cell block samples of cell lines were prepared as described previously.25
Quantitative real-time PCR (qPCR)
Total RNA was isolated using RNAiso Plus (Takara Bio Inc.) and reverse transcribed using a PrimeScript RT Reagent Kit (Takara Bio Inc.). qPCR was performed using TaqMan polymerase with SYBR Green Fluorescence (Takara Bio Inc.) and an ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA). All primer sets were purchased from Takara Bio Inc. Primer sequences are as follows: IL-34; (F) 5’-TGTTCAGAATCGCCAACGTC-3’, (R) 5’-GCTCACCAAGACCCACAGATAC-3’, M-CSF; (F) 5’-GCTGAAGAGCTGCTTCACCAA-3’, (R) 5’-CATTCTTGACCTTCTCCAGCAA-3’, β-actin; (F) 5’-CTGTGGCATCCACGAAACTAC-3’, (R) 5’-CTGATCCACATCTGCTGGAAG-3’.
Immunocytostaining (IC)
ATL-T cells were fixed with 2% paraformaldehyde for 5 min, and then incubated with anti-cleaved caspase-3 antibody (clone 5A1E, Cell Signaling Tech., Danvers, MA, USA) with CanGet signal solution A (Toyobo, Tokyo, Japan). HRP-labeled anti-rabbit immunoglobulin antibody (Nichirei) was used as secondary antibody, and the reaction was visualized with DAB.
For staining of macrophages, the cells were detached and plated on coverslips in a 12-well culture plate (50,000 cells/well). Cells were treated with CM and PLX3397 (or DMSO as control) for 24 h and then fixed with 1% paraformaldehyde. Cells were dried and then reacted with anti-programmed death-1 ligand (PD-L1, CD274) and PD-L2 (CD273) antibody (Clone 29E.2A3 and 24F.10C12, BioLegend, San Diego, CA, USA). HRP-labeled anti-mouse immunoglobulin antibody (Nichirei) was used as secondary antibody, and the reaction was visualized with DAB. For quantification of positive signals, a cell-enzyme linked immunosorbent assay (cell-ELISA) was performed as described previously.26 In brief, cells were plated in a 96-well plate (5,000 cells/well) and treated with CM and inhibitor. Cells were then fixed with 1% paraformaldehyde, reacted with anti-PD-L1 and PD-L2 antibody, and incubated with secondary antibody. Reactions were visualized with TMB solution (Thermo Fisher Scientific, Waltham, MA, USA).
Western blot analysis
To detect cell apoptosis, cells were treated according to the manufacturer’s protocol and reacted with anti-caspase-3 (#19677-1-AP, Peprotech), cleaved caspase-3 (clone D3E9, Cell Signaling Tech.), and Poly(ADP-ribose)polymerase (PARP)(#9542, Cell Signaling Tech.) antibodies. Visualization was performed as described previously.26
Macrophage culture
Peripheral blood mononuclear cells were obtained from healthy volunteer donors who each provided written informed consent for the use of their cells in accordance with the study protocols approved by the Kumamoto University Hospital Review Board (#1169). Monocytes were isolated using RosettSep cocktail (StemCell Tech., Vancouver, Canada), plated in UpCELL culture plates (CellSeed, Tokyo, Japan), and cultured in 2% human serum, 1 ng/mL granulocyte macrophage-colony stimulating factor (WAKO), and 50 ng/mL macrophage-colony stimulating factor (WAKO) for 7 days to induce macrophage differentiation.
Flow cytometry
Flow cytometry of PD-L1 and PD-L2 was performed as described previously.27
Statistical analysis
Student’s t-test was used for statistical analysis of in vitro data. A P value <0.05 was considered to be statistically significant. All data from cell culture studies are representative of at least two independent experiments. All error bars indicate the standard deviation.
RESULTS
ATLL cells express M-CSFR
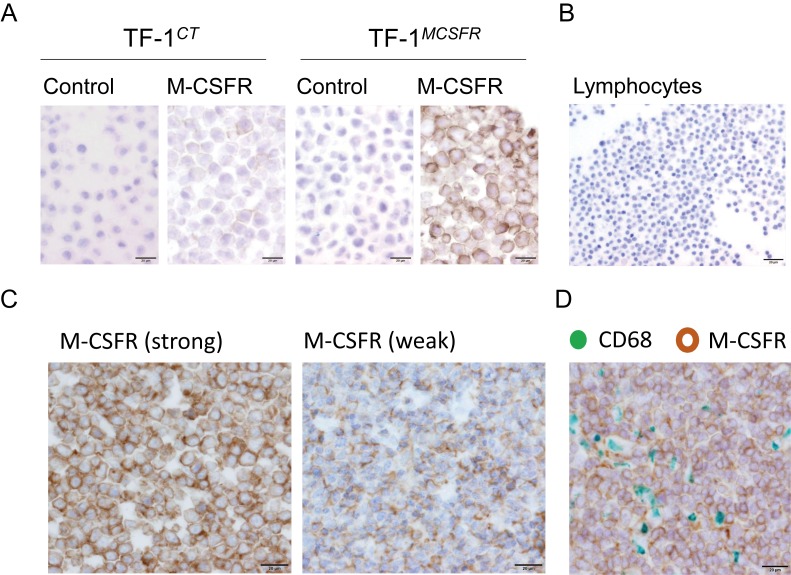
IHC for M-CSFR was performed using cell blocks of TF-1CT and TF-1MCSFR to confirm the specificity of the anti-M-CSFR antibody. Strong positive signals were seen on TF-1MCSFR, whereas no or weak signals were detected on TF-1CT (Figure 1A). Normal lymphocytes were negative for M-CSFR (Figure 1B). These data indicate the specificity of this antibody for the M-CSFR protein. Next, IHC for M-CSFR was performed using 13 ATLL tissue sections, and positive signals were detected in all cases. Strong circumferential membrane staining of M-CSFR on lymphoma cells was seen in 11 cases (85%), and weak incomplete membrane staining of M-CSFR was seen in two cases (Figure 1C). Double IHC with anti-CD68 antibody indicated that M-CSFR was detected on lymphoma cells that were negative for macrophage marker (Figure 1D).
Fig. 1.
IHC for M-CSFR. IHC for M-CSFR was performed with cell block specimens of TF-1CT and TF-1MCSFR cell lines (A), cell blocks of lymphocytes (B), and biopsy sections from patients diagnosed with ATLL (C). Double IHC for CD68 (pan-macrophage marker, green) and M-CSFR (brown) (D).
M-CSFR signaling stimulates lymphoma cell growth
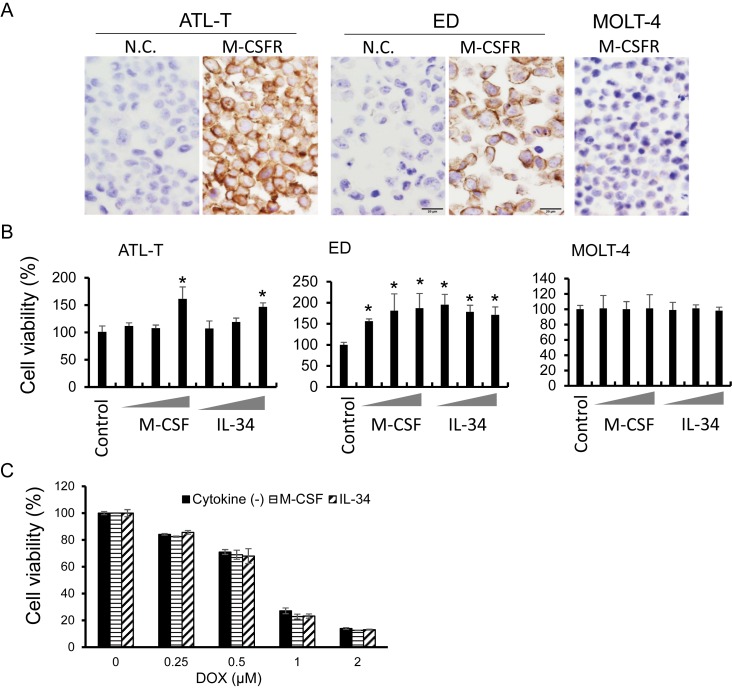
Next, M-CSFR expression was examined in cell lines. Two ATLL cell lines, ATL-T and ED cells, expressed M-CSFR, whereas no expression of M-CSFR was seen on MOLT-4 cells (Figure 2A). Recombinant M-CSF and IL-34 protein induced lymphoma cell growth in ATL-T and ED cells, but no effect was observed in MOLT-4 cells (Figure 2B). M-CSF and IL-34 did not affect the sensitivity of ATLL cells to doxorubicin (Figure 2C).
Fig. 2.
Effect of M-CSF and IL-34 on cell lines. IHC for M-CSFR was performed using cell block specimens of ATL-T, ED, and MOLT-4 cell lines (A). Cell lines were cultured with or without M-CSF (1.0, 10, 100 μg/mL) or IL-34 (0.1, 1.0, 10 μg/mL) for 3 days in a 96-well plate, and then cell viability was tested with the WST assay (n = 5) (B). ED cells were cultured with doxorubicin and M-CSF or IL-34 for 2 days, and then cell viability was tested with the WST assay (n = 5) (C). *P < 0.05. N.C.; negative control.
ATLL cells produce M-CSF and IL-34
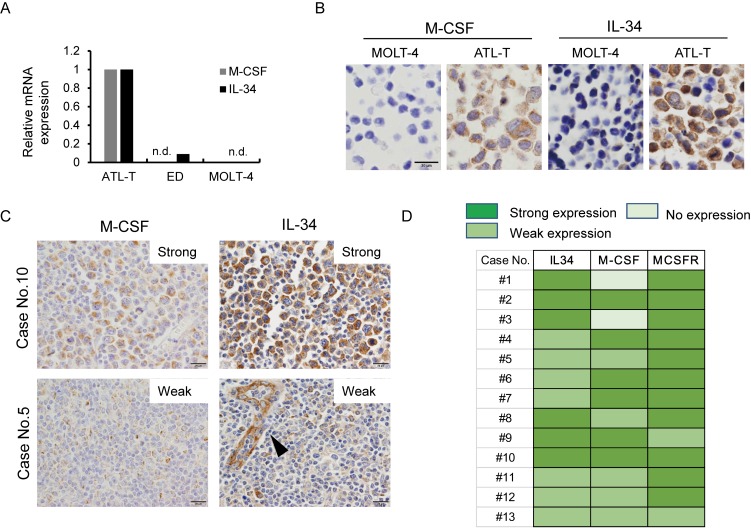
We next used qPCR to test whether ATLL cell lines express M-CSF, IL-34, and M-CSFR. ATL-T cells expressed M-CSF and IL-34 mRNA, whereas no mRNA expression of either ligand was seen in MOLT-4 cells (Figure 3A). ED cells expressed low levels of IL-34 mRNA (Figure 3A). Then we tested the protein expression of M-CSF and IL-34 in cell lines and tissue sections. Consistent with the qPCR data, ATL-T cells were positive for M-CSF and IL-34, whereas MOLT-4 cells were negative (Figure 3B). M-CSF expression in lymphoma cells was strong in six cases (46%), weak in five cases (38%), and negative in two cases (Figure 3C, 3D). Strong and weak IL-34 expression in lymphoma cells was seen in six (46%) and seven cases (54%), respectively (Figure 3C, 3D). Notably, IL-34 expression was also detected in endothelial cells (arrowhead, Figure 3C).
Fig. 3.
M-CSF and IL-34 expression in ATLL. The expression of M-CSF and IL-34 mRNA was determined with qPCR (A). The expression of M-CSF and IL-34 protein was determined with IHC using cell block specimens of ATL-T and MOLT-4 cell lines (B). IHC for M-CSF and IL-34 was performed using biopsy sections of ATLL (C). The arrowhead indicates positive staining for IL-34 in endothelial cells (C). Summary of M-CSF, IL-34, and M-CSFR expression in ATLL cases (D). n.d.; not detected.
An M-CSFR inhibitor induced apoptosis of ATL-T cells
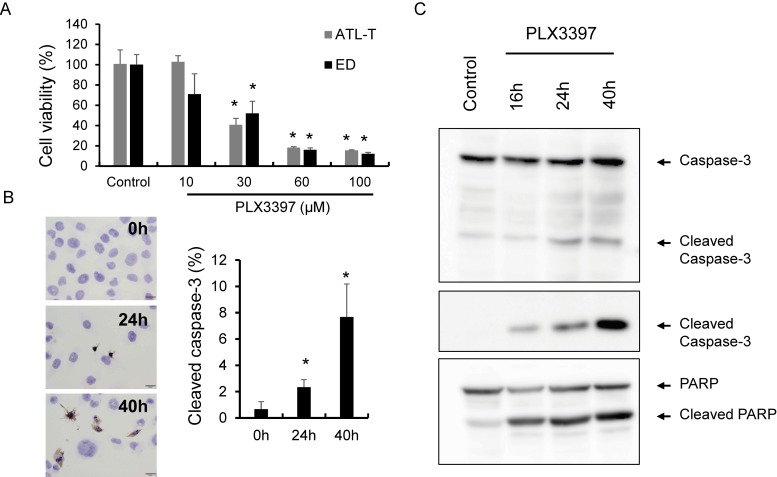
PLX3397, an M-CSFR inhibitor, significantly inhibited ATL-T and ED cell proliferation in a dose-dependent manner (Figure 4A). IC for cleaved caspase-3 showed that PLX3397 induced apoptosis in lymphoma cells after 24 h of incubation (Figure 4B). Western blot analysis using anti-caspase 3, anti-cleaved caspase 3 and anti-PARP antibodies showed similar results with regard to apoptosis in lymphoma cells. Cleavage of caspase 3 and PARP was seen in 16h-treatment and the densities were dependent on stimulation time (Figure 4C).
Fig. 4.
Effect of PLX3397 in ATLL cell lines. ATL-T and ED cell lines were cultured with or without the M-CSFR inhibitor, PLX3397, for 2 days, and cell viability was tested with the WST assay (A). IC for cleaved caspase-3 was performed using ATL-T cells cultured with PLX3397 (100 μM), and apoptotic cells positive for cleaved caspase-3 were counted (n = 3) (B). Western blot analysis of caspase-3, cleaved caspase-3, and PARP using cell lysates of ATL-T cells treated with PLX3397 (100 μM) (C).
The M-CSFR inhibitor abrogates PD-L1/L2 expression in macrophages and ATL-T cells.
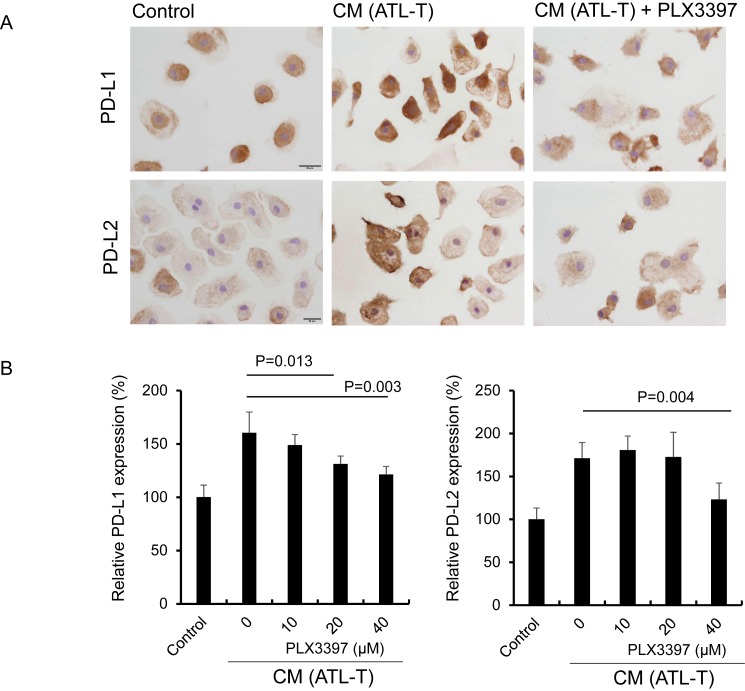
PD-L1 and PD-L2 expression on macrophages is associated with immune suppression in the tumor microenvironment.31 We previously showed that PD-L1 and PD-L2 expression on macrophages is significantly elevated by CM from ATL-T cells.29 IC and cell-ELISA were performed to test if PLX3397 abrogated PD-L1 and PD-L2 expression on macrophages stimulated with CM. We observed suppression of PD-L1 and PD-L2 by PLX3397 (Figure 5A and 5B).
Fig. 5.
Effect of PLX3397 on PD-L1 and PD-L2 expression on macrophages. Macrophages were plated in a 24-well plate and cultured with CM from ATL-T cells and PLX3397 (40 μM) for 1 day (A). Macrophages were plated in a 96-well plate and cultured with CM from ATL-T cells and PLX3397 for 1 day, and the expression of PD-L1 and PD-L2 was determined with a cell-ELISA (n = 3) (B).
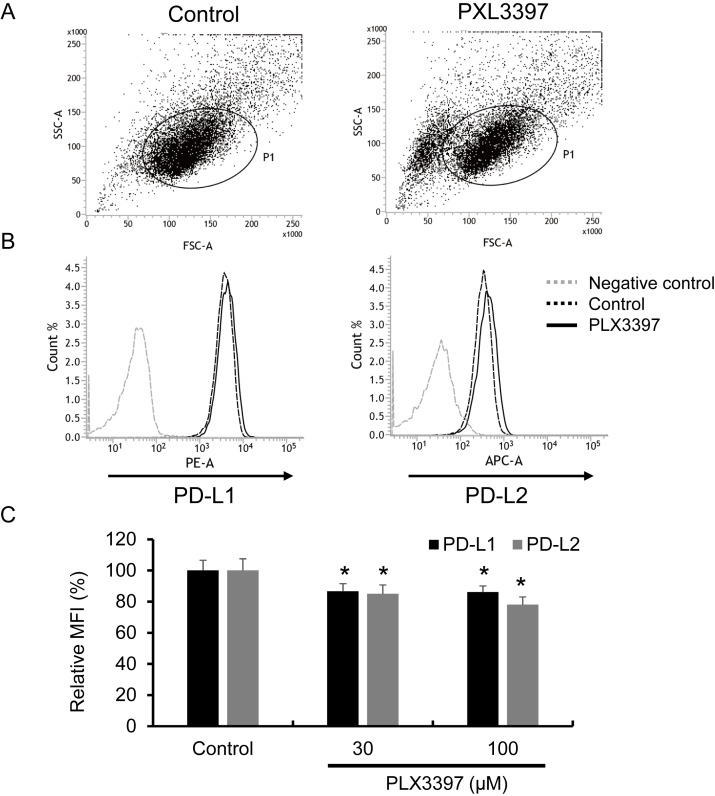
Finally, because ATL-T cells also express PD-L1 and PD-L2, we tested the effect of PLX3397 on PD-L1 and PD-L2 expression in ATL-T cells and found that these proteins were slightly but significantly suppressed by PLX3397 (Figure 6A-6C).
Fig. 6.
Effect of PLX3397 on PD-L1 and PD-L2 expression in the ATL-T cell line. The expression of PD-L1 and PD-L2 was tested with flow cytometry, and a dot blot of SSC/FSC (A) and a histogram of PD-L1 and PD-L2 staining (B) are presented. Mean fluorescent intensity (MFI) of PD-L1 and PD-L2 is presented (C). *P < 0.05 (n = 3).
DISCUSSION
In the present study, we showed high expression of M-CSFR in ATLL cells in pathological specimens and cell lines. An in vitro study using ATLL cell lines revealed that stimulation of M-CSFR with M-CSF or IL-34 increased ATLL cell proliferation. We believe that the present study is the first report to describe the function of M-CSFR in ATLL.
M-CSFR activates signaling pathways involving Stat3, ERK, and AKT, and affects survival, proliferation, migration, adhesion, and production of several cytokines in tumor cells.5 We did not investigate the correlation between M-CSFR and the details of the signaling pathway in ATLL in the present study. Further investigation is needed to uncover the importance of M-CSFR in ATLL.
ATLL is generally resistant to chemotherapy.29 Therefore, we tested if M-CSFR signaling affects the sensitivity to a chemotherapeutic drug. However, we found no correlation between M-CSFR signaling and sensitivity to doxorubicin. We previously demonstrated that a high density of tumor-associated macrophages (TAMs) in ATLL tissues is correlated with worse clinical prognosis,30 and co-culture with macrophages induces chemo-resistance in ATLL cells.31 However, the detailed mechanisms of chemo-resistance in ATLL cells have not been determined. Unknown factors derived from the microenvironment may be involved in chemo-resistance of ATLL cells.
An IHC study using biopsy samples showed that ATLL cells expressed both M-CSF and IL-34 at high frequency. IL-34 protein is expressed in keratinocytes of the skin and neurons of the brain in healthy conditions,32 and IL-34 mRNA is expressed in various tissues such as the spleen, kidney, liver, lung, testis, ovary, and intestines.5 IL-34 expression is elevated by several pathological conditions including autoimmune diseases and neurological disorders.33-35 At the cellular level, immune cells, synovial fibroblasts, endothelial cells, epithelial cells, and adipocytes also express IL-34.5 Overexpression of IL-34 was recently suggested to be related to tumor progression or chemo-resistance in lung cancer, breast cancer, osteosarcoma, colorectal cancer, and melanoma.21,36-39 Co-expression of M-CSF and IL-34 is associated with the worst clinical course in lung cancer.21 In our present study, many cases (11 of 13) expressed both M-CSF and IL-34, and co-expression of these molecules may be related to the aggressiveness of ATLL.
Recent advances in immunotherapy indicate that blocking immune checkpoint molecules improves the clinical course in several cancers including Hodgkin lymphoma.40 PD-L1 expression in lymphoma cells is observed in 7% of ATLL patients and is correlated with a poor clinical course; however, PD-L1 expression in TAMs is correlated with a better clinical course.41 We suggested that PD-L1 expression in TAMs is mediated by Stat3 activation in the microenvironment and that IL-27 is a candidate molecule related to Stat3 activation.42 In the present study, we found that inhibition of M-CSFR suppressed PD-L1 and PD-L2 expression in CM-stimulated macrophages. M-CSFR signaling activates Stat3 signaling,27 and the M-CSFR-Stat3 pathway may be related to the overexpression of PD-1 ligands in TAMs. We also previously showed the importance of Stat3 in PD-L1 and PD-L2 expression in ATL-T cells.42 The expression of PD-1 ligands in ATL-T cells was down-regulated by an M-CSFR inhibitor, although the decrease was slight as compared to that of Stat3 inhibitor. Unknown mechanisms other than M-CSFR signal might be related to Stat3 activation in lymphoma cells. Disruption of the 3′-region of the PD-L1 gene is related to overexpression of PD-L1 in ATLL.43 Mechanisms other than M-CSFR signaling may be linked to overexpression of PD-1 ligands in the ATL-T cell line.
In conclusion, overexpression of M-CSFR and its ligands, M-CSF and IL-34, was observed in ATLL cells. The M-CSFR inhibitor induced apoptosis in ATLL cell lines, and also suppressed the expression of PD-1 ligands in lymphoma cells and macrophages. Inhibition of M-CSFR may affect the immunosuppressive microenvironment and the anti-tumor environment. An M-CSFR inhibitor may be useful as additional therapy against ATLL via direct and indirect mechanisms.
ACKNOWLEDGMENTS
We thank Ms. Ikuko Miyakawa and Mr. Takenobu Nakagawa for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 16H05162 and 16K15503).
Footnotes
CONFLICT OF INTEREST: None of the authors have any conflicts of interest in association with this manuscript.
REFERENCES
- 1.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991; 79: 428-437. 10.1111/j.1365-2141.1991.tb08051.x [DOI] [PubMed] [Google Scholar]
- 2.Katsuya H, Ishitsuka K, Utsunomiya A, et al. ATL-Prognostic Index Project . Treatment and survival among 1594 patients with ATL. Blood. 2015; 126: 2570-2577. 10.1182/blood-2015-03-632489 [DOI] [PubMed] [Google Scholar]
- 3.Murata K, Yamada Y. The state of the art in the pathogenesis of ATL and new potential targets associated with HTLV-1 and ATL. Int Rev Immunol. 2007; 26: 249-268. 10.1080/08830180701709817 [DOI] [PubMed] [Google Scholar]
- 4.Hume DA, MacDonald KPA. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012; 119: 1810-1820. 10.1182/blood-2011-09-379214 [DOI] [PubMed] [Google Scholar]
- 5.Baghdadi M, Umeyama Y, Hama N, et al. Interleukin-34, a comprehensive review. J Leukoc Biol. 2018; 104: 931-951. 10.1002/JLB.MR1117-457R [DOI] [PubMed] [Google Scholar]
- 6.Lin H, Lee E, Hestir K, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008; 320: 807-811. 10.1126/science.1154370 [DOI] [PubMed] [Google Scholar]
- 7.Rahmati M, Petitbarat M, Dubanchet S, et al. Colony Stimulating Factors 1, 2, 3 and early pregnancy steps: from bench to bedside. J Reprod Immunol. 2015; 109: 1-6. 10.1016/j.jri.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Kascinski B. Expression of CSF-1 and its receptor CSF-1R in non-hematopoietic neoplasms. Cancer Treat Res. 2002; 107: 285-292. [DOI] [PubMed] [Google Scholar]
- 9.Ries CH, Hoves S, Cannarile MA, Rüttinger D. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol. 2015; 23: 45-51. 10.1016/j.coph.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional Relationship between Tumor-Associated Macrophages and Macrophage Colony-Stimulating Factor as Contributors to Cancer Progression. Front Immunol. 2014; 5: 489. 10.3389/fimmu.2014.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills K. Gene expression profiling for the diagnosis and prognosis of acute myeloid leukaemia. Front Biosci. 2008; Volume: 4605-4616. 10.2741/3026 [DOI] [PubMed] [Google Scholar]
- 12.Gruss HJ, Brach MA, Drexler HG, et al. Expression of cytokine genes, cytokine receptor genes, and transcription factors in cultured Hodgkin and Reed-Sternberg cells. Cancer Res. 1992; 52: 3353-3360. [PubMed] [Google Scholar]
- 13.Nosaka K, Miyamoto T, Sakai T, et al. Mechanism of hypercalcemia in adult T-cell leukemia: overexpression of receptor activator of nuclear factor kappa B ligand on adult T-cell leukemia cells. Blood. 2002; 99: 634-640. 10.1182/blood.V99.2.634 [DOI] [PubMed] [Google Scholar]
- 14.El-Gamal MI, Anbar HS, Yoo KH, Oh CH. FMS Kinase Inhibitors: Current Status and Future Prospects. Med Res Rev. 2013; 33: 599-636. 10.1002/med.21258 [DOI] [PubMed] [Google Scholar]
- 15.Burns CJ, Wilks AF. c-FMS inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:147-165. [DOI] [PubMed]
- 16.Butowski N, Colman H, De Groot JF, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-oncol. 2016; 18: 557-564. 10.1093/neuonc/nov245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011; 1: 54-67. 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dammeijer F, Lievense LA, Kaijen-Lambers ME, et al. Depletion of Tumor-Associated Macrophages with a CSF-1R Kinase Inhibitor Enhances Antitumor Immunity and Survival Induced by DC Immunotherapy. Cancer Immunol Res. 2017; 5: 535-546. 10.1158/2326-6066.CIR-16-0309 [DOI] [PubMed] [Google Scholar]
- 19.Stafford JH, Hirai T, Deng L, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-oncol. 2016; 18: 797-806. 10.1093/neuonc/nov272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa T, Ohnishi K, Kosaki Y, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. J Clin Exp Hematop. 2017; 57: 31-36. 10.3960/jslrt.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baghdadi M, Endo H, Takano A, et al. High co-expression of IL-34 and M-CSF correlates with tumor progression and poor survival in lung cancers. Sci Rep. 2018; 8: 418. 10.1038/s41598-017-18796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor GP, Matsuoka M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene. 2005; 24: 6047-6057. 10.1038/sj.onc.1208979 [DOI] [PubMed] [Google Scholar]
- 23.Chihara T, Suzu S, Hassan R, et al. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010; 17: 1917-1927. 10.1038/cdd.2010.60 [DOI] [PubMed] [Google Scholar]
- 24.Hide T, Komohara Y, Miyasato Y, et al. Oligodendrocyte Progenitor Cells and Macrophages/Microglia Produce Glioma Stem Cell Niches at the Tumor Border. EBioMedicine. 2018; 30: 94-104. 10.1016/j.ebiom.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010; 101: 2128-2136. 10.1111/j.1349-7006.2010.01652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara Y, Komohara Y, Ikeda T, Takeya M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011; 102: 206-211. 10.1111/j.1349-7006.2010.01772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horlad H, Ma C, Yano H, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016; 107: 1696-1704. 10.1111/cas.13065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau J, Cheung J, Navarro A, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017; 8: 14572. 10.1038/ncomms14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsuya H, Ishitsuka K. Treatment advances and prognosis for patients with adult T-cell leukemia-lymphoma. J Clin Exp Hematop. 2017; 57: 87-97. 10.3960/jslrt.17008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komohara Y, Niino D, Saito Y, et al. Clinical significance of CD163 + tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013; 104: 945-951. 10.1111/cas.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horlad H, Ohnishi K, Ma C, et al. TIM-3 expression in lymphoma cells predicts chemoresistance in patients with adult T-cell leukemia/lymphoma. Oncol Lett. 2016; 12: 1519-1524. 10.3892/ol.2016.4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H, Lee E, Hestir K, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008; 320: 807-811. 10.1126/science.1154370 [DOI] [PubMed] [Google Scholar]
- 33.Zhou RP, Wu XS, Xie YY, et al. Functions of interleukin-34 and its emerging association with rheumatoid arthritis. Immunology. 2016; 149: 362-373. 10.1111/imm.12660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chitu V, Gokhan Ş, Nandi S, Mehler MF, Stanley ER. Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends Neurosci. 2016; 39: 378-393. 10.1016/j.tins.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang EJ, Lee SK, Song YS, et al. IL-34 is associated with obesity, chronic inflammation, and insulin resistance. J Clin Endocrinol Metab. 2014; 99: E1263-E1271. 10.1210/jc.2013-4409 [DOI] [PubMed] [Google Scholar]
- 36.Ségaliny AI, Mohamadi A, Dizier B, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer. 2015; 137: 73-85. 10.1002/ijc.29376 [DOI] [PubMed] [Google Scholar]
- 37.Zins K, Heller G, Mayerhofer M, Schreiber M, Abraham D. Differential prognostic impact of interleukin-34 mRNA expression and infiltrating immune cell composition in intrinsic breast cancer subtypes. Oncotarget. 2018; 9: 23126-23148. 10.18632/oncotarget.25226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franzè E, Dinallo V, Rizzo A, et al. Interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Oncotarget. 2017; 9: 3432-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giricz O, Mo Y, Dahlman KB, et al. The RUNX1/IL-34/CSF-1R axis is an autocrinally regulated modulator of resistance to BRAF-V600E inhibition in melanoma. JCI Insight. 2018; 3: e120422; Epub ahead of print. 10.1172/jci.insight.120422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015; 372: 311-319. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi H, Kiyasu J, Kato T, et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood. 2016; 128: 1374-1381. 10.1182/blood-2016-02-698936 [DOI] [PubMed] [Google Scholar]
- 42.Ma C, Horlad H, Pan C, et al. Stat3 inhibitor abrogates the expression of PD-1 ligands on lymphoma cell lines. J Clin Exp Hematop. 2017; 57: 21-25. 10.3960/jslrt.17006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016; 534: 402-406. 10.1038/nature18294 [DOI] [PubMed] [Google Scholar]