Abstract
Context: Calenduloside E (CE), one of the primary natural products found in Aralia elata (Miq.) Seem. (Araliaceae), possesses prominent anti-apoptotic potential. A previous study found that one of the anti-apoptotic CE targets is heat shock protein 90 AB1 (Hsp90AB1) by probe CE-P, while the other targets of CE still need to be identified with more efficient probes.
Objective: This study investigates CE analogue (CEA) as one clickable activity-based probe for use in exploring anti-apoptotic CE targets.
Materials and methods: Pretreatment of HUVECs with CEA (1.25 μM) for 8 hr, followed by ox-LDL stimulation for 24 h. Flow cytometry analysis and JC-1 staining assays were performed The kinetic constant measurements were tested by the Biacore T200, CM5 Sensor Chip which was activated by using sulpho-NHS/EDC. Ligands were dissolved and injected with a concentration of 12.5, 6.25, 3.125, 1.56, 0.78 and 0 μM.
Results: CEA was confirmed to possess an anti-apoptotic effect. The probable targets of CE/CEA were calculated, and as one of the higher scores proteins (Fit values: 0.88/0.86), Hsp90 properly got our attention. Molecular modelling study showed that both CE and CEA could bind to Hsp90 with the similar interaction, and the docking scores (S value) were −7.61 and −7.33. SPR assay provided more evidence to prove that CEA can interact with Hsp90 with the KD value 11.7 µM.
Discussion and conclusions: Our results suggest that clickable probe CEA could alleviate ox-LDL induced apoptosis by a similar mechanism of anti-apoptotic CE, and afforded the possibility of identifying additional anti-apoptotic targets of CE.
Keywords: Aralia elata, natural products, CE analogue, molecular modelling, SPR, Hsp90
Introduction
Target identification of natural products is one of the most crucial aspects and greatest challenges for innovative drug R&D. Many biologically active natural products were delayed on the steps of being candidate drug, due to insufficient information of exact functional target (Overington et al. 2006; Eder et al. 2014). Increasing evidence illustrates that chemical proteomics is a powerful strategy commonly used for identification of natural products target (Guiffant et al. 2007; Yi et al. 2012; Cui et al. 2015; Wang et al. 2015, 2016). Among them, click chemistry activity-based protein profiling (CC-ABPP) strategies, as a more efficient chemical proteomic method could avoid influencing cell permeability by the bulky tag and promote the active group entering the active site (Speers and Cravatt 2009; Li et al. 2012; Martell and Weerapana 2014).
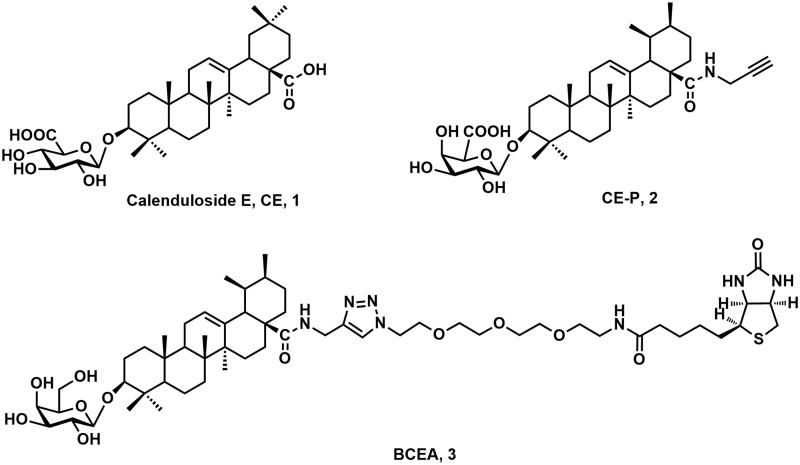
Calenduloside E (CE, 1, Figure 1) is one of the primary natural pentacyclic triterpenoid saponins extracted from Aralia elata (Miq) Seem (Araliaceae), (AS), which is commonly employed as an antiarrhythmic, antihypertensive and antidiabetic agent in traditional Chinese medicine (Nhiem et al. 2011; Wang et al. 2014). We previously demonstrated that the proteomic profiling of CE targets associated with anti-apoptosis effect by using the whole probe biotin-conjugated CE analogue (BCEA, 2, Figure 1) in HUVEC (Tian, Wang, et al. 2017). We also reported that CE and its analogues could provide protection to H9c2 cardiomyocytes against H2O2-induced apoptosis, most likely via anti-apoptotic mechanism (Tian, Du, et al. 2017, 2018). Moreover, we successfully founded that Hsp90AB1, as a familiar protein associated with anti-apoptosis effect, was proved to be one of the CE targets by a clickable CE probe (CE-P, 3, Figure 1) in our recent report (Wang et al. 2018). Though CE proved to be a multi-mechanism and multi-target natural product, the other targets of CE still need to be identified with more efficient probes.
Figure 1.
The structures of calenduloside E (CE), the clickable probe CE-P and the whole probe BCEA of CE.
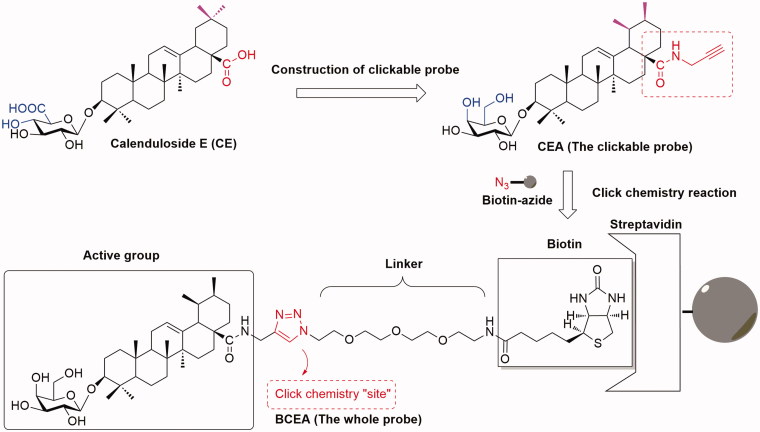
Herein, we describe another available clickable probe CEA (Figure 2), which could be introduced the tag ‘Biotin-azide’ to be an integral probe BCEA in the physiological environment via click chemistry reaction and avoid the steric hindrance like BCEA with bulky tag moiety. Compared with CE, the novel probe CEA exhibit a similar protective and anti-apoptotic effect in HUVEC. Virtual target fishing and molecular modelling research showed that Hsp90 might be the probable target of CE/CEA, and both CE and CEA could bind to Hsp90 with the similar mode. In addition, the SPR assay showed that CEA could interact with Hsp90. Our data revealed that CEA could also attenuate apoptosis by the similar anti-apoptotic mechanism with CE, and provided the possibility for detection and identification of more anti-apoptotic targets of CE.
Figure 2.
The construction of clickable probe CEA from lead compound CE.
Materials and methods
All the reagents were used without further purification unless otherwise specified. Solvents were dried and redistilled in the usual manner prior to use. Analytical TLC was performed using silica gel HF254. Preparative column chromatography was performed with silica gel H. 1H- and 13C-NMR spectra were recorded on a Bruker Advance III 600 MHz spectrometer. HRMS were obtained on a Thermo Fisher LTQ-Orbitrap XL. CE was provided by the Institute of Medicinal Plant Development (Beijing, China) (Tian, Wang, et al. 2017). Cell culture products were purchased from Gibco BRL (Grand Island, NY).
Chemistry
The chemical methods and data were described in our previous studies (Tian, Du, et al. 2017; Tian, Wang, et al. 2017; Tian et al. 2018; Wang et al. 2018).
Evaluation of the biological activity
Cell preparation and culture
HUVECs were isolated from fresh human umbilical veins using 0.1% collagenase I, as previously described (Qin et al. 2015). After dissociation, the cells were collected and cultured in VascuLife® VEGF Endothelial Cell Culture Medium (Lifeline Cell Technology, MD) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin. The cell cultures were kept in a humidified atmosphere containing 5% CO2 at 37°C. Cells at passages 3–7 were used in subsequent experiments. Neonatal umbilical cords were donated by the Maternal and Child Care Service Center in Beijing, China.
Cell viability assay
Cell viability was determined using the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium, Amresco, 0973] assay as previously described (Tian, Wang, et al. 2017). Briefly, HUVECs were plated on 96-well plates at a density of 8 × 104 cells/well and then grown at 37 °C for 24 h. The treatment group cells were pretreated with CE/CEA for 8 h, followed by treatment with ox-LDL (80 μg/mL, 24 h), the control group was pretreated with vehicle for 8 h then exposed without ox-LDL. To measure cell viability, 1 mg/mL MTT assay solution was added and the plates were incubated for an additional 4 h. The supernatant was removed and the formazan crystals were dissolved in 100 μL of DMSO. Then, the absorbance was measured at 570 nm on a microplate reader (Tecan, Switzerland). The ratio of living cells was calculated by the ratio of optical density compared with that of the normal wells.
Assessment of cell apoptosis
HUVECs were incubated with ox-LDL (80 μg/mL, 24 h) and pretreated with CEA for 8 h prior to the apoptosis assay. Double fluorescence staining was performed using an Annexin V-FITC/PI apoptosis staining kit (Molecular Probes™, V13241) according to the manufacturer’s instructions to detect early apoptotic and necrotic cells. Cellular fluorescence was measured using flow cytometry with a FACS Calibur Flow Cytometer (BD Biosciences, USA).
Determination of ΔΨm
JC-1, Beyotime Biotechnology, (C2005) was used to measure the changes in mitochondrial transmembrane potential. Briefly, the HUVECs cells were harvested and washed with phosphate buffered saline (PBS). Then, the cells were stained with 2 μM JC-1 working solution for 30 min in the dark at 37 °C, photographed and analyzed using an EVOS® FL fluorescence microscope (Thermo Fisher Scientific, USA).
Targets predicted by discovery Studio 2016
The molecular targets of CE/CEA were predicted by Discovery Studio 2016 (BIOVIA Software Inc., San Diego, CA), a software suite for performing computational analysis of data relevant to Life Sciences research (Wang et al. 2018). To predict the possible targets of CE/CEA, the Ligand Profiler protocol that maps a set of pharmacophores, including Pharma DB by default was employed. The ligands CE/CEA were prepared by the Specifying Ligands parameter protocol. After inputting all parameters, the job was run and all the results could be monitored from the Jobs Explorer.
Molecular docking
The potential interacting mode of CE/CEA with the Hsp90 protein (PDB code: 1AMW), a molecular modelling study was explored and performed by the docking program named Induced-Fit, a refinement method in another software MOE (Wang et al. 2018). To eliminate any bond length and bond angle biases, the ligands (CE/CEA) were subjected to an ‘energy minimize’ prior to docking. The binding affinities ‘S’ values in MOE were used to evaluate the interactions between Hsp90 and CE/CEA. The scores (binding affinities) were obtained based on the virtual calculation of various interactions of the ligands with the targeted receptor.
Surface-plasmon resonance (SPR)
The interaction detection and kinetic constant measurement of molecules and proteins were examined by the Biacore T200 System (Wang et al. 2018). CM5 Sensor Chip was activated by using sulpho-NHS/EDC chemistry in a buffer consisting of 2.7 mM KCl 137 mM NaCl, 0.05% (v/v) surfactant P20, pH 7.4. The chip was subsequently immobilized with the recombinant human Hsp90AB1 protein at a concentration of 37 μg/mL in sodium acetate, pH 4.5 and then blocked with 1 M ethanolamine, pH 8.0. Ligands were dissolved to 10 mM in 100% DMSO and then 50-fold into running buffer without DMSO then diluted 2-fold by running buffer into 12.5, 6.25, 3.125, 1.56, 0.78 and 0 μM before injection. The optical interference pattern was recorded as a change in optical path difference in units of nm. Data were analyzed with Biacore T200 Evaluation Software.
Statistical analysis
Data are presented as the means ± standard deviation (SD) of three independent experiments. The groups were compared using one-way ANOVA followed by Tukey's multiple comparison tests using the statistics module of Graph Pad Prism 5.0. A value of p < 0.05 was considered statistically significant.
Results and discussion
Chemistry
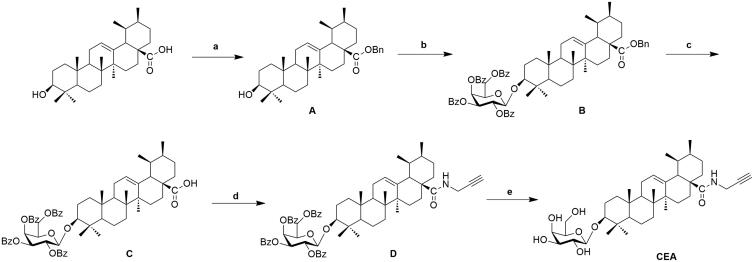
Abundant natural ursolic acid was treated with benzyl bromide (BnBr), potassium carbonate solution (K2CO3), and tetrabutyl ammonium bromide (TBAB) in dichloromethane (DCM) to produce intermediate A, in Scheme 1. Galactosyl trichloroacetimidate was obtained from monose employing general procedures which published previously by Schmidt and Michel (1980). Compound A coupled with galactosyl trichloroacetimidate in Lewis acidic catalytic conditions trimethylsilyl trifluoro methanesulfonate (TMSOTf) to gained intermediate B, which was subsequently hydrogenated to obtain intermediate C in existing of catalytic amount of Pd-C. Intermediate D was prepared via acylation with propargyl amine at C-28 carboxyl of CE, and deprotected benzoyl groups on the glycosyl-groups by NaOMe/MeOH solution to obtain clickable activity-based probe CEA. The chemical methods shown above, were described in our former studies which illustrated potent saponin analogues associated with cardioprotective activity (Tian, Du, et al. 2017; Tian, Wang, et al. 2017; Tian et al. 2018; Wang et al. 2018).
Scheme 1.
Synthesis of clickable activity-based probe CEA. Reagents and conditions: (a) BnBr, K2CO3, TBAB; (b) Galactosyl trichloroacetimidate, TMSOTf, 4 Å MS; (c) H2, Pd-C (10%); (d) HOBt, EDCI, Propargylamine; (e) NaOMe, MeOH.
Biological results and discussion
Clickable probe CEA protects HUVEC against ox-LDL-mediated injury
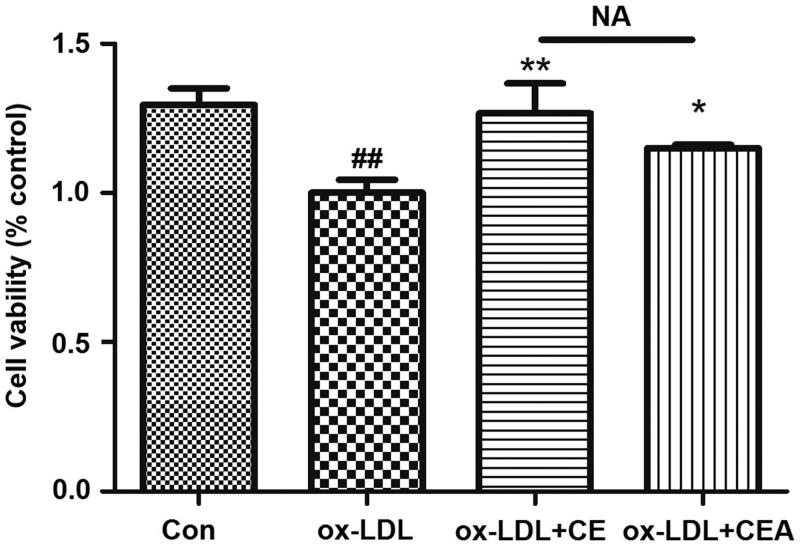
Oxidized low-density lipoprotein (ox-LDL) leads to lesion and damage that is one pathogenetic cause for atherosclerosis generation and also can be employed for evaluating small molecules (Wang et al. 2018). In the current study, we assessed the protective potential of saponin CE and the clickable probe CEA on ox-LDL mediated cells injury. In Figure 3, pre-treatment of HUVECs with compounds (CE/CEA, 1.25 μM) for 8 h, then followed ox-LDL stimulation for 24 h, could both produced prominent protective effects. Compared to ox-LDL treated group, both saponin prototype and probe could promote the viability of HUVECs, while compared to prototype CE group, clickable probe CEA group showed no significant difference on cell viability that means CEA could also keep the protective function in HUVECs.
Figure 3.
Protective effects of CE/CEA on ox-LDL induced HUVEC injury. Cell viability of HUVEC cells incubated with 1.25 μM concentration of CE/CEA for 8 h. Pretreatment with probe could significantly alleviate ox-LDL-induced cell damage. ## p < 0.01 versus control, **p < 0.01 versus ox-LDL, *p < 0.05 versus ox-LDL.
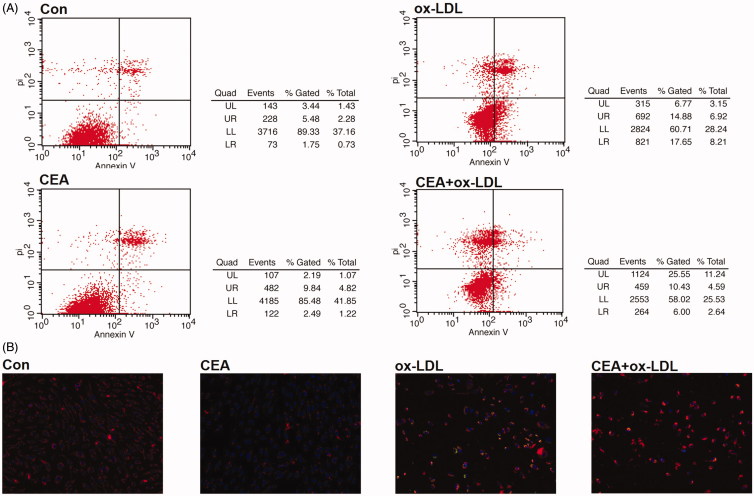
Clickable probe CEA attenuates ox-LDL-treated apoptosis
Generally occurring during the early stage of apoptosis, phosphatidylserine (PS) that exposure on the external surface of cytomembrane can be detected by flow cytometry with Annexin V–PI double staining (Tian, Wang, et al. 2017). In the present research, compared with the control group, apoptotic cells were remarkably increased after ox-LDL exposure, which was significantly descended by treatment with CEA group (Figure 4(A)). As an early event in the apoptotic cascade, the disruption of mitochondrial membrane potential (ΔΨm) could be detected by using JC-1 assay. Ox-LDL treated group showed an enhancement in green fluorescence intensity, while the CEA treated group exhibited more red fluorescence, indicating that the clickable probe CEA could suppress the ox-LDL mediated mitochondrial dissipation and apoptosis (Figure 4(B)).
Figure 4.
CEA attenuates ox-LDL mediated HUVEC apoptosis. (A) Annexin V-PI double staining was employed for detecting a protective effect of CEA; (B) JC-1 staining was employed for detecting mitochondrial membrane potential changes in CEA-treated HUVECs during ox-LDL mediated apoptosis.
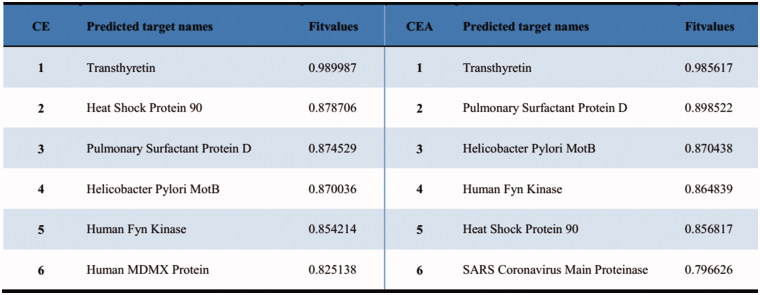
Target prediction of the clickable probe CEA and CE
Virtual target fishing strategy has been a useful technique for prediction and revelation of the potential targets of bioactive natural products (Rollinger et al. 2009; Lomenick et al. 2011). Therefore, the Discovery Studio 2016 software was employed for predicting the probable molecular targets of CE and its clickable probe CEA. Our findings showed that CE and the probe could modulate a series of functional targets. According to the Fit values data arranged from higher to lower, the top six predicted targets of CE and CEA were chosen (Figure 5). Among them, heat shock protein 90 (Hsp90), an apoptosis-related significant target that existed higher on both the lists of predicted targets names of CE and CEA was aroused our attention.
Figure 5.
The predicted targets names of CE and CEA.
Previous studies have demonstrated that the proteomic profiling of CE targets associated with anti-apoptosis effect, likely through the interaction of the glycosyl group of saponin with the apoptosis-associated proteins such as Hsp90 (Tian, Wang, et al. 2017; Wang et al. 2018). In this current research, probable targets which have crucial relevance with the endothelial protective effects of CE were predicted through virtual target fishing technique. Among the top six predicted candidates with higher Fit values, both the lists of two compounds involved Hsp90 that one of the familiar apoptosis-related targets. On top of that, Hsp90 has been proved to be one of the CE targets in our recent report. Therefore, Hsp90 was inferred as one of the apoptosis-related targets of both CE and its probe CEA but still need more experiments to identify.
Molecular modelling of the anti-apoptotic targets of CE and clickable probe CEA
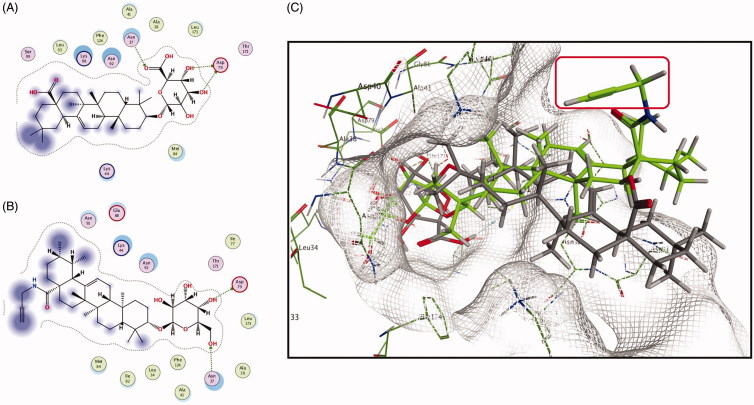
Molecular modelling is a commonly and frequently applied technique to predict the specific positioning of a ligand in the active site of a protein (Wang et al. 2018). Due to one of the apoptosis-related targets was predicted as Hsp90, molecular docking was performed to investigate the possible binding mode of probe CEA to protein Hsp90 compared with CE.
Molecular Operating Environment (MOE; Chemical Computing Group, Montreal, Quebec, Canada) software package was employed for simulating the possible interaction sites between the predicted target Hsp90 receptor (PDB ID: 1AMW) and small molecules CE/CEA. The estimated values always exhibited in the ‘S’ field, and these S values were ranked through the scores from GBVI/WSA binding free energy calculation of the binding action of CE/CEA with Hsp90 protein. The S value of CE was −7.61, while CEA was −7.33, a little weaker than CE. Molecular docking results indicated that both CE and CEA can bind into the Hsp90 active pocket, which located to the N-terminal domain.
As shown in Figure 6(A), the glycosyl moieties of saponin CE could form three significant hydrogen bonds with critical amino acid residues Asn 37 and Asp 79, while the glycosyl moieties of probe CEA could come out two (Figure 6(B)). This simulated data also illustrated and explained that the above cell viability and cell apoptosis assay results and the active moiety of CE might be located in the glycosyl group but not carboxyl.
Figure 6.
Molecular docking of CE/CEA binding with Hsp90. (A) 2D ligand interaction diagram of CE and Hsp90; (B) CEA and Hsp90. (C) 3D poses of CE/CEA binding with Hsp90.
In Figure 6(C), both CE (grey) and CEA (green) could bind in the active pocket of Hsp90, the glycosyl groups were responsible essential for binding to amino acid residues Asn 37 and Asp 79. Compared to prototype CE, the probe CEA showed a similar binding pose in the active site of the protein, and its propargyl segment (in red frame) that exposing external of the pocket was constructed for introducing the biotin or fluorescence tags conveniently via ‘clicking chemistry’ reaction. Molecular modeling assay found that both CE and CEA could bind with Hsp90 with the similar interaction mode, and it might be the indirect evidence that CE/CEA could affect the function of Hsp90, and CEA could be a useful probe for searching the other targets of CE.
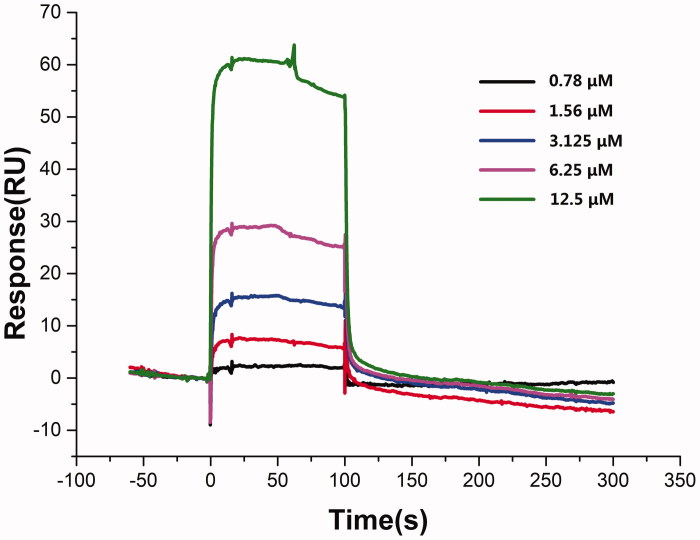
SPR assay analysis of clickable probe CEA binding to apoptosis-related protein Hsp90
Optical biosensors based on surface plasmon resonance (SPR) detection emerged as an efficient approach to obtain the interactional information about the binding of small ligands to target proteins. The detection principle, based solely on refractivity at the surface of a sensor chip, does not require any pre-labelling of molecules, avoiding time- and money-consuming steps, which could also potentially affect the interaction (Cannon et al. 2004; Nguyen et al. 2015).
Based on our previous research, one of the targets of anti-apoptotic CE was proved to be Hsp90AB1 by another clickable probe CE-P and further confirmed through SPR assay. Therefore, Biacore T200 instrument (General Electric Company, Boston, MA, USA) was employed for confirming the interaction situation and binding affinity of CEA with Hsp90AB1. As shown in Figure 7, SPR data analysis revealed that CEA could bind with Hsp90AB1 in a dose-dependent manner. KD value of CEA binding to Hsp90AB1 was 11.7 μM, while CE was 2.43 μM and CE-p was 23.4 μM (Wang et al. 2018). By means of SPR analysis results, we found that CEA (11.7 μM) showed a relatively weaker affinity than CE (2.43 μM) and a slightly better affinity than another clickable probe CE-p (23.4 μM), and still held capacity binding with Hsp90AB1. This result showed that CEA, compared to previous probe CE-p, could be a more efficient clickable probe of CE, and could provide the possibility for the fishing and identification of other anti-apoptotic targets of CE.
Figure 7.
SPR analyses of CEA binding to Hsp90AB1.
Conclusions
In summary, our present research indicates that a clickable activity-based probe CEA and its prototype CE showed similar endothelial protective and anti-apoptotic potential with ox-LDL treated HUVEC injury. Through virtual target fishing, molecular modelling and SPR affinity kinetic assay, our data reveal that anti-apoptotic mechanism contributes to the protective effect of CEA and CE, at least in part, by the similar anti-apoptotic target such as Hsp90. As an efficient clickable probe, CEA could provide the basis for the further identification of the other anti-apoptotic targets of CE. The related targets fishing experiments are ongoing and will be described in subsequent reports.
Funding Statement
This work was supported by the Natural Sciences Foundation of Beijing [Grant No. 7144225], the National Natural Sciences Foundation of China [Grant No. 81302656 and 81502929], the National Science and Technology Major Project [Grant No. 2015ZX09501004-001-003], and the CAMS Innovation Fund for Medical Science (CIFMS) [Grant No. 2016-I2M-1-012].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cannon MJ, Papalia GA, Navratilova I, Fisher RJ, Roberts LR, Worthy KM, Stephen AG, Marchesini GR, Collins EJ, Casper DH, et al. 2004. Comparative analyses of a small molecule/enzyme interaction by multiple users of Biacore technology. Anal. Biochem. 330:98–113. [DOI] [PubMed] [Google Scholar]
- Cui GZ, Shan L, Chu IK, Li GH, Leung GPH, Wang YQ, Kwan YW, Chan SW, Hoi MPM, Lee SMY. 2015. Identification of disulfide isomerase ERp57 as a target for small molecule cardioprotective agents. RSC Adv. 5:74605–74610. [Google Scholar]
- Eder J, Sedrani R, Wiesmann C. 2014. The discovery of first-in-class drugs: origins and evolution. Nat Rev Drug Discov. 13:577–587. [DOI] [PubMed] [Google Scholar]
- Guiffant D, Tribouillard D, Gug F, Galons H, Meijer L, Blondel M, Bach S. 2007. Identification of intracellular targets of small molecular weight chemical compounds using affinity chromatography. Biotechnol J. 2:68–75. [DOI] [PubMed] [Google Scholar]
- Li N, Overkleeft HS, Florea BI. 2012. Activity-based protein profiling: an enabling technology in chemical biology research. Curr Opin Chem Biol. 16:227–233. [DOI] [PubMed] [Google Scholar]
- Lomenick B, Olsen RW, Huang J. 2011. Identification of direct protein targets of small molecules. ACS Chem Biol. 6:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell J, Weerapana E. 2014. Applications of copper-catalyzed click chemistry in activity-based protein profiling. Molecules. 19:1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhiem NX, Lim HY, Kiem PV, Minh CV, Thu VK, Tai BH, Quang TH, Song SB, Kim YH. 2011. Oleanane-type triterpene saponins from the bark of Aralia elata and their NF-κB inhibition and PPAR activation signal pathway. Bioorganic Med Chem Lett. 21:6143–6147. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, Park J, Kang S, Kim M. 2015. Surface plasmon resonance: a versatile technique for biosensor applications. Sensors (Basel, Switzerland). 15:10481–10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington JP, AL-Lazikani B, Hopkins AL. 2006. How many drug targets are there? Nat Rev Drug Discov. 5:993–996. [DOI] [PubMed] [Google Scholar]
- Qin M, Luo Y, Meng XB, Wang M, Wang HW, Song SY, Ye JX, Pan RL, Yao F, Wu P, et al. 2015. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: an insight into PI3K/Akt activation and STAT3 signaling pathways. Vasc Pharmacol. 70:23–34. [DOI] [PubMed] [Google Scholar]
- Rollinger JM, Schuster D, Danzl B, Schwaiger S, Markt P, Schmidtke M, Gertsch J, Raduner S, Wolber G, Langer T, et al. 2009. In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens . Planta Medica. 75:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RR, Michel J. 1980. Facile synthesis of α- and β-O-glycosyl imidates: preparation of glycosides and disaccharides. Angewandte Chemie Int Ed. 19:731–732. [Google Scholar]
- Speers AE, Cravatt BF. 2009. Activity-based protein profiling (ABPP) and click chemistry (CC)-ABPP by MudPIT mass spectrometry. Curr Protoc Chem Biol. 1:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Wang S, Shang H, Wang M, Sun GB, Xu XD, Sun XB. 2017. The proteomic profiling of calenduloside E targets in HUVEC: design, synthesis and application of biotinylated probe BCEA. RSC Adv. 7:6259–6265. [Google Scholar]
- Tian Y, Du YY, Shang H, Wang M, Sun ZH, Wang BQ, Deng D, Shan W, Xu XD, Sun GB, et al. 2017. Calenduloside E analogues protecting H9c2 cardiomyocytes against H2O2-induced apoptosis: design, synthesis and biological evaluation. Front Pharmacol. 8:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Sun ZH, Wang WQ, Shang H, Wang BQ, Deng D, Ma GX, Wu HF, Liang ZN, Xu XD, et al. 2018. Semisynthesis and biological evaluation of oleanolic acid 3-O-β-d-glucuronopyranoside derivatives for protecting H9c2 cardiomyoblasts against H2O2-induced injury. Molecules. 23:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Xu XD, Xu HB, Wen FC, Zhang XP, Sun H, Yao F, Sun GB, Sun XB. 2014. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J Ethnopharmacol. 155:240–247. [DOI] [PubMed] [Google Scholar]
- Wang JG, Zhang CJ, Zhang JB, He YK, Lee YM, Chen SB, Lim TK, Ng SK, Shen HM, Lin QS. 2015. Mapping sites of aspirin-induced acetylations in live cells by quantitative acid-cleavable activity-based protein profiling (QA-ABPP). Sci Rep. 5:7896–7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JG, Zhang JB, Zhang CJ, Wong YK, Lim TK, Hua ZC, Liu B, Tannenbaum SR, Shen HM, Lin QS. 2016. In situ proteomic profiling of curcumin targets in HCT116 colon cancer cell line. Sci Rep. 6:22146–22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tian Y, Zhang JY, Xu HB, Zhou P, Wang M, Lu SB, Luo Y, Wang M, Sun GB, et al. 2018. Targets fishing and identification of calenduloside E as Hsp90AB1: design, synthesis, and evaluation of clickable activity-based probe. Front Pharmacol. 9:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Zhong B, Smith KM, Geldenhuys WJ, Feng Y, Pink JJ, Dowlati A, Xu Y, Zhou A, Su B. 2012. Identification of a class of novel tubulin inhibitors. J Med Chem. 55:3425–3435. [DOI] [PubMed] [Google Scholar]