Abstract
Purpose
High-dose chemotherapy with autologous stem cell transplantation (ASCT) is considered to be the only curative treatment option for patients with refractory or relapsed diffuse large B-cell lymphoma (DLBCL). Due to toxicity, not all patients are eligible for this treatment leading to different treatment intensities. Here, we aim to analyze the impact of treatment intensity on survival in patients previously treated with rituximab and chemotherapy, and, furthermore, to analyze the association between socioeconomic position and treatment intensity, defined as palliation, non-salvage, and salvage regimens.
Materials and methods
We identified patients with refractory or relapsed DLBCL diagnosed in 2000–2015 in the Danish National Lymphoma Registry (n=1,228). We analyzed the impact of treatment intensity on survival in patients previously treated with rituximab (n=277) using a Cox proportional hazards model. Multinomial regression analyses were performed to identify associations between socioeconomic factors and treatment intensity for the entire cohort.
Results
In the rituximab era, the 5-year overall survival (OS) was 31% for patients receiving salvage regimens (n=194), and 17% for patients receiving non-salvage regimens (n=83). In the adjusted analysis, HR was 1.88, 95% CI: 0.9–3.9 for patients receiving salvage regimens. Patients living alone were significantly less likely to receive salvage regimens, as were patients with two or more comorbidities.
Conclusion
We observed a better OS in patients treated with salvage regimens compared with non-salvage regimens; however, the adjusted analysis contradicts this. Furthermore, our results indicate that there is a chance of remission for patients not eligible for ASCT.
Keywords: non-Hodgkin lymphoma, chemotherapy, epidemiology, stem cell transplantation, socioeconomic status, education, income
Introduction
The outcome for patients with diffuse large B-cell lymphoma (DLBCL) after first-line treatment has improved substantially, particularly due to the introduction of the monoclonal anti-CD20 antibody rituximab. A long-term follow-up of the GELA trial, in which 399 patients with DLBCL were randomized between CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab added to CHOP (R-CHOP), confirmed the survival benefit, with a 10-year overall survival (OS) of 44% for the R-CHOP group compared with 28% for CHOP alone.1 Although relapse rates have decreased, one-third of patients will have primary refractory disease or develop a subsequent relapse.1–3 The standard treatment for patients with relapsed or refractory DLBCL is salvage chemotherapy followed by high-dose therapy with autologous stem cell transplantation (ASCT). The PARMA trial included 215 patients with relapsed non-Hodgkin lymphoma (NHL), and the 109 patients who responded after two cycles of salvage therapy with DHAP (dexamethasone, cisplatin, and cytarabine) were randomized to either conventional therapy (four additional cycles of DHAP) or ASCT. A significant survival benefit for ASCT was demonstrated, with a 5-year OS of 53% for the patients undergoing transplantation vs 32% for those receiving conventional therapy.4 ASCT is normally offered to younger, fit patients without comorbidities.5,6 However, a significant number of patients are not eligible for ASCT because of age and/or comorbidity and no standardized chemotherapy salvage regimens are available in this setting.
The role of ASCT after the introduction of rituximab has been debated.7 In the CORAL study, 396 patients with relapsed or refractory DLBCL were randomized to DHAP or ICE (ifosfamide, carboplatin, etoposide) as salvage therapy before ASCT. Prior rituximab exposure was associated with impaired survival, with a 3-year OS of 40% vs 66% for rituximab-naïve patients.8 The European Group for Blood and Marrow Transplantation analyzed 470 patients receiving ASCT for relapsed DLBCL and demonstrated that the remission after ASCT was significantly longer compared with that achieved following the initial first-line treatment (median disease-free survival of 51 months vs 11 months, P<0.001) irrespective of prior rituximab exposure.9 Thus, the effect of ASCT is still significant in the rituximab era, but the question remains whether some patients might benefit from a less intensive regimen. No randomized study investigating the efficacy of ASCT has been conducted since the PARMA trial.4 Currently, all patients with refractory or relapsed DLBCL will have received rituximab as part of first-line treatment,10 and to our knowledge, there are no studies comparing the outcome between patients treated with less intensive (non-salvage) regimens and those with more intensive salvage regimens.
Another factor of interest is the impact of the socioeconomic position on the choice of treatment intensity. In a population-based study among 6,234 patients in Denmark diagnosed with NHL in 2000–2008, all-cause mortality was increased by 63% for patients with a short education compared with patients with higher education, and there was a significant higher frequency of intensive treatment in patients with higher education.11 In line with these findings, Hong et al showed in a retrospective single-center study among 687 patients with NHL in Ohio that a high socioeconomic position was associated with a significantly better OS (HR 0.74, 95% CI: 0.58–0.95).12 Investigations of the impact of socioeconomic factors on treatment intensity in the relapse setting are scarce. The patients’ awareness and self-care are important in order to decrease the diagnostic delay at the time of relapse. Furthermore, better communication skills and knowledge of treatment options might be more prevalent among patients with a higher socioeconomic position.
Here, we analyze OS in patients with refractory or relapsed DLBCL and compare treatment intensities and the impact of socioeconomic position on treatment intensity using data from the Danish nationwide population-based lymphoma database (LYFO).
Materials and methods
Patients
Patients diagnosed with de novo DLBCL during the period 2000–2015 were identified in the LYFO (N=5,816). The LYFO contains detailed information on both clinical and treatment-related parameters on all lymphoma patients in Denmark.13,14 Patients registered with relapse, or no response to first-line treatment, were included. Furthermore, patients who died with progressive disease within 6 months or with active or progressive disease later than 6 months from diagnosis were deemed as primary refractory or relapse, respectively. Patients with primary or secondary central nervous system lymphoma, primary mediastinal DLBCL, or a non-DLBCL lymphoma subtype at the time of relapse were excluded (n=432), as were patients with missing information on relapse treatment (n=48).
Treatment regimens were categorized as salvage regimens, non-salvage regimens, or no/palliative treatment (Table 1). Patients >70 years of age were included in the non-salvage group when treated with salvage regimens, since they most likely received a lower dose without the intent of conducting ASCT.
Table 1.
Categorization of treatment regimens
| Categorization | Chemo regimens |
|---|---|
|
| |
| Salvage | DHAP |
| ICE | |
| MIME | |
| GDP | |
| dexaBEAM | |
| MaxiMIME | |
| MiniBEAM | |
| Cyclophosphamide | |
|
| |
| Non-salvage | CHOP |
| CHOEP | |
| COPE | |
| PREBEN | |
| BFM | |
| FCD | |
| Gemcitabine | |
|
| |
| None/palliation | No treatment |
| Radiation therapy alone | |
| Rituximab alone | |
| CVP | |
| COP | |
| Bendamustine | |
| Fludarabine | |
| Bleomycin | |
| CCVP | |
| Chlorambucil | |
Abbreviations: ASCT, autologous stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DHAP, dexamethasone, cisplatin, and cytarabine; ICE, ifosfamide, carboplatin, etoposide; DHAP, dexamethasone, high-dose cyrabine, cisplatin; ICE, ifosfamide, carpoplatin, etoposide; MIME, mitoguazone, ifosfamide, methotrexate, etoposide; GDP dexaBEAM, gemcitabin, cisplatine, dexamethasone + BEAM; MiniBEAM, camustine, etoposide, cytarabine, melphalan; CHOEP, cyclophosphamide, doxorubicin, vincristin, etoposide, prednisone; COPE, cyclophosphamide, vincristin, prednisone, etoposide; PREBEN, pixantrone, rituximab, etoposide, bendamustine; BFM, cyclophosphamide, adriamycine, oncovin, methothrexate, cytarabine; FCD, fludarabine, cyclophosphamide, doxorubicin; CVP, cyclophosphamide, oncovin, prednisone; COP, cyclophosphamide, oncovin, prednisone; CCVP, vinorelbine, cyclophosphamide, prednisone.
Only patients who received rituximab as part of their treatment, and who received non-salvage or salvage regimens were included in the survival analysis (n=277).
Comorbidity
To estimate the burden of comorbidity, all somatic diagnoses other than lymphoma were retrieved from the Danish National Patient Registry (DNPR).15 Comorbidity was measured as the number of comorbidities, using the 19 disorders specified in the Charlson Comorbidity Index (CCI),16 grouped into no comorbidities, one, and two or more.
Socioeconomic factors
Information on socioeconomic factors was obtained from population-based administrative registries at Statistics Denmark.17,18 We obtained information on highest attained educational level until 1 October the year before relapse, and categorized it as short (7–9 years of education), medium (10–12 years of education), and higher education (>12 years of education). Disposable income the year before relapse was grouped into quintiles on the basis of that of the entire population, adjusted for sex and age. Disposable income was categorized as low (first quintile), medium (second – third quintile) and high (fourth – fifth quintile). Information on cohabitation status was obtained until 1 January of the year of relapse and defined as living alone or with a partner.
Statistical analysis
Patient baseline characteristics were analyzed descriptively and compared by chi-squared test. For the survival analyses, the log-rank test was used for comparisons of OS. Cox proportional hazards models were used to find an association between treatment intensity and survival, when adjusting for possible confounders (age, sex, period, educational level, comorbidities, and cohabitation status). The proportional hazards assumption was tested and fulfilled for all models using the function cox.zph in R. Patients were followed from the date of relapse or the date primary refractory disease was identified until death or 31 December 2015, whichever came first. ASCT was modeled as a time-dependent variable, when testing survival within the patient group receiving salvage regimens.
Using multinomial regression analyses, we performed bivariate comparisons to determine significant associations between treatment intensity and socioeconomic factors, reported as ORs. Within the multinomial logistic regression, treatment categories were compared using non-salvage treatment as reference. Multinomial logistic regression was chosen for its added efficiency over individual binomial logistic regression models after we had considered and then rejected the use of an ordinal logistic regression model for not meeting the proportional odds assumption. Multivariable multinomial logistic regression was conducted to provide adjustment for possible confounding variables for each analysis. Analyses were made for the entire cohort and for the subpopulation of patients with relapsed disease since patients with primary refractory disease normally would not receive non-salvage treatment regimens.
Values were regarded as statistically significant if P<0.05. All analyses were conducted using R 3.2.2 with survival and nnet packages.19,20
Ethics
The LYFO is approved by the National Board of Health and the Danish Data Protection Agency as a nationwide clinical database. This project was approved by the Danish Data Protection Agency (file number RH-2016–18).
Results
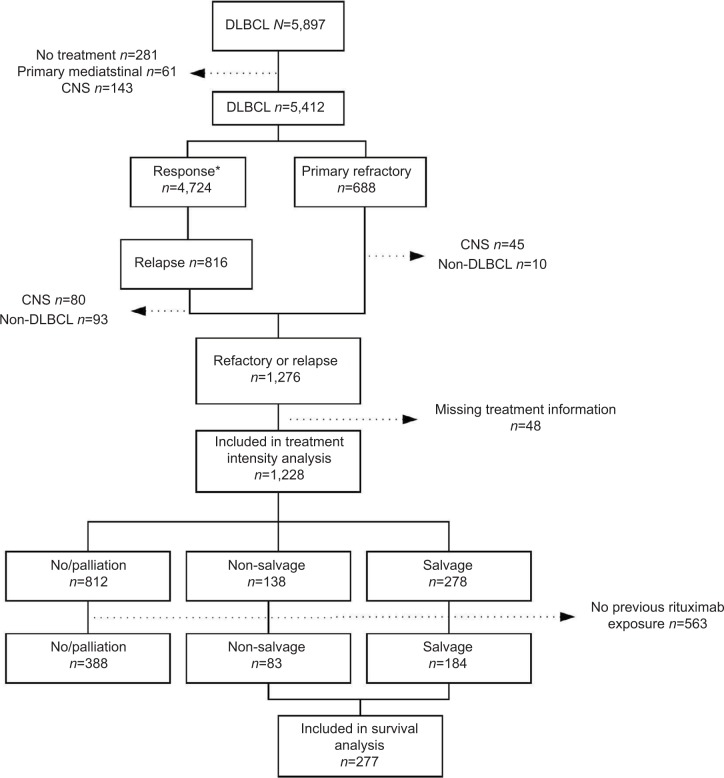
A total of 1,228 patients with either primary refractory or relapsed disease were included in the analyses (Figure 1). Median age at the time of relapse or refractory disease was 71 years (range 18–100), and 54% were male (Table 2). The median time to relapse was 348 days (range 30–4,593).
Figure 1.
Flowchart of the study cohorts of patients with primary refractory or relapsed DLBCL in the LYFO database in Denmark, 2000–2015.
Note: *Response defined as complete or partial remission.
Abbreviations: CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma.
Table 2.
Baseline characteristics of the 1,228 patients with primary refractory or relapsed diffuse large B-cell lymphoma in the LYFO database in Denmark, 2000–2015, by treatment category
| Baseline characteristics | Total | None/palliation | Non-salvage | Salvage | P-value |
|---|---|---|---|---|---|
| 1,228 | 812 | 138 | 278 | ||
|
| |||||
| Age, years (median [range]) | 72 (18–100) | 75 (22–100) | 73 (27–92) | 60 (18–70) | |
|
| |||||
| Age, years | <0.001 | ||||
| <65 | 388 (32) | 173 (21) | 17 (12) | 198 (71) | |
| ≥65 | 840 (68) | 639 (79) | 121 (88) | 80 (29) | |
|
| |||||
| Sex | 0.1 | ||||
| Male | 667 (54) | 423 (52) | 79 (57) | 165 (59) | |
| Female | 561 (46) | 389 (48) | 59 (43) | 113 (41) | |
|
| |||||
| Period | <0.001 | ||||
| 2000–2004 | 360 (29) | 277 (34) | 24 (17) | 59 (21) | |
| 2005–2009 | 426 (35) | 243 (30) | 68 (49) | 115 (41) | |
| 2010–2015 | 442 (36) | 292 (36) | 46 (33) | 104 (37) | |
|
| |||||
| Previous rituximab | <0.001 | ||||
| No | 563 (46) | 424 (52) | 55 (40) | 84 (30) | |
| Yes | 665 (54) | 388 (48) | 83 (60) | 194 (70) | |
|
| |||||
| Primary refractory disease | <0.001 | ||||
| No | 610 (50) | 315 (39) | 110 (80) | 185 (66) | |
| Yes | 618 (50) | 497 (61) | 28 (20) | 93 (34) | |
|
| |||||
| Cohabitation status | <0.001 | ||||
| Single | 518 (42) | 391 (48) | 63 (46) | 64 (23) | |
| Couple | 710 (58) | 421 (52) | 75 (54) | 214 (77) | |
|
| |||||
| Educational level | <0.001 | ||||
| Short | 525 (43) | 363 (45) | 74 (54) | 88 (32) | |
| Medium | 404 (33) | 249 (31) | 37 (27) | 118 (42) | |
| Long | 179 (14) | 94 (11) | 21 (15) | 64 (23) | |
| Unknown | 120 (10) | 106 (13) | 6 (4) | 8 (3) | |
|
| |||||
| Disposable income | 0.3 | ||||
| Low | 287 (23) | 200 (25) | 34 (25) | 53 (19) | |
| Medium | 504 (41) | 334 (41) | 52 (38) | 118 (42) | |
| High | 437 (36) | 278 (34) | 52 (38) | 107 (39) | |
|
| |||||
| Comorbidities | <0.001 | ||||
| 0 | 459 (37) | 256 (32) | 49 (36) | 154 (55) | |
| 1 | 399 (33) | 269 (33) | 46 (33) | 84 (30) | |
| ≥2 | 370 (30) | 287 (35) | 43 (31) | 40 (14) | |
|
| |||||
| ASCT | |||||
| No | 1,254 | // | // | 133 (48) | |
| Yes | 144 | // | // | 144 (52) | |
Note: // Information is not relevant in the columns.
Abbreviation: ASCT, autologous stem cell transplant.
Treatment intensity analysis
A total of 812 (66%) patients received either palliative treatment or no relapse treatment, 138 (11%) patients received non-salvage regimens, and 278 (23%) received salvage regimens. Patients with two or more comorbidities were less likely to receive non-salvage regimens compared with no/palliative treatment, (OR 0.60, 95% CI: 0.4–0.9). A similar association was seen for patients with relapsed disease (OR 0.49, 95% CI: 0.3–0.8). Also patients with two or more comorbidities were less likely to receive salvage regimens compared with non-salvage regimens for the entire cohort (OR 0.52, 95% CI: 0.3–0.9), and for patients with relapsed disease (OR 0.34, 95% CI: 0.2–0.8). Patients living with a partner were more likely to receive salvage regimens compared with non-salvage regimens (OR 2.20, 95% CI: 1.3–3.7), which was also true among patients with relapsed disease (OR 2.25, 95% CI: 1.2–4.4) (Table 3).
Table 3.
Adjusted ORs (with accompanying 95% CIs) for the choice of treatment-intensity (non-salvage vs no/palliative and salvage vs non-salvage) for the total cohort, and the subgroup of 610 patients with relapsed disease in the LYFO database in Denmark, 2000–2015
| Variables | Total cohort (n= 1,228)
|
Patients with relapse (n= 610)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Non-salvage vs no/palliative
|
Salvage vs non-salvage
|
Non-salvage vs no/palliative
|
Salvage vs non-salvage
|
|||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
|
| ||||||||
| Cohabitation status | ||||||||
| Single | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Couple | 1.04 (0.7–1.6) | 0.8 | 2.20 (1.3–3.7) | 0.003 | 1.15 (0.7–1.9) | 0.5 | 2.25 (1.2–4.4 | 0.02 |
|
| ||||||||
| Educational level | ||||||||
| Short | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Medium | 0.78 (0.5–1.2) | 0.3 | 1.34 (0.8–2.4) | 0.3 | 0.83 (0.5–1.44) | 0.5 | 1.46 (0.7–3.0) | 0.3 |
| Long | 1.12 (0.6–2.0) | 0.7 | 1.54 (0.8–3.1) | 0.2 | 1.46 (0.7–2.9) | 0.3 | 1.33 (0.6–3.1) | 0.5 |
| Unknown | 0.26 (0.1–0.6) | 0.002 | 2.14 (0.6–7,7) | 0.2 | 0.30 (0.1–0.7) | 0.01 | 1.37 (0.3–6.5) | 0.7 |
|
| ||||||||
| Disposable income | ||||||||
| low | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Medium | 0.84 (0.5–1.4) | 0.5 | 1.91 (1.1–3.6) | 0.05 | 1.00 (0.5–1.8) | 0.9 | 2.2 (0.9–5.1) | 0.06 |
| High | 0.95 (0.6–1.6) | 0.9 | 1.89 (1.0–3.7) | 0.06 | 1.07 (0.6–2.0) | 0.8 | 1.9 (0.8–4.6) | 0.1 |
|
| ||||||||
| Comorbidities | ||||||||
| 0 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| 1 | 0.75 (0.5–1.2) | 0.2 | 0.85 (0.5–1.5) | 0.6 | 0.61 (0.3–1.1) | 0.1 | 0.88 (0.4–1.8) | 0.7 |
| ≥2 | 0.60 (0.4–0.9) | 0.03 | 0.52 (0.3–0.9) | 0.04 | 0.49 (0.3–0.8) | 0.02 | 0.34 (0.2–0.8)* | 0.01 |
Notes: All analyses are adjusted for sex, age, and period. Cohabitation: Also adjusted for educational level. Disposable income: Also adjusted for educational level, cohabitation and the number of comorbidities Comorbidities: Also adjusted for educational level.
Survival analysis
A total of 277 patients were included in the survival analysis (Figure 1) (Table 4). The majority of these patients had received R-CHOP-like treatment as first-line treatment.
Table 4.
Baseline characteristics of the 277 patients with refractory or relapsed DLBCL previously treated with rituximab and receiving treatment for relapsed disease in the LYFO database in Denmark, 2000–2015
| Characteristics | Previous rituximab | Non-salvage | Salvage
|
||
|---|---|---|---|---|---|
| No ASCT | ASCT | P-value | |||
|
| |||||
| 277 | 83 | 104 | 90 | ||
|
| |||||
| Age, years (median [range]) | 65 (18–92) | 74 (27–92) | 62 (30–70) | 60 (18–69) | |
|
| |||||
| Age, years* | <0.001 | ||||
| <65 | 136 (49) | 9 (11) | 61 (59) | 66 (73) | |
| ≥65 | 141 (51) | 74 (89) | 43 (41) | 24 (27) | |
|
| |||||
| Sex | 0.4 | ||||
| Male | 164 (59) | 45 (54) | 66 (63) | 53 (59) | |
| Female | 113 (41) | 38 (46) | 38 (37) | 37 (41) | |
|
| |||||
| Period | 0.3 | ||||
| 2000–2009 | 147 (53) | 50 (60) | 56 (54) | 41 (39) | |
| 2009–2015 | 130 (47) | 33 (40) | 48 (46) | 49 (54) | |
|
| |||||
| Primary refractory disease* | <0.001 | ||||
| No | 190 (69) | 65 (78) | 54 (52) | 71 (80) | |
| Yes | 87 (31) | 18 (22) | 50 (48) | 19 (20) | |
|
| |||||
| Cohabitation status | <0.001 | ||||
| Single | 81 (29) | 38 (46) | 20 (19) | 23 (26) | |
| Couple | 196 (71) | 45 (54) | 84 (81) | 67 (74) | |
|
| |||||
| Educational level* | 0.003 | ||||
| Short | 104 (38) | 43 (52) | 40 (39) | 21 (23) | |
| Medium | 110 (40) | 26 (31) | 46 (44) | 38 (42) | |
| Long | 53 (19) | 11 (13) | 15 (14) | 27 (30) | |
| Unknown | 10 (3) | 3 (4) | 3 (2) | 4 (5) | |
|
| |||||
| Disposable income | 0.4 | ||||
| Low | 60 (21) | 20 (24) | 26 (25) | 14 (15 | |
| Medium | 115 (42) | 30 (36) | 44 (42) | 41 (46) | |
| High | 102 (37) | 33 (40) | 34 (33) | 35 (39) | |
|
| |||||
| Comorbidities* | <0.001 | ||||
| 0 | 125 (45) | 23 (28) | 48 (46) | 54 (60) | |
| 1 | 90 (33) | 29 (35) | 31 (30) | 30 (33) | |
| ≥2 | 62 (22) | 31 (37) | 25 (24) | 6 (7) | |
Abbreviations: ASCT, autologous stem cell transplantation; DLBCL, diffuse large B-cell lymphoma.
Five-year OS for patients treated with non-salvage regimens (n=83) was 17% (95% CI: 10%–28%), and the median survival was 332 days. For patients treated with salvage regimens (n=194), the 5-year OS was 31% (95% CI: 35%–39%), and the median survival was 435 days. Patients who received salvage regimens had a crude HR of 0.75 (95% CI: 0.6–1.0) compared with patients receiving non-salvage regimens (Figure 2). In the adjusted analysis, we found an HR of 1.9 for patients receiving salvage regimens compared with patients receiving non-salvage regimen (adjusted HR, 1.88 [95% CI: 0.9–3.9]) (Table 5).
Figure 2.
Survival of patients with primary refractory or relapsed diffuse large B-cell lymphoma previously exposed to rituximab according to relapse regimens in the LYFO database in Denmark, n=277.
Abbreviation: OS, overall survival.
Table 5.
Adjusted HRs and 5-year OS rates (with accompanying CIs) for the 277 patients with refractory or relapsed DLBCL previously treated with rituximab who received relapse treatment in the LYFO database in Denmark, 2000–2015
| Treatment category | N | Events | Person years | Adj. HR (95% CI) | 3-year OS (95% CI) | 5-year OS (95% CI) |
|---|---|---|---|---|---|---|
|
| ||||||
| Non-salvage | 83 | 69 | 173 | 1.00 (ref) | 27% (19%–38%) | 17% (10%–28%) |
| Salvage | 194 | 137 | 509 | 1.88 (0.9–3.9) | 39% (32%–46%) | 31% (25%–39%) |
|
| ||||||
| No ASCT | 104 | 88 | 238 | 1.00 (ref) | 25% (18%–35%) | 18 (12%–28%) |
| ASCTb | 90 | 49 | 270 | 0.65 (0.4–1.0) | 51% (42%–69%) | 46% (37%–59%) |
Notes: All analyses adjusted for sex, age, period, educational level, number of comorbidities, cohabitation status, and primary refractory disease.
For ASCT, adjusted HR is calculated with ASCT as a time-dependent variable, overall survival from the day of stem cell reinfusion.
Abbreviations: ASCT, autologous stem cell transplantation; DLBCL, diffuse large B-cell lymphoma.
For patients who received salvage regimens (n=194), we analyzed survival according to ASCT. For patients receiving ASCT, the 5-year OS from the time of stem cell reinfusion was 46% (95% CI: 37%–59%), and the median survival 1,172 days. The survival for patients who received ASCT was significantly better, with an unadjusted HR of 0.58 (95% CI: 0.4–0.9), adjusted HR 0.65 (95% CI: 0.4–1.0) (Table 5).
Discussion
In this population-based study of patients with relapsed/refractory DLBCL, socioeconomic position, measured as highest attained educational level and disposable income, was not associated with treatment intensity. However, patients living alone were less likely to receive salvage regimens. Previously, a population-based study among 6,234 patients with NHL found similar results, with patients living alone being less likely to receive any of the treatment modalities, eg, OR for receiving chemotherapy was 0.79 (95% CI: 0.65–0.97) for patients living alone compared with patients living with a partner.11 The more aggressive salvage regimens will normally be planned with the intent of ASCT. Due to the toxicity of this treatment, it might be a consideration for the clinician that patients will benefit from the social support of a spouse during and after treatment, which might explain our findings regarding cohabitation status. Patients with comorbidities have a poorer outcome after ASCT. Therefore, it is not surprising that the number of comorbidities affects treatment intensity for patients with refractory or relapsed DLBCL in patients with two or more comorbidities. In this study, about 30% of patients had two or more comorbidities; nevertheless, 7% were treated with non-salvage regimens and 15% with salvage regimens.
A relatively high proportion of patients were treated with a palliative strategy. These patients were older, and more patients had primary refractory disease. Supposedly, a more aggressive treatment was not chosen due to frailty.
In our study of patients with relapsed/refractory DLBCL who had received R-CHOP-like treatment as first-line treatment (n=277), we found that for patients receiving salvage treatment, only 46% were able to proceed to ASCT, and the 5-year OS was 31%. For patients with a non-salvage strategy, 17% were alive after 5 years.
There are some possible explanations for these results when comparing our findings with major studies in the field. In the PARMA study, eligibility was restricted to patients <60 years of age and with a previous response to first-line chemotherapy.4 With the advent of improved supportive care, ASCT is now also an option for older patients with higher stage disease. Furthermore, ASCT is also an accepted treatment for patients with primary refractory disease who have not sufficiently responded to initial therapy.21,22 In the present study, we included all patients receiving salvage regimens (n=194), including patients who were aged ≥65 years (35%), and patients with primary refractory disease (n=69, 36%). Of the 194 patients receiving salvage regimens, almost half (46%) also received ASCT. This is consistent with the CORAL study, in which 52% of patients received ASCT.8 Both in the CORAL and PARMA trials, the most common reason for not proceeding to ASCT was insufficient response to salvage therapy, with other reasons being toxicity or death.4,8 We were not able to obtain information on reasons not to proceed to ASCT in our study, but we found that patients >65 years of age were less likely to proceed to ASCT (P=0.04), as were patients with primary refractory disease (P<0.001), and patients with two or more comorbidities (P=0.004) (data not shown). Several studies have analyzed the impact of comorbidity on outcomes after ASCT. Wildes et al found that a higher CCI score was negatively associated with survival after ASCT among 159 patients with NHL.23 Graf et al showed that higher hematopoietic cell transplantation-specific comorbidity index scores predicted non-relapse mortality after ASCT among 754 lymphoma patients (HR 1.94, 95% CI: 1.0–3.7).24 Primary refractory disease has also been shown to be associated with impaired survival after ASCT; in the CORAL study, 3-year OS was 39% and 64% for patients with refractory and relapsed disease, respectively.8 In the present study, we were able to adjust for the number of comorbidities, age, and primary refractory disease, as well as socioeconomic factors. The crude analysis shows a 5-year OS of 31% for patients treated with salvage regimens and 17% for patients treated with a non-salvage strategy, however, after taking the mentioned factors into account, our results indicate an almost twofold increased mortality for patients receiving salvage regimens compared with those receiving non-salvage regimens. However, more patients in the salvage group had primary refractory disease, 36%, compared with 22% in the non-salvage group (Table 4), and 84% of the patients in the non-salvage group were >70 years of age (data not shown), which might explain the results in the adjusted analysis. Unfortunately, we were not able to adjust for more disease-specific factors, such as performance status at time of treatment decision nor the stage of the disease. Within the salvage group, there was a survival benefit regarding the receipt of ASCT, however, with quite wide CIs. No difference was found in the sub-analyses among patients with refractory and relapsed disease. However, study numbers are small, and again, a larger study population might have yielded significant results. A 5-year OS of 17% for patients receiving non-salvage regimens together with a 5-year OS of 18% for patients in the salvage group not receiving ASCT indicates indicate a non-negligible chance of survival, even for patients not eligible for ASCT. However, newer targeted treatment options are needed in order to improve the outcome for these patients.
Since the Danish population is entitled to free access to tax-supported medical care provided by the public health care system, our findings of socioeconomic position might not be generalizable to other populations. However, even with free access to medical care, we do find an association between socioeconomic position and treatment intensity, and we believe that this association may only be stronger in populations where medical care is not free of charge.
A strength of this study is the nationwide inclusion of patients identified in the LYFO, which is population-based and includes patients from all Danish hematological departments with a high coverage.13,14 This allows for an analysis of survival in an unselected population, reflecting clinical practice, and permits an adjustment for known confounders, such as age, sex, and socioeconomic position. The use of population-based registries minimizes loss to follow-up and reduces the risk of recall and selection bias. However, comorbidity was measured using data from the DNPR, and therefore, only comorbidities severe enough to lead to a hospital contact are included in the analyses. Furthermore, there might be a risk of misclassification of outcome in terms of treatment intensity in case of erroneous registration. Registration in the LYFO is, however, mandatory, and data have been shown to be highly valid.14 We believe that the potential misclassification will have limited influence on the validity of our estimates or underestimate the true risk. Finally, the registries used are designed for administrative purposes, which limited our possibility to obtain more detailed clinical information. This leaves us with residual confounding since we are not able to adjust for factors, such as performance status and Ann Arbor staging. Especially in the question on survival depending on treatment intensity, these factors may influence the results, since they would have an impact on both exposure and outcome. However, it is not possible to predict in which way the results would differ. In daily practice, the higher the performance status, age, disease stage, and number of comorbidity, the less likely it is to receive salvage treatment. On the contrary, one could also imagine, especially for younger patients, salvage treatment being planned, since it is the only known curative treatment regimen regardless of the clinical factors.
The information in these registries were created for administrative purposes, independent of the study hypothesis, and were established before this study was conceptualized, limiting information bias and excluding recall and selection bias completely.
Despite these advantages, our sample size is small and, thus, leads to broader CIs, which illustrates the need for larger studies in this area. Furthermore, using historical data in the comparison of different treatment strategies is not optimal.25 Treatment recommendations should not be based on the results of observational data, and the aim of this study was merely to describe the outcome of different treatment strategies in the care of relapsed or refractory DLBCL. However, we have included ASCT as a time-dependent variable in order to take into account the risk of immortal time bias.
Conclusion
Our analysis is, to our knowledge, the first to compare survival according to treatment intensity in patients with relapsed or refractory DLBCL in the rituximab era. It is a retrospective, population-based, non-randomized study, which included all relevant patients in all age groups with relapsed or refractory DLBCL in Denmark in the defined period. Our data indicate that mortality is increased for patients treated with salvage regimens compared with non-salvage regimens, when age, comorbidity, educational level, and primary refractory disease are taken into account. The patients in the non-salvage group were older than in the salvage group, and for these patients, the less intensive treatment regimens might be the best choice. However, a 5-year OS of 17% for patients in the non-salvage group together with a 5-year OS of 18% for patients in the salvage group without ASCT indicates a hope of durable remissions, even if ASCT is not an option.
Acknowledgments
This work was supported by grants from The Danish Cancer Society Scientific Committee, The Danish Lymphoma Group, and The Højmosegaard Grant.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 4.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011(1):498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 6.Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146–171. doi: 10.1016/j.critrevonc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Gisselbrecht C. Is there any role for transplantation in the rituximab era for diffuse large B-cell lymphoma? Hematology Am Soc Hematol Educ Program. 2012;2012:410–416. doi: 10.1182/asheducation-2012.1.410. [DOI] [PubMed] [Google Scholar]
- 8.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mounier N, Canals C, Gisselbrecht C, et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European blood and marrow transplantation registry. Biol Blood Marrow Transplant. 2012;18(5):788–793. doi: 10.1016/j.bbmt.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Lukenbill J, Hill B. Relapsed/refractory diffuse large B-cell lymphoma: review of the management of transplant-eligible patients. Leuk Lymphoma. 2015;56(2):293–300. doi: 10.3109/10428194.2014.910660. [DOI] [PubMed] [Google Scholar]
- 11.Frederiksen BL, Dalton SO, Osler M, Steding-Jessen M, de Nully Brown P. Socioeconomic position, treatment, and survival of non-Hodgkin lymphoma in Denmark – a nationwide study. Br J Cancer. 2012;106(5):988–995. doi: 10.1038/bjc.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Rybicki L, Abounader DM. Association of socioeconomic status with autologous hematopoietic cell transplantation outcomes for lymphoma. Nat Publ Gr. 2016;51(9):1191–1196. doi: 10.1038/bmt.2016.107. [DOI] [PubMed] [Google Scholar]
- 13.Arboe B, Josefsson P, Jørgensen J, et al. Danish national lymphoma registry. Clin Epidemiol. 2016;8:577–581. doi: 10.2147/CLEP.S99470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arboe B, El-Galaly TC, Clausen MR, et al. The Danish national lymphoma registry: coverage and data quality. PLoS One. 2016;11(6):e0157999. doi: 10.1371/journal.pone.0157999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 Suppl):91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 18.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7 Suppl):103–105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 19.Therneau TM. A package for survival analysis in R. 2015. [Accessed December 7, 2018]. Available from: https://cran.r-project.org/package=survival.
- 20.Ripley B, Venables W. Modern Applied Statistics with S Fourth Edition. New York: Springer; 2002. [Accessed December 7, 2018]. Available from: http://www.stats.ox.ac.uk/pub/MASS4. [Google Scholar]
- 21.Smith SD, Bolwell BJ, Rybicki LA, et al. Comparison of outcomes after auto-SCT for patients with relapsed diffuse large B-cell lymphoma according to previous therapy with rituximab. Bone Marrow Transplant. 2011;46(2):262–266. doi: 10.1038/bmt.2010.95. [DOI] [PubMed] [Google Scholar]
- 22.Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1729–1736. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wildes TM, Augustin KM, Sempek D, et al. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(7):840–846. doi: 10.1016/j.bbmt.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Graf SA, Vaughn JE, Chauncey TR, et al. Comorbidities, alcohol use disorder, and age predict outcomes after autologous hematopoietic cell transplantation for Lymphoma. Biol Blood Marrow Transplant. 2016;22(9):1582–1587. doi: 10.1016/j.bbmt.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green SB, Byar DP. Using observational data from registries to compare treatments: the fallacy of omnimetrics. Stat Med. 1984;3(4):361–370. doi: 10.1002/sim.4780030413. [DOI] [PubMed] [Google Scholar]