Abstract
Background
The primary objective of this study was to evaluate the effect of specific direct and indirect factors that accounted, in trauma patients, for the development of acute respiratory distress syndrome (ARDS) and mortality in patients with ARDS.
Methods
We performed a retrospective cohort study of patients from the National Trauma Data Bank. Multilevel mixed-effects logistic regression was used with the development of ARDS as the primary and mortality in patients with ARDS as the secondary outcome measures. We compared trauma patients with versus without thoracic (direct) and extrathoracic (indirect) risk factors, using patient demographics, physiologic, and anatomic injury severity as covariates. Subset analysis was performed for patients with trauma-induced lung contusion (TILC) and for patients with minor (Injury Severity Score [ISS] ≤15) injury.
Results
A total of 2 998 964 patients were studied, of whom 28 597 developed ARDS. From 2011 to 2014, the incidence of ARDS decreased; however, mortality in patients with ARDS has increased. Predictors of ARDS included direct thoracic injury (TILC, multiple rib fractures, and flail chest), as well as indirect factors (increased age, male gender, higher ISS, lower Glasgow Coma Scale motor component score, history of cardiopulmonary or hematologic disease, and history of alcoholism or obesity). Patients with ARDS secondary to direct thoracic injury had a lower risk of mortality compared with patients with ARDS due to other mechanisms.
Discussion
Despite the decreasing incidence of trauma-induced ARDS, mortality in patients with ARDS has increased. Direct thoracic injury was the strongest predictor of ARDS. Knowing specific contributors to trauma-induced ARDS could help identify at-risk patients early in their hospitalization and mitigate the progression to ARDS and thereby mortality.
Level of evidence
Prognostic study, level III.
Keywords: acute respiratory distress syndrome, lung contusion, trauma, acute lung injury
Background
Worldwide, for people under 45, trauma is the leading cause of death and disability.1 In patients who develop multiple organ failure, acute respiratory distress syndrome (ARDS) is the most frequent manifestation. Despite improvements in respiratory intensive care during the past several decades, ARDS is still associated with substantial mortality (25%–60% ARDS alone and 50%–80% when associated with multiple organ failure), morbidity, and costs.2–10
Far fewer investigations have studied the risk factors and prognosis in patients with trauma-induced ARDS, as compared with medical or sepsis-affiliated ARDS.11 The vast majority of investigations that have studied trauma-induced ARDS were either single-institution studies and/or used pre-2007 data sources, prior to widespread adoption of ARDSNet (short for the multicenter ARDS Network) and fluid resuscitation best practices.1 12–24 Moreover, less is known regarding the risk factors for the development of ARDS in non-severely (Injury Severity Score [ISS] ≤15) injured trauma patients as these patients have frequently been excluded from previous studies.22 25–32 Furthermore, existing studies of predictors of trauma-induced ARDS have reported conflicting findings. One example is older age, which is a consistent risk factor for the development of ARDS; however, after accounting for injury severity and comorbidities, its independent association has been inconsistent.1 17 29 33 34 Additionally, reports of the relationship between trauma-induced ARDS and mortality have been inconsistent.1 3 13 35 Patients with ARDS have a higher raw mortality rate, but typically are also more severely injured and have additional comorbidities. Two retrospective single-institution studies called into question the effect of ARDS on trauma mortality, highlighting a key unknown in our field.13 35 The question of high mortality in patients with ARDS and its relation to the trauma itself or to extrathoracic factors (such as overall injury severity or patient comorbidities) remains unanswered.
Given the significant impact of ARDS on clinical outcomes and costs, recent calls have been made for large observational studies to assess the potential predictors for ARDS.36 A more complete understanding could facilitate early identification and prompt treatment to improve survival. In this study, we sought to evaluate predictors of trauma-induced ARDS and mortality in patients with ARDS, using a large national database of US trauma patients from 2009 through 2014. We hypothesized that trauma-induced ARDS occurs along the entire burden of injury severity, and direct thoracic risk factors (vs. extrathoracic risk factors) are most strongly associated with developing trauma-induced ARDS.
Methods
Data collection
The data source for this study was the American College of Surgeons National Trauma Data Bank (NTDB), which is the largest trauma registry in the USA.37 It includes patient and hospital data on traumatic injuries and clinical outcomes for more than 700 trauma centers.
Participants
Patient-level data were obtained from the NTDB from January 1, 2009 through December 31, 2014. The inclusion criteria were as follows: age ≥16 years, at least one valid International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) trauma code (range, 800–959.9), and admission to a US trauma center for definitive care. The exclusion criteria included patients with no signs of life at initial evaluation (emergency department [ED] systolic blood pressure [SBP]=0, pulse=0, Glasgow Coma Scale [GCS] score=3) or patients transferred for definitive care.
Measures
We used the NTDB data dictionary’s definition of acute lung injury (ALI)/ARDS.38 Briefly, the definition encompassed sudden severe lung failure characterized by an arterial oxygen tension:fractional inspired oxygen (PaO2:FiO2) ratio of <300 mm Hg, bilateral fluffy infiltrates seen on frontal chest radiography, and the absence of clearly demonstrable volume overload (as signified by pulmonary wedge pressure, if measured, of 18 mm Hg, or by a similar surrogate such as echocardiography). Because our study’s data period began before the adoption of the Berlin definition of ARDS in 2012, we elected to use the American-European consensus conference definition of ARDS.39
For rib fractures, we used the following ICD-9 codes: rib fracture not otherwise specified—807, 807.0, 807.00, 807.1, 807.10, 807.09, 807.19; one rib fracture—807.01, 807.11; two rib fractures—807.02, 807.12; three rib fractures—807.03, 807.13; four rib fractures—807.04, 807.14; five rib fractures—807.05, 807.15; six rib fractures—807.06, 807.16; seven rib fractures—807.07, 807.17; and eight or more rib fractures—807.08, 807.18. For flail chest, we used ICD-9 code 807.4. Trauma-induced lung contusion (TILC) was defined as patients having an Abbreviated Injury Scale (AIS) code that began with the prefix 4414.
We obtained information regarding patient characteristics, thoracic and extrathoracic risk factors, and comorbidities. Patient characteristics included age (at time of ED visit), gender, race and ethnicity, and insurance status. Age was categorized as 16 to 25, 26 to 45, 46 to 65, 66 to 75, and greater than 75 years. Race and ethnicity were categorized as white, black, Hispanic or Latino, and other. Insurance status was recorded as Medicaid, Medicare, private, and self-pay.
Direct thoracic risk factors included the number of rib fractures, presence of flail chest, and TILC. Indirect extrathoracic risk factors included preinjury alcohol use and drug (prescription and illicit) use, time (minutes) from dispatch of emergency medical services to arrival in the trauma bay, ISS, lowest ED SBP, respiratory rate, oxygen saturation, temperature, and GCS motor component score. Alcohol use was recorded as not tested, no-confirmed, below 0.08, and above 0.08. Drug use was recorded as not tested, no-confirmed, yes-prescription, and yes-illegal. Preinjury alcohol and drug use were of interest because it is possible that, due to concerns for withdrawal, these patients had prolonged hospital stays and were at higher than average risk for oversedation and pulmonary complications. The most aberrant ED vital signs during the first hour of ED arrival are a marker for disease severity, and therefore these were evaluated for their association with subsequent development of ARDS. GCS motor was used instead of GCS total as a large portion of the NTDB is missing GCS verbal, and therefore it is common practice when conducting NTDB observational studies to use GCS motor. A prolonged time from dispatch of emergency medical service to arrival in the trauma bay may be a marker for rurality or prolonged prehospital interventions, signifying more severely injured patients, and therefore was included in the analysis. Finally, ISS is a marker of disease severity and thus of interest.
Comorbidities of interest included medical history of alcoholism,20 40 bleeding disorder,1 14 32 congestive heart failure (CHF),41 42 current smoker,40 diabetes mellitus,43 44 history of angina within 30 days,43 history of myocardial infarction,41 43 hypertension requiring medications,44 obesity,16 24 and history of respiratory disease.45
Statistical methods
A retrospective cohort study was performed using multilevel logistic regression to ascertain factors associated with the development of ARDS. For descriptive purposes, data were expressed as the mean and SD for continuous variables and as percentages for categorical variables. Student’s t-tests and Pearson χ2 tests were used in the preliminary analyses. For our main analysis, the primary outcome was development of ARDS. A multilevel mixed-effects logistic model was used, which accounted for hospital-level random effects. To account for missing data, we used the multiple imputation (mi) suite of commands, with five imputations for each missing value. We excluded variables with more than 35% missing values.46 To assess for potential effect modification, we considered all clinically relevant pairwise interaction terms, that is, TILC and history of respiratory disease. Subgroup analysis included stratification by TILC. We set alpha at 0.05, two-tailed. To assess model performance, we used the C-statistic. For all statistical analyses, we used Stata MP V.15.
The secondary outcome was all-cause in-hospital mortality. Predictors of mortality in patients with ARDS were investigated by generating a multilevel mixed-effects logistic model with hospital-level random effects.
Results
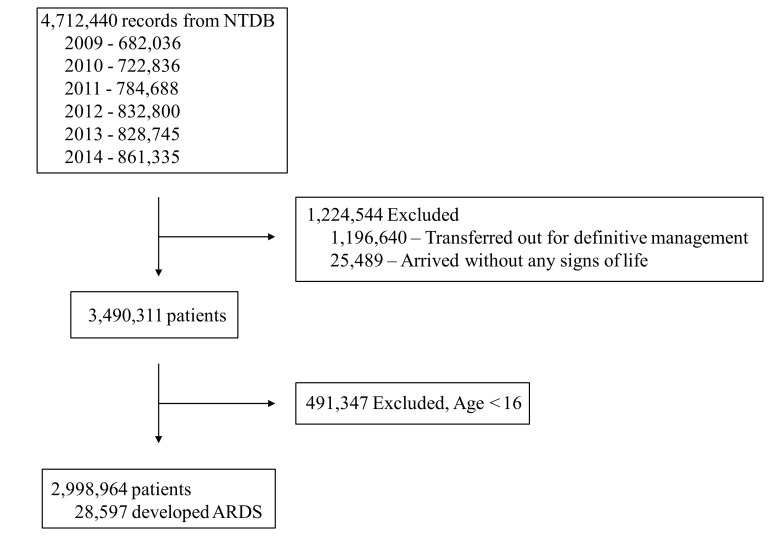
Within the 2009 to 2014 NTDB database, records were available for 4 712 440 patients (figure 1). After we applied our inclusion and exclusion criteria, 2 998 964 patients were eligible for the study, of whom 28 597 (1%) developed ARDS. Of these patients, 5888 had an ISS ≤10, 8011 had an ISS ≤15, and 20 586 had an ISS >15. The incidence of trauma-induced ARDS was greatest during 2009 to 2011 (1.3%–1.4%) and decreased thereafter to 0.5% in 2014. Yet mortality in patients with ARDS increased during the study period, from 21.2% in 2009 to 27.8% in 2014.
Figure 1.
Study diagram detailing the selection of patients in the 2009 to 2014 National Trauma Data Bank. ARDS, acute respiratory distress syndrome; NTDB, National Trauma Data Bank.
The characteristics of patients who developed ARDS are shown in table 1. The age category with the highest percentage of ARDS was 46 to 65 years. Men were considerably more likely to develop ARDS than women, with 73.5% of the cases. Whites were more likely to develop ARDS, as were patients with Medicaid and private insurance. Extrathoracic risk factors associated with ARDS were acute intoxication on arrival in the trauma bay, preinjury illegal and prescription drug use, higher ISS, ED hypotension, ED hypothermia, and lower GCS motor component score. Thoracic risk factors that were more prevalent in patients with ARDS were multiple rib fractures, TILC, and flail chest. Comorbidities associated with ARDS included history of chronic alcoholism, cardiopulmonary disease, hematologic disease, obesity, and diabetes mellitus. Patients with (vs. without) ARDS were less likely to currently smoke.
Table 1.
Characteristics of patients who developed ARDS
| No ARDS | ARDS | P value | |
| Demographics | |||
| Age, n (%) | |||
| 16–25 | 536 163 (19.7) | 5109 (17.9) | <0.001 |
| 26–45 | 757 781 (27.8) | 7654 (26.8) | |
| 46–65 | 743 021 (27.3) | 8887 (31.1) | |
| 66–75 | 257 950 (9.5) | 3063 (10.7) | |
| >75 | 428 854 (15.7) | 3884 (13.6) | |
| Male, n (%) | 1 710 390 (62.8) | 21 002 (73.5) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 1 797 453 (70.4) | 19 879 (72.9) | |
| Black | 411 673 (16.1) | 4525 (16.6) | |
| Hispanic or Latino | 261 519 (10.3) | 2001 (7.3) | |
| Other | 81 848 (3.2) | 859 (3.2) | |
| Insurance, n (%) | <0.001 | ||
| Medicaid | 343 047 (17.4) | 4238 (19.8) | |
| Medicare | 613 508 (31.1) | 6025 (28.1) | |
| Private | 539 732 (27.4) | 6619 (30.9) | |
| Self-pay | 476 434 (24.2) | 4554 (21.2) | |
| Extrathoracic risk factors | |||
| EtOH, n (%) | <0.001 | ||
| Not tested | 1 195 385 (49.4) | 9821 (38.4) | |
| No-confirmed | 741 053 (30.6) | 9387 (36.7) | |
| Below 0.08 | 128 126 (5.3) | 1511 (5.9) | |
| Above 0.08 | 356 037 (14.7) | 4838 (18.9) | |
| Drugs, n (%) | <0.001 | ||
| Not tested | 1 495 965 (68.0) | 10 859 (51.0) | |
| No-confirmed | 340 618 (15.5) | 4472 (21.0) | |
| Yes-prescription | 87 440 (4.0) | 1520 (7.1) | |
| Yes-illegal | 274 913 (12.5) | 4426 (20.8) | |
| Injury Severity Score, mean (SD) | 10.9 (8.7) | 23.3 (13.3) | <0.001 |
| Lowest ED SBP, mean (SD) | 138.7 (28.6) | 130.5 (36.6) | <0.001 |
| Temperature (°C), mean (SD) | 36.5 (1.7) | 36.1 (2.1) | <0.001 |
| GCS motor score, mean (SD) | 5.7 (1.1) | 4.3 (2.2) | <0.001 |
| Thoracic risk factors | |||
| Presence of rib fracture, n (%) | <0.001 | ||
| No rib fracture | 2 330 056 (85.6) | 17 395 (60.1) | |
| 1 rib fracture | 86 130 (3.2) | 1681 (5.9) | |
| 2 rib fractures | 60 549 (2.2) | 1189 (4.2) | |
| 3 rib fractures | 46 196 (1.7) | 784 (2.7) | |
| 4 rib fractures | 48 317 (1.8) | 1229 (4.3) | |
| 5 rib fractures | 26 295 (1.0) | 647 (2.3) | |
| 6 rib fractures | 19 593 (0.7) | 568 (2.0) | |
| 7 rib fractures | 13 873 (0.5) | 491 (1.7) | |
| ≥8 rib fractures | 31 803 (1.2) | 1615 (5.7) | |
| Rib fracture NOS | 46 745 (1.7) | 1759 (6.2) | |
| Flail chest | 14 212 (0.5) | 1239 (4.3) | |
| Trauma-induced lung contusion, n (%) | 151 216 (5.6) | 6182 (21.6) | <0.001 |
| Comorbidities, n (%) | |||
| Alcoholism | 215 944 (8.2) | 4427 (16.5) | <0.001 |
| Bleeding disorder | 117 319 (4.5) | 2264 (8.4) | <0.001 |
| Congestive heart failure | 73 357 (2.8) | 1623 (6.1) | <0.001 |
| Current smoker | 368 726 (14.0) | 3622 (13.5) | 0.02 |
| Diabetes mellitus | 282 705 (10.8) | 3771 (14.1) | <0.001 |
| History of angina within 30 days | 4186 (0.2) | 292 (1.1) | <0.001 |
| History of myocardial infarction | 31 434 (1.2) | 673 (2.5) | <0.001 |
| Hypertension requiring medications | 689 740 (26.2) | 8471 (31.6) | <0.001 |
| Obesity | 125 396 (4.8) | 2415 (9.0) | <0.001 |
| History of respiratory disease | 185 996 (7.1) | 3057 (11.4) | <0.001 |
ARDS, acute respiratory distress syndrome; ED, emergency department; EtOH, preinjury alcohol consumption; GCS, Glasgow Coma Scale; NOS, not otherwise specified; SBP, systolic blood pressure.
Risk factors for ARDS
On multivariable analysis, patients with ARDS were more likely to have the following risk factors (per the various categories): increased age, male gender, white race, and Medicaid insurance (patient characteristics); pre-injury illegal drug use, higher ISS, and lower GCS motor component score (extrathoracic); multiple rib fractures, TILC, and flail chest (direct thoracic); and history of chronic alcoholism, cardiopulmonary disease, hematologic disease, obesity, and diabetes mellitus (comorbidities) (table 2). The top three predictors for ARDS were flail chest (OR 2.6, 95% CI 2.42 to 2.80, p<0.001), history of angina within 30 days (OR 2.21, 95% CI 1.93 to 2.53, p<0.001), and history of CHF (OR 1.87, 95% CI 1.76 to 1.99, p<0.001). Patients with (vs. without) ARDS were less likely to currently smoke. ED hypotension, ED hypothermia, and preinjury prescription drug use were not associated with the development of ARDS.
Table 2.
Multivariable analysis of predictors for acute respiratory distress syndrome in trauma patients*
| OR | 95% CI | P value | |
| Demographics | |||
| Age | |||
| 16–25 | Baseline | ||
| 26–45 | 1.06 | 1.02 to 1.10 | 0.002 |
| 46–65 | 1.19 | 1.15 to 1.24 | <0.001 |
| 66–75 | 1.43 | 1.34 to 1.52 | <0.001 |
| >75 | 1.48 | 1.39 to 1.58 | <0.001 |
| Male | 1.35 | 1.31 to 1.39 | <0.001 |
| Race | |||
| White | Baseline | ||
| Black | 0.96 | 0.93 to 1.00 | 0.046 |
| Hispanic or Latino | 0.91 | 0.86 to 0.97 | 0.003 |
| Other | 1.05 | 0.97 to 1.13 | 0.2 |
| Insurance | |||
| Medicaid | Baseline | ||
| Medicare | 0.89 | 0.84 to 0.94 | <0.001 |
| Private | 0.93 | 0.89 to 0.96 | <0.001 |
| Self-pay | 0.71 | 0.67 to 0.75 | <0.001 |
| Extrathoracic risk factors | |||
| EtOH | |||
| No-confirmed | Baseline | ||
| Not tested | 0.75 | 0.71 to 0.78 | <0.001 |
| Below 0.08 | 0.90 | 0.85 to 0.96 | 0.001 |
| Above 0.08 | 0.86 | 0.82 to 0.89 | <0.001 |
| Drugs | |||
| No-confirmed | Baseline | ||
| Not tested | 0.77 | 0.72 to 0.83 | <0.001 |
| Yes-prescription | 1.05 | 0.98 to 1.11 | 0.1 |
| Yes-illegal | 1.27 | 1.20 to 1.35 | <0.001 |
| Injury Severity Score | 1.05 | 1.05 to 1.05 | <0.001 |
| Lowest ED systolic blood pressure | 1.00 | 1.00 to 1.00 | 0.7 |
| Temperature | 1.00 | 1.00 to 1.01 | 0.3 |
| GCS motor component score | 0.73 | 0.72 to 0.73 | <0.001 |
| Thoracic risk factors | |||
| Trauma-induced lung contusion | 1.46 | 1.40 to 1.51 | <0.001 |
| Traumatic rib fracture | |||
| No rib fractures | Baseline | ||
| 1 rib fracture | 1.45 | 1.37 to 1.54 | <0.001 |
| 2 rib fractures | 1.46 | 1.37 to 1.56 | <0.001 |
| 3 rib fractures | 1.31 | 1.21 to 1.41 | <0.001 |
| 4 rib fractures | 1.50 | 1.40 to 1.60 | <0.001 |
| 5 rib fractures | 1.55 | 1.42 to 1.69 | <0.001 |
| 6 rib fractures | 1.71 | 1.55 to 1.87 | <0.001 |
| 7 rib fractures | 1.83 | 1.65 to 2.02 | <0.001 |
| ≥8 rib fractures | 1.84 | 1.73 to 1.95 | <0.001 |
| Rib fracture (NOS) | 1.80 | 1.70 to 1.90 | <0.001 |
| Flail chest | 2.60 | 2.42 to 2.80 | <0.001 |
| Comorbidities | |||
| Alcoholism | 1.72 | 1.65 to 1.80 | <0.001 |
| Bleeding disorder | 1.62 | 1.53 to 1.70 | <0.001 |
| Congestive heart failure | 1.87 | 1.76 to 1.99 | <0.001 |
| Current smoker | 0.92 | 0.88 to 0.96 | <0.001 |
| Diabetes mellitus | 1.19 | 1.14 to 1.24 | <0.001 |
| History of angina within 30 days | 2.21 | 1.93 to 2.53 | <0.001 |
| History of myocardial infarction | 1.14 | 1.04 to 1.25 | 0.005 |
| Hypertension requiring medications | 1.25 | 1.21 to 1.30 | <0.001 |
| Obesity | 1.64 | 1.56 to 1.72 | <0.001 |
| History of respiratory disease | 1.69 | 1.62 to 1.76 | <0.001 |
*C-statistic=0.85.
ED, emergency department; EtOH, preinjury alcohol consumption; GCS, Glasgow Coma Scale; NOS, not otherwise specified.
Sensitivity and subgroup analyses
Risk factors for ARDS in patients with TILC
A subgroup analysis was conducted evaluating the risk factors for the development of ARDS in patients with TILC (table 3). In patients with TILC, five or fewer rib fractures no longer represented a significant risk factors for the development of trauma-induced ARDS. For the remainder of risk factors, associations were similar for the development of trauma-induced ARDS in patients with TILC (vs. without) (table 3).
Table 3.
Multivariable analysis of predictors for acute respiratory distress syndrome in patients with trauma-induced lung contusion*
| OR | 95% CI | P value | |
| Demographics | |||
| Age | |||
| 16–25 | Baseline | ||
| 26–45 | 1.05 | 0.97 to 1.13 | 0.2 |
| 46–65 | 1.15 | 1.05 to 1.25 | 0.002 |
| 66–75 | 1.36 | 1.18 to 1.57 | <0.001 |
| >75 | 1.58 | 1.35 to 1.84 | <0.001 |
| Male | 1.31 | 1.23 to 1.40 | <0.001 |
| Race | |||
| White | Baseline | ||
| Black | 0.96 | 0.88 to 1.05 | 0.4 |
| Hispanic or Latino | 0.97 | 0.86 to 1.09 | 0.6 |
| Other | 1.00 | 0.85 to 1.19 | 0.96 |
| Insurance | |||
| Medicaid | Baseline | ||
| Medicare | 0.85 | 0.76 to 0.96 | 0.008 |
| Private | 0.94 | 0.86 to 1.02 | 0.2 |
| Self-pay | 0.72 | 0.64 to 0.81 | <0.001 |
| Extrathoracic risk factors | |||
| EtOH | |||
| No-confirmed | Baseline | ||
| Not tested | 0.90 | 0.82 to 0.99 | 0.03 |
| Below 0.08 | 0.84 | 0.74 to 0.96 | 0.01 |
| Above 0.08 | 0.93 | 0.85 to 1.01 | 0.09 |
| Drugs | |||
| No-confirmed | Baseline | ||
| Not tested | 0.83 | 0.75 to 0.92 | 0.001 |
| Yes-prescription | 1.06 | 0.94 to 1.21 | 0.3 |
| Yes-illegal | 1.15 | 1.05 to 1.26 | 0.003 |
| Injury Severity Score | 1.04 | 1.04 to 1.04 | <0.001 |
| Lowest ED systolic blood pressure | 1.00 | 1.00 to 1.00 | 0.5 |
| Temperature | 1.01 | 1.00 to 1.02 | 0.2 |
| GCS motor score | 0.79 | 0.78 to 0.81 | <0.001 |
| Thoracic risk factors | |||
| Traumatic rib fracture | |||
| No rib fractures | Baseline | ||
| 1 rib fracture | 1.09 | 0.98 to 1.21 | 0.1 |
| 2 rib fractures | 1.08 | 0.96 to 1.21 | 0.2 |
| 3 rib fractures | 0.85 | 0.74 to 0.99 | 0.04 |
| 4 rib fractures | 1.16 | 1.03 to 1.30 | 0.1 |
| 5 rib fractures | 1.15 | 0.99 to 1.34 | 0.06 |
| 6 rib fractures | 1.35 | 1.16 to 1.56 | <0.001 |
| 7 rib fractures | 1.50 | 1.28 to 1.76 | <0.001 |
| ≥8 rib fractures | 1.51 | 1.37 to 1.67 | <0.001 |
| Rib fracture (NOS) | 1.39 | 1.26 to 1.55 | <0.001 |
| Flail chest | 2.09 | 1.87 to 2.33 | <0.001 |
| Comorbidities | |||
| Alcoholism | 1.53 | 1.38 to 1.68 | <0.001 |
| Bleeding disorder | 1.79 | 1.56 to 2.05 | <0.001 |
| Congestive heart failure | 1.42 | 1.17 to 1.73 | <0.001 |
| Current smoker | 0.97 | 0.89 to 1.05 | 0.4 |
| Diabetes mellitus | 1.30 | 1.17 to 1.44 | <0.001 |
| History of angina within 30 days | 1.82 | 1.16 to 2.83 | 0.009 |
| History of myocardial infarction | 1.01 | 0.77 to 1.31 | 0.96 |
| Hypertension requiring medications | 1.24 | 1.15 to 1.35 | <0.001 |
| Obesity | 1.77 | 1.60 to 1.95 | <0.001 |
| History of respiratory disease | 1.53 | 1.37 to 1.70 | <0.001 |
*C-statistic=0.85.
ED, emergency department; EtOH, preinjury alcohol consumption; GCS, Glasgow Coma Scale; NOS, not otherwise specified.
Risk factors for ARDS in patients with minor versus major trauma
Patients who developed ARDS were dichotomized into two groups, patients with minor injury (ISS ≤15) compared with patients with major (ISS >15) injury. Risk factors for ARDS were significantly different between the patients with minor and major trauma. In general, patients who developed ARDS after major trauma were more likely to have direct thoracic injury (multiple rib fractures [p<0.001], flail chest [p<0.001], or lung contusions [p<0.001]) or traumatic brain injury (TBI) (p<0.001). Patients who developed ARDS after minor trauma were more likely to be older (>46 years old, p0.006), have Medicare insurance (p=0.047), be legally intoxicated at the time of their injury (p<0.001), and more likely to have multiple comorbidities (p≤0.009) (table 4).
Table 4.
Multivariable analysis of predictors for acute respiratory distress syndrome in patients with major (ISS >16) vs. minor (ISS ≤15) trauma
| OR | 95% CI | P value | |
| Demographics | |||
| Age | |||
| 16–25 | Baseline | ||
| 26–45 | 1.0 | 0.87 to 1.07 | 0.5 |
| 46–65 | 0.83 | 0.74 to 0.92 | <0.001 |
| 66–75 | 0.80 | 0.68 to 0.93 | 0.01 |
| >75 | 0.75 | 0.63 to 0.9 | <0.001 |
| Male | 1.14 | 1.05 to 1.2 | <0.001 |
| Race | |||
| White | Baseline | ||
| Black | 1.11 | 1.001 to 1.21 | 0.05 |
| Hispanic or Latino | 0.98 | 0.82 to 1.18 | 0.9 |
| Other | 1.09 | 0.95 to 1.25 | 0.2 |
| Insurance | |||
| Medicaid | Baseline | ||
| Medicare | 0.76 | 0.66 to 0.87 | <0.001 |
| Private | 1.12 | 0.97 to 1.29 | 0.1 |
| Self-pay | 0.93 | 0.82 to 1.05 | 0.2 |
| Extrathoracic risk factors | |||
| EtOH | |||
| No-confirmed | Baseline | ||
| Not tested | 0.56 | 0.51 to 0.61 | <0.001 |
| Below 0.08 | 0.86 | 0.74 to 0.99 | 0.04 |
| Above 0.08 | 0.79 | 0.71 to 0.89 | <0.001 |
| Drugs | |||
| No-confirmed | Baseline | ||
| Not tested | 0.84 | 0.75 to 0.95 | 0.01 |
| Yes-prescription | 0.73 | 0.62 to 0.87 | <0.001 |
| Yes-illegal | 0.87 | 0.78 to 0.97 | 0.02 |
| Lowest ED systolic blood pressure | 0.99 | 0.99 to 0.99 | <0.001 |
| Temperature | 0.93 | 0.91 to 0.95 | <0.001 |
| GCS motor score | 0.78 | 0.76 to 0.79 | <0.001 |
| Thoracic risk factors | |||
| Trauma-induced lung contusion | 2.16 | 1.96 to 2.38 | <0.001 |
| Traumatic rib fracture | |||
| No rib fractures | Baseline | ||
| 1 rib fracture | 2.20 | 1.89 to 2.56 | <0.001 |
| 2 rib fractures | 1.80 | 1.55 to 2.07 | <0.001 |
| 3 rib fractures | 1.69 | 1.43 to 1.98 | <0.001 |
| 4 rib fractures | 2.30 | 1.88 to 2.8 | <0.001 |
| 5 rib fractures | 3.40 | 2.8 to 4.1 | <0.001 |
| 6 rib fractures | 3.49 | 2.7 to 4.44 | <0.001 |
| 7 rib fractures | 3.42 | 2.63 to 4.44 | <0.001 |
| ≥8 rib fractures | 4.41 | 3.23 to 6.01 | <0.001 |
| Rib fracture (NOS) | 5.36 | 4.40 to 6.51 | <0.001 |
| Flail chest | 8.48 | 6.33 to 11.34 | <0.001 |
| Comorbidities | |||
| Alcoholism | 0.71 | 0.64 to 0.78 | <0.001 |
| Bleeding disorder | 1.28 | 1.13 to 1.44 | <0.001 |
| Congestive heart failure | 0.69 | 0.59 to 0.79 | <0.001 |
| Current smoker | 0.87 | 0.78 to 0.96 | 0.01 |
| Diabetes mellitus | 0.99 | 0.89 to 1.08 | 0.77 |
| History of angina within 30 days | 0.88 | 0.66 to 1.15 | 0.36 |
| History of myocardial infarction | 0.76 | 0.62 to 0.91 | <0.001 |
| Hypertension requiring medications | 0.87 | 0.8 to 0.94 | <0.001 |
| Obesity | 0.82 | 0.73 to 0.91 | <0.001 |
| History of respiratory disease | 0.60 | 0.55 to 0.66 | <0.001 |
ED, emergency department; EtOH, pre-injury alcohol consumption; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; NOS, not otherwise specified.
Independent predictors of mortality
Of the 28 597 patients with ARDS, 6221 (22%) subsequently died while hospitalized. On multivariable analysis, patients who died were more likely to be older and male (table 5). Age was the strongest predictor of mortality identified. Extrathoracic predictors of mortality included higher ISS, ED hypothermia, and lower GCS motor component score. Comorbidities associated with mortality were history of CHF, history of bleeding disorder, and current diabetes mellitus. In patients who died (vs. did not die) while hospitalized, acute intoxication and use of prescription or illegal drugs were more common in those patients who did not die while hospitalized. In addition, TILC (OR 0.91, 95% CI 0.84 to 0.99, p=0.025), one or more rib fracture (OR range 0.68–0.76, p<0.007), and flail chest (OR 0.56, 95% CI 0.48 to 0.66, p<0.001) were less frequent in patients who died. Patients with (vs. without) hypertension requiring medications were more likely to survive.
Table 5.
Predictors of mortality for patients with acute respiratory distress syndrome after trauma*
| OR | 95% CI | P value | |
| Demographics | |||
| Age | |||
| 16–25 | Baseline | ||
| 26–45 | 1.20 | 1.08 to 1.33 | 0.001 |
| 46–65 | 2.10 | 1.89 to 2.34 | <0.001 |
| 66–75 | 3.60 | 3.08 to 4.20 | <0.001 |
| >75 | 6.73 | 5.79 to 7.81 | <0.001 |
| Male | 1.12 | 1.03 to 1.21 | 0.005 |
| Race | |||
| White | Baseline | ||
| Black | 1.08 | 0.98 to 1.18 | 0.1 |
| Hispanic or Latino | 1.04 | 0.92 to 1.19 | 0.5 |
| Other | 0.90 | 0.75 to 1.09 | 0.3 |
| Insurance | |||
| Medicaid | Baseline | ||
| Medicare | 1.35 | 1.19 to 1.52 | <0.001 |
| Private | 0.93 | 0.84 to 1.03 | 0.1 |
| Self-pay | 1.71 | 1.52 to 1.93 | <0.001 |
| Extrathoracic risk factors | |||
| EtOH | |||
| No-confirmed | Baseline | ||
| Not tested | 0.97 | 0.88 to 1.06 | 0.5 |
| Below 0.08 | 0.96 | 0.83 to 1.12 | 0.6 |
| Above 0.08 | 0.73 | 0.66 to 0.82 | <0.001 |
| Drugs | |||
| No-confirmed | Baseline | ||
| Not tested | 1.07 | 0.96 to 1.18 | 0.2 |
| Yes-prescription | 0.71 | 0.60 to 0.83 | <0.001 |
| Yes-illegal | 0.83 | 0.74 to 0.93 | 0.002 |
| Injury Severity Score | 1.05 | 1.04 to 1.05 | <0.001 |
| Lowest ED systolic blood pressure | 1.00 | 1.00 to 1.00 | <0.001 |
| Temperature | 0.96 | 0.95 to 0.98 | <0.001 |
| GCS motor score | 0.79 | 0.78 to 0.81 | <0.001 |
| Thoracic risk factors | |||
| Trauma-induced lung contusion | 0.91 | 0.84 to 0.99 | 0.025 |
| Traumatic rib fracture | |||
| No rib fractures | Baseline | ||
| 1 rib fracture | 0.68 | 0.59 to 0.78 | <0.001 |
| 2 rib fractures | 0.73 | 0.61 to 0.88 | 0.001 |
| 3 rib fractures | 0.64 | 0.52 to 0.78 | <0.001 |
| 4 rib fractures | 0.81 | 0.69 to 0.94 | 0.007 |
| 5 rib fractures | 0.57 | 0.46 to 0.71 | <0.001 |
| 6 rib fractures | 0.54 | 0.42 to 0.68 | <0.001 |
| 7 rib fractures | 0.43 | 0.33 to 0.56 | <0.001 |
| ≥8 rib fractures | 0.51 | 0.44 to 0.59 | <0.001 |
| Rib fracture (NOS) | 0.76 | 0.66 to 0.87 | <0.001 |
| Flail chest | 0.56 | 0.48 to 0.66 | <0.001 |
| Comorbidities | |||
| Alcoholism | 0.92 | 0.82 to 1.03 | 0.2 |
| Bleeding disorder | 1.50 | 1.34 to 1.68 | <0.001 |
| Congestive heart failure | 1.22 | 1.07 to 1.39 | 0.004 |
| Current smoker | 0.78 | 0.70 to 0.88 | <0.001 |
| Diabetes mellitus | 1.17 | 1.06 to 1.29 | 0.001 |
| History of angina within 30 days | 0.97 | 0.73 to 1.30 | 0.9 |
| History of myocardial infarction | 1.18 | 0.98 to 1.42 | 0.09 |
| Hypertension requiring medications | 0.90 | 0.83 to 0.97 | 0.009 |
| Obesity | 1.00 | 0.87 to 1.13 | 0.9 |
| History of respiratory disease | 1.08 | 0.96 to 1.20 | 0.2 |
*C-statistic=0.94.
ED, emergency department; EtOH, preinjury alcohol consumption; GCS, Glasgow Coma Scale; NOS, not otherwise specified.
Discussion
To our knowledge, ours is the largest study evaluating the predictors of developing ARDS and mortality in trauma patients, and one of a few studies evaluating trauma-induced ARDS across multiple institutions using post-2007 data.1 12–24 Furthermore, this is one of the few studies investigating ARDS in patients with an ISS ≤15. Using a large national trauma database, we identified and characterized patients who developed ARDS after a traumatic injury. Our key findings are as follows. First, the overall incidence of ARDS has decreased; however, mortality in patients with ARDS has actually increased in trauma patients. Second, ARDS is not a disease process of severely injured patients alone; in our study, nearly a third of the patients with ARDS had an ISS ≤15. Third, the strongest risk factors for the development of ARDS were direct thoracic injury and a history of cardiopulmonary or hematologic disease and alcoholism. Fourth, although TILC was an independent risk factor for the development of ARDS, on subgroup analysis, patients with or without TILC had relatively similar risk factors for the development of ARDS (table 3). Fifth and finally, mortality in patients with ARDS was due not to direct thoracic injury but rather to patient-level factors such as older age, male gender, more severe injury, and certain comorbidities (table 5).
In our study, we identified a 1% overall incidence of ARDS in US trauma centers. Some reports have indicated that the incidence has remained unchanged during the past decade. But according to the majority of the literature, the incidence of trauma-induced ARDS has decreased—a finding that aligns with our clinical experience and findings in this study.23 47–49 The decreased incidence is likely due to improved resuscitative and critical care strategies developed during the past two decades. Key areas of clinical improvement include widespread adoption of lung-protective strategies for mechanical ventilation and of restrictive fluid strategies for resuscitation. Our data strengthen the claim that trauma-induced ARDS incidence is decreasing over time in the USA.
ARDS is not a disease process of just the severely injured; we observed nearly one-third of patients who developed ARDS had an ISS ≤15. Given this finding, we included patients with an ISS ≤15 in our analysis, which also accounts for the low incidence of ARDS identified in this study. This finding is important, as previous studies on trauma-induced ARDS have excluded this population from analysis.22 25–32 Risk factors were significantly different for developing ARDS in patients with minor (vs. major) injury. Patients who developed ARDS with major injury were more likely to have direct thoracic injury, perhaps contributing to their elevated ISS via an increased AIS chest score. Surprisingly, patients who developed ARDS with minor injuries were more likely to be older (>46 years old) and have multiple comorbidities. There is much interest in better understanding the role patient comorbidities play in the development and management of patients with ARDS, as nearly all ARDS clinical trials and epidemiologic studies since 1998 have excluded patients with major comorbidities.50 In this study we identified that patients with a history of alcoholism, bleeding disorders, and cardiopulmonary comorbidities after minor trauma were more likely to develop trauma-induced ARDS than patients after major trauma (table 4). Future research is needed to investigate the effect of patient comorbidities on the development of trauma ARDS and how the presence of specific patient comorbidities influences current ARDS prevention strategies.
Central to this study’s objective, we have identified that the direct thoracic factors—flail chest, multiple rib fractures, and TILC—are significant contributors to the development of ARDS. Multiple studies have documented an inconsistent correlation between the volume of TILC and the development of ARDS.51 These suggest interactions with other factors occurring around the time of injury may be involved in the development of ARDS. In our subgroup analysis, stratified by TILC, we found that patients with or without TILC had relatively similar risk factors for the development of ARDS. The presence of significant injury burden, represented by an elevated ISS and low GCS motor component score, was identified as an important extrathoracic predictor of ARDS. It is likely that contribution from a robust systemic inflammatory response resulted in the deterioration of lung function, as observed with experimental data in animals and in human subjects with additional risk factors.52 53 Similarly, the possibility of additional insults to the lung such as aspiration-induced lung injury seen frequently as a complication of low GCS score from TBI accounts for the deterioration of lung function to a full-blown ARDS. Our laboratory has shown in small animals a combination of aspiration-induced lung injury when added to TILC produces a synergistic increase in lung injury and inflammation.54 Additionally, elderly age, male sex, illegal drug use, history of alcoholism, bleeding disorder, cardiac history, obesity, and respiratory disease were the most significant predictors for the development of ARDS in trauma patients. These findings supplement those of previous studies that also aimed to identify risk factors in trauma patients.1 13 17 29 33 35 Previous studies have suffered from many limitations. For example, the majority of multi-institutional studies performed used pre-2007 accrual periods, before the widespread implementation of critical care practices devised by ARDSNet (short for the multicenter ARDS Network initiated by the National Heart, Lung, and Blood Institute of the National Institutes of Health), with the majority of studies published using post-2007 patient data being single institution in nature.1 12–24 Additionally, previous studies included a relatively limited number of patients with ARDS, as compared with our study’s 28 597 patients.1 13 17 29 33 35
In our study, 22% of the patients with ARDS died while hospitalized. The precise incidence of mortality in patients with ARDS has generated much controversy.13 15 35 55 We found that mortality in patients with ARDS has increased over time in the USA, strengthening the claim that mortality is not declining. Although ARDS is considered an independent risk factor for mortality in trauma patients, the contribution of associated injuries and comorbidities to mortality is unclear.13 35 Predictors of mortality in our study patients included extrathoracic risk factors as a higher ISS and a lower GCS motor component score. Patient characteristics associated with higher mortality included increased age, male gender, and non-Medicaid insurance status. Comorbidities associated with higher mortality include a history of bleeding disorder, CHF, and diabetes mellitus. We found that patients with ARDS secondary to direct thoracic injury (including TILC, flail chest, and rib fractures) had improved survival, as compared with ARDS due to other mechanisms. This finding suggests that the nature of the inflammatory response to direct thoracic injury (vs. to extrathoracic risk factors) is distinctly different. It is well known that in basic research of direct and indirect risk factors, the mechanisms, nature of inflammatory attribute, type of cellular and cytokine response, and healing are entirely different.56 57 We propose using the stratification of direct thoracic and extrathoracic risk factors as a methodology in the future to evaluate risk factors in studies involving trauma-induced ARDS.
This study has several limitations, the major one being its retrospective study design. We were also limited by the elements within the database, which did not include such relevant information as the units of blood products, the volume of crystalloid solutions, or the patient’s cause of death. Additionally, the NTDB does not differentiate between ALI and ARDS; thus, this study may overestimate the clinical burden of ARDS as it includes patients with a PaO2:FiO2 ratio of 200 to 300 mm Hg in the definition of ARDS. Although in-hospital mortality was reported, no follow-up information was available regarding patient survival after hospital discharge.
Conclusion
ARDS is a complex disease process occurring across the entire spectrum of injury burden. Despite the decreasing incidence of ARDS, mortality in patients with ARDS has increased. In our study, direct thoracic injury was the strongest predictor of trauma-induced ARDS. Distinct demographic, comorbid, extrathoracic, and thoracic predictors were identified for trauma-induced ARDS and mortality in patients with ARDS. The recognition of specific thoracic risk factors in trauma-induced ARDS has the potential to identify at-risk patients early in their hospitalization and mitigate the progression to ARDS and thereby mortality. Future research should be directed at implementing therapeutic strategies to mitigate the development of ARDS among subsets of trauma patients with identifiable risk factors.
Acknowledgments
The authors thank Mary Knatterud, PhD, medical editor with the University of Minnesota, Department of Surgery for proof-reading this article.
Footnotes
Presented at: This article was accepted for oral presentation in the Scientific Form program at the American College of Surgeons Clinical Congress, October 2018 meeting in Boston, Massachusetts.
Contributors: Concept and design: CJT, MAMR, KR. Acquisition, analysis, or interpretation of data: all authors. Drafting of the article: all authors. Critical revision of the article for important intellectual content: all authors. Statistical analysis: CJT, MAMR, MRH. Supervision: MAMR, MRH, KR.
Funding: KR was supported by R-01- HL102013 from the National Institutes of Health.
Competing interests: MRH receives support from Blue Cross Blue Shield of Michigan and Blue Care Network (a non-profit mutual company) for the conduct of the Michigan Trauma Quality Improvement Program with a Collaborative Quality Initiatives grant.
Patient consent for publication: Not required.
Ethics approval: The University of Michigan Medical School Institutional Review Board gave this study a determination of “not regulated” status. This study was approved by the University of Minnesota Institutional Review Board (STUDY00001489). The need for individual patient consent was waived because all data used in this study were already de-identified in the NTDB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg 2002;68:845–50. [PubMed] [Google Scholar]
- 2. Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma 2003;55:608–16. 10.1097/01.TA.0000092378.10660.D1 [DOI] [PubMed] [Google Scholar]
- 3. Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151(2 Pt 1):293–301. 10.1164/ajrccm.151.2.7842182 [DOI] [PubMed] [Google Scholar]
- 4. MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care 2005;11:43–9. 10.1097/00075198-200502000-00007 [DOI] [PubMed] [Google Scholar]
- 5. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–93. 10.1056/NEJMoa050333 [DOI] [PubMed] [Google Scholar]
- 6. Estenssoro E, Dubin A, Laffaire E, Canales H, Sáenz G, Moseinco M, Pozo M, Gómez A, Baredes N, Jannello G, et al. . Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med 2002;30:2450–6. 10.1097/00003246-200211000-00008 [DOI] [PubMed] [Google Scholar]
- 7. Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, et al. . The alien study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932–41. 10.1007/s00134-011-2380-4 [DOI] [PubMed] [Google Scholar]
- 8. Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, Raymondos K, Rios F, Nin N, Apezteguía C, et al. . Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220–30. 10.1164/rccm.201212-2169OC [DOI] [PubMed] [Google Scholar]
- 9. Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, Bernard GR, Matthay MA, Ware LB, Kangelaris KN. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 2014;40:388–96. 10.1007/s00134-013-3186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788–800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 11. Bakowitz M, Bruns B, McCunn M. Acute lung injury and the acute respiratory distress syndrome in the injured patient. Scand J Trauma Resusc Emerg Med 2012;20:54 10.1186/1757-7241-20-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bone LB, Johnson KD, Weigelt J, Scheinberg R. Early versus delayed stabilization of femoral fractures. A prospective randomized study. J Bone Joint Surg Am 1989;71:336–40. 10.2106/00004623-198971030-00004 [DOI] [PubMed] [Google Scholar]
- 13. Treggiari MM, Hudson LD, Martin DP, Weiss NS, Caldwell E, Rubenfeld G. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med 2004;32:327–31. 10.1097/01.CCM.0000108870.09693.42 [DOI] [PubMed] [Google Scholar]
- 14. Croce MA, Tolley EA, Claridge JA, Fabian TC. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma 2005;59:19–24. 10.1097/01.TA.0000171459.21450.DC [DOI] [PubMed] [Google Scholar]
- 15. Martin M, Salim A, Murray J, Demetriades D, Belzberg H, Rhee P. The decreasing incidence and mortality of acute respiratory distress syndrome after injury: a 5-year observational study. J Trauma 2005;59:1107–13. 10.1097/01.ta.0000188633.94766.d0 [DOI] [PubMed] [Google Scholar]
- 16. Plurad D, Martin M, Green D, Salim A, Inaba K, Belzberg H, Demetriades D, Rhee P. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: the potential role of lung protective ventilation and conservative transfusion practice. J Trauma 2007;63:1–8. 10.1097/TA.0b013e318068b1ed [DOI] [PubMed] [Google Scholar]
- 17. Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, Rivara FP. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009;110:351–60. 10.1097/ALN.0b013e3181948a97 [DOI] [PubMed] [Google Scholar]
- 18. DuBose JJ, Teixeira PG, Shiflett A, Trankiem C, Putty B, Recinos G, Inaba K, Belzberg H. American College of surgeons trauma centre designation and mechanical ventilation outcomes. Injury 2009;40:708–12. 10.1016/j.injury.2008.09.015 [DOI] [PubMed] [Google Scholar]
- 19. Haider AH, Crompton JG, Oyetunji T, Stevens KA, Efron DT, Kieninger AN, Chang DC, Cornwell EE, Haut ER. Females have fewer complications and lower mortality following trauma than similarly injured males: a risk adjusted analysis of adults in the National trauma data bank. Surgery 2009;146:308–15. 10.1016/j.surg.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 20. Aziz H, Siordia JA, Rhee P, Pandit V, O'Keeffe T, Kulvatunyou N, Joseph B. Analyzing the effects of alcohol on adolescent trauma using the National trauma data bank. J Trauma Acute Care Surg 2015;79:463–7. 10.1097/TA.0000000000000777 [DOI] [PubMed] [Google Scholar]
- 21. Vande Vusse LK, Caldwell E, Tran E, Hogl L, Dinwiddie S, López JA, Maier RV, Watkins TR. The epidemiology of Transfusion-related acute lung injury varies according to the applied definition of lung injury onset time. Ann Am Thorac Soc 2015;12:1328–35. 10.1513/AnnalsATS.201504-246OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reilly JP, Meyer NJ, Shashaty MGS, Feng R, Lanken PN, Gallop R, Kaplan S, Herlim M, Oz NL, Hiciano I, et al. . ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest 2014;145:753–61. 10.1378/chest.13-1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fahr M, Jones G, O'Neal H, Duchesne J, Tatum D. Acute respiratory distress syndrome incidence, but not mortality, has decreased nationwide: a National trauma data bank study. Am Surg 2017;83:323–31. [PubMed] [Google Scholar]
- 24. O'Leary MP, Keeley JA, Yule A, Suruki C, Plurad DS, Moazzez A, Neville AL, Putnam BA, Kim DY. Clinical predictors of early acute respiratory distress syndrome in trauma patients. Am J Surg 2016;212:1096–100. 10.1016/j.amjsurg.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 25. Hildebrand F, Stuhrmann M, van Griensven M, Meier S, Hasenkamp S, Krettek C, Pape H-C. Association of IL-8-251A/T polymorphism with incidence of acute respiratory distress syndrome (ARDS) and IL-8 synthesis after multiple trauma. Cytokine 2007;37:192–9. 10.1016/j.cyto.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 26. Hildebrand F, Kalmbach M, Kaapke A, Krettek C, Stuhrmann M. No association between CALCA polymorphisms and clinical outcome or serum procalcitonin levels in German polytrauma patients. Cytokine 2009;47:30–6. 10.1016/j.cyto.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 27. Ciesla DJ, Moore EE, Johnson JL, Cothren CC, Banerjee A, Burch JM, Sauaia A. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery 2006;140:640–8. 10.1016/j.surg.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 28. Dicker RA, Morabito DJ, Pittet JF, Campbell AR, Mackersie RC. Acute respiratory distress syndrome criteria in trauma patients: why the definitions do not work. J Trauma 2004;57:522–8. 10.1097/01.TA.0000135749.64867.06 [DOI] [PubMed] [Google Scholar]
- 29. Navarrete-Navarro P, Rivera-Fernández R, Rincón-Ferrari MD, García-Delgado M, Muñoz A, Jiménez JM, Ortega FJ, García DM, GITAN multicenter project . Early markers of acute respiratory distress syndrome development in severe trauma patients. J Crit Care 2006;21:253–8. 10.1016/j.jcrc.2005.12.012 [DOI] [PubMed] [Google Scholar]
- 30. Reilly JP, Bellamy S, Shashaty MG, Gallop R, Meyer NJ, Lanken PN, Kaplan S, Holena DN, May AK, Ware LB, et al. . Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc 2014;11:728–36. 10.1513/AnnalsATS.201308-280OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med 2008;36:2309–15. 10.1097/CCM.0b013e318180dc74 [DOI] [PubMed] [Google Scholar]
- 32. Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma 2005;59:717–23. [PubMed] [Google Scholar]
- 33. Watkins TR, Nathens AB, Cooke CR, Psaty BM, Maier RV, Cuschieri J, Rubenfeld GD. Acute respiratory distress syndrome after trauma: development and validation of a predictive model. Crit Care Med 2012;40:2295–303. 10.1097/CCM.0b013e3182544f6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manzano F, Yuste E, Colmenero M, Aranda A, García-Horcajadas A, Rivera R, Fernández-Mondéjar E. Incidence of acute respiratory distress syndrome and its relation to age. J Crit Care 2005;20:274–80. 10.1016/j.jcrc.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 35. Salim A, Martin M, Constantinou C, Sangthong B, Brown C, Kasotakis G, Demetriades D, Belzberg H. Acute respiratory distress syndrome in the trauma intensive care unit: Morbid but not mortal. Arch Surg 2006;141:655–8. 10.1001/archsurg.141.7.655 [DOI] [PubMed] [Google Scholar]
- 36. Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al. . Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med 2010;181:1121–7. 10.1164/rccm.201001-0024WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haider AH, Saleem T, Leow JJ, Villegas CV, Kisat M, Schneider EB, Haut ER, Stevens KA, Cornwell EE, MacKenzie EJ, et al. . Influence of the National trauma data bank on the study of trauma outcomes: is it time to set research best practices to further enhance its impact? J Am Coll Surg 2012;214:756–68. 10.1016/j.jamcollsurg.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliphant BW, Tignanelli CJ, Napolitano LM, Goulet JA, Hemmila MR. ACS-COT verification level affects trauma center management of pelvic ring injuries and patient mortality. J Trauma Acute Care Surg 2018. [DOI] [PubMed] [Google Scholar]
- 39. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European consensus Conference on ARDS. definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149(3 Pt 1):818–24. 10.1164/ajrccm.149.3.7509706 [DOI] [PubMed] [Google Scholar]
- 40. Moazed F, Calfee CS. Environmental risk factors for acute respiratory distress syndrome. Clin Chest Med 2014;35:625–37. 10.1016/j.ccm.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saguil A, Fargo M. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician 2012;85:352–8. [PubMed] [Google Scholar]
- 42. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 43. de Prost N, Pham T, Carteaux G, Mekontso Dessap A, Brun-Buisson C, Fan E, Bellani G, Laffey J, Mercat A, Brochard L, et al. . Etiologies, diagnostic work-up and outcomes of acute respiratory distress syndrome with no common risk factor: a prospective multicenter study. Ann Intensive Care 2017;7:69 10.1186/s13613-017-0281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Magazine R, Rao S, Chogtu B, Venkateswaran R, Shahul HA, Goneppanavar U. Epidemiological profile of acute respiratory distress syndrome patients: a tertiary care experience. Lung India 2017;34:38–42. 10.4103/0970-2113.197097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo-shou Z, Xiang-jun B, Cheng-ye Z. Analysis of high risk factors related to acute respiratory distress syndrome following severe thoracoabdominal injuries. Chin J Traumatol 2007;10:275–8. [PubMed] [Google Scholar]
- 46. Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley, 1987:14–278. [Google Scholar]
- 47. O'Toole RV, O'Brien M, Scalea TM, Habashi N, Pollak AN, Turen CH. Resuscitation before stabilization of femoral fractures limits acute respiratory distress syndrome in patients with multiple traumatic injuries despite low use of damage control orthopedics. J Trauma 2009;67:1013–21. 10.1097/TA.0b013e3181b890be [DOI] [PubMed] [Google Scholar]
- 48. Vallier HA, Wang X, Moore TA, Wilber JH, Como JJ. Timing of orthopaedic surgery in multiple trauma patients: development of a protocol for early appropriate care. J Orthop Trauma 2013;27:543–51. 10.1097/BOT.0b013e31829efda1 [DOI] [PubMed] [Google Scholar]
- 49. Pfeifer R, Heussen N, Michalewicz E, Hilgers RD, Pape HC. Incidence of adult respiratory distress syndrome in trauma patients: a systematic review and meta-analysis over a period of three decades. J Trauma Acute Care Surg 2017;83:496–506. 10.1097/TA.0000000000001571 [DOI] [PubMed] [Google Scholar]
- 50. Azoulay E, Lemiale V, Mourvillier B, Garrouste-Orgeas M, Schwebel C, Ruckly S, Argaud L, Cohen Y, Souweine B, Papazian L, et al. . Management and outcomes of acute respiratory distress syndrome patients with and without comorbid conditions. Intensive Care Med 2018;44:1050–60. 10.1007/s00134-018-5209-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock 2009;32:122–30. 10.1097/SHK.0b013e31819c385c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg 1982;144:124–30. 10.1016/0002-9610(82)90612-2 [DOI] [PubMed] [Google Scholar]
- 53. Perl M, Gebhard F, Brückner UB, Ayala A, Braumüller S, Büttner C, Kinzl L, Knöferl MW. Pulmonary contusion causes impairment of macrophage and lymphocyte immune functions and increases mortality associated with a subsequent septic challenge. Crit Care Med 2005;33:1351–8. 10.1097/01.CCM.0000166352.28018.A9 [DOI] [PubMed] [Google Scholar]
- 54. Raghavendran K BAD, Huebschmann JC, Helinski JD, Hutson AD, Dayton MT. Superimposed gastric aspiration increases the severity of inflammation and permeability injury in a rat model of lung contusion. J Surg Research:20081–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 2014;20:3–9. 10.1097/MCC.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 56. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379–L399. 10.1152/ajplung.00010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. B Moore B, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 2013;49:167–79. 10.1165/rcmb.2013-0094TR [DOI] [PMC free article] [PubMed] [Google Scholar]