Abstract
Reactive oxygen species (ROS) are a group of unstable and highly reactive molecules or free radicals typically generated as by-products of cellular processes involving molecular oxygen. In vascular cells, the excessive ROS generation results in the initiation and progression of cardiovascular diseases (CVD). Therefore, a dynamic, robust and accurate ROS detection method in the blood vessels is essential for patho-physiological research studies of the cardiovascular system.
In this chapter, we describe a fluorescence dye-based detection method for assaying superoxide and mitochondrial superoxide in mouse aorta using dihydroethidium (DHE) and MitoSOX. The protocol includes preparation of frozen aortic tissue sections, monitoring DHE oxidation-derived fluorescence by fluorescence microscopy, and HPLC-based analysis of MitoSOX and its oxidation products. For studying the role of AMP-activated protein kinase (AMPK) in the redox regulation, we employed AMPKα2 knockout mice and observed increased superoxide and mitochondrial superoxide levels in the aorta of AMPK knockout mice relative to the Wild-type group. This novel ROS detection method will be valuable for investigating the roles of cellular and/or mitochondrial ROS in the pathogenesis of CVDs.
Keywords: ROS, Mitochondria, HPLC, AMP-activated protein kinase, Cardiovascular disease
1. Introduction
Reactive oxygen species (ROS) are high reactive oxygen-containing molecules, and include superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (OH•), as well as hydroxyl ion (OH−). Under physiological conditions, low ROS concentrations regulate essential cellular functions and signaling transduction pathways to maintain vascular homeostasis. However, when endothelial cells are exposed to pathological stimuli, such as hyperglycemia or hyperlipidemia, excessive ROS are continuously generated as the natural by-product of aerobic metabolic reactions in mitochondria. Aberrant ROS production causes oxidative modification on proteins and/or nucleic acids (such as DNA). Heightened oxidative stress is widely considered as common pathways for the initiation and progression of CVD, including atherosclerosis, hypertension, and heart failure [1]. Therefore, alterations to physiologic ROS levels represents a common and potent mediator of pathogenic risk factors associated with cardiovascular dysfunction. Accurate and dynamic quantification of cellular ROS levels is therefore essential for providing clarity with respect to mechanistic links to CVDs.
1.1. O2•− detection in mammalian cells using dihydroethidium.
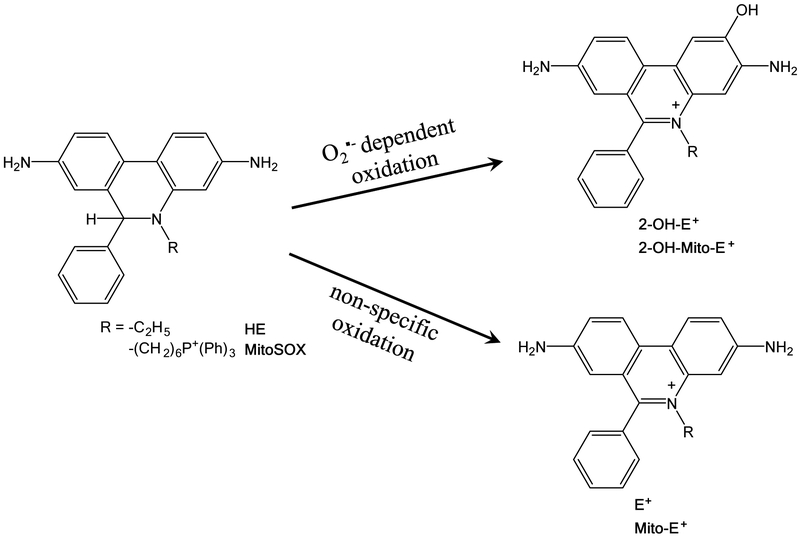
During the past decade, several fluorescent dyes have been widely used to quantify cellular O2•− and H2O2, for example by dihydroethidium (DHE) or dichlorodihydrofluorescein diacetate (DCFH-DA) [2]. DHE can freely permeate cell membrane and be oxidized by cellular O2•− to produce two red fluorescent products, namely ethidium (E+), which is typically formed by a non-specific redox reaction, and 2-hydroxyethidium (2-OH-E+), a specific adduct of cellular O2•− (Figure 1). The fluorescent spectrum of 2-OH-E+ (Ex 500–530 nm/Em 590–620 nm) and E+ (Ex 520 nm/Em 610 nm) is very similar. Thus, specific detection of 2-OH-E+ is a challenge due to overlapping fluorescence of 2-OH-E+ and E+ spectrum. Scientists routinely employed a modified high-performance liquid chromatography (HPLC) method to separate two fluorescent products and directly quantify 2-OH-E+ in cells in vitro. However, the HPLC method has big limitation to distinguish the type of cells that contributes to ROS production in tissue responses to pathological stimuli, e.g. tumor, atherosclerosis lesion, in vivo. Recently, many investigators use fluorescent microscopy to measure DHE-derived fluorescence in the artery from diabetes [3], hypertension [4] and atherosclerosis [5]. In Method 3.1 to 3.2, we describe a specific method for detection of O2•− level in mice aorta using cryosectioning technique and fluorescence microscopy.
Figure 1.
Superoxide anions (O2•−)-specific hydroxylation and non-specific oxidation of DHE and mitoSOX.
1.2. Mitochondrial O2•− detection in mammalian cells using MitoSOX.
Mitochondrion is an important source of ROS produced by abnormalities of electron transport chain in cardiovascular cells, e.g. endothelial cells (EC) and vascular smooth muscle cell. Excessive production of mitochondrial O2•− initiates endothelial dysfunction and atherosclerosis [6,7]. MitoSOX Red indicator is a modified DHE analog derived by the addition of a triphenylphosphonium group, which specifically targets to mitochondria. MitoSOX is oxidized to form two fluorescent products, mito-ethidium (mito-E+) and 2-hydroxy-mito-ethidium (2-OH-mito-E+). 2-OH-mito-E+ is the sole reaction product of mitoSOX with mitochondrial O2•−. Investigators perform double staining using MitoSOX Red and Mitotracker green and measure mitochondrialO2•− using confocal fluorescent microscopy in live cells [8,9]. However, it possesses serious disadvantages in tissue due to tissue autofluorescence interference. Recently, the mitoSOX fluorescence/HPLC combination is the most accepted measure of qualitative mitochondrialO2•− production in biological systems [10–12]. In Method 3.3, we show new optimized HPLC-fluorescence protocol that allows specific measurement of mitochondrialO2•− using MitoSOX. 2-OH-mito-E+ is identified and quantified by HPLC.
1.3. Role of AMP-activated protein kinase on cellular redox homeostasis
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that is composed of catalytic α-subunit and regulatory β and γ subunits. AMPK functions as a regulator of cellular energy metabolism and redox homeostasis. Accumulating evidence suggest cardiovascular protective effects of AMPK via the regulation of the redox system [13]. In diabetes mellitus, the activation of AMPK results in decreased mitochondrial ROS production in endothelial cells via the upregulation of mitochondrial anti-oxidative enzymes expression [14], acceleration of mitochondrial turnover [15], and maintenance of normal mitochondrial morphology [7]. In addition, AMPK activation reduces hyperglycemia-induced NADPH oxidase expression and NADPH oxidase-derived superoxide production in endothelial cells (EC). Conversely, AMPKα2 deficiency causes generation of excess superoxide and NADPH oxidase from the mitochondria, resulting in acceleration of atherosclerotic lesion formation [16]. Therefore, we employed the use of AMPKα2 KO mice as a positive model to measure cellular ROS and mitochondrial ROS.
In this paper, we describe a novel fluorescence dye-based O2•− and mitochondrial O2•− measurement method in blood vessels using a combination of HPLC and fluorescence microscopy. Employing blood vessels of AMPKα2 KO mice, we demonstrate that this method successfully and accurately measures cellular and mitochondrial O2•− levels. In summary, this method provides a valuable tool for investigations related to the roles of cellular or mitochondrial ROS in the pathogenesis of CVD.
2. Materials
2.1. Measurement of O2•− in mouse aorta
2–3 month-old male C57BL/6J wild-type (WT) mice and AMPKα2 knockout (AMPKα2 KO) mice are housed in humidity- and temperature-controlled (20–22°C) environments using a 12-hours light, 12-hours dark cycle. Mice are fed with autoclaved water and regular rodent chow diet.
70% Ethanol: Ethanol diluted in deionized H2O 70% (v/v).
Phosphate-buffered saline (PBS): 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 in deionized H2O, adjust the pH to 7.4 with HCl, autoclave and store at room temperature.
Optimal cutting temperature (OCT) compound.
DHE staining solution: 5 μM DHE dissolved in Milli-Q pure H2O.
Carbon dioxide (CO2) tank.
Dry ice.
Surgical tools: Polycarbonate surgery board, Dissecting scissors (one Straight, one Curved), Spring scissors, Graefe forceps (Curved), Dumont forceps (two Angled), Sterile syringe (1 ml and 10 ml), Needle (25G × 5/8, 26G × 1/2).
Sterile gauze pad.
100 mm × 20 mm tissue culture dish.
Surgical microscopy.
Cryostat.
−80 °C Ultra deep freezer.
Fluorescence microscopy with green and red fluorescence filter.
ImageJ software.
2.2. Measurement of mitochondrialO2•− in mouse aorta
Krebs buffer: 118.3 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, 0.026 mM EDTA, and 11 mM glucose in deionized H2O.
MitoSOX staining solution: 2 μM MitoSOX dissolved in Krebs buffer. (See Note 1).
50 % Methanol: Methanol diluted in Milli-Q pure H2O 50% (v/v).
Bicinchoninic acid assay (BCA) protein assay reagent.
HClO4 protein precipitation solution: 0.2 M HClO4 diluted in methanol.
MitoTEMPO: 5 μM MitoTEMPO dissolved in Krebs buffer.
HPLC mobile phase A: 0.1 % Trifluoroacetic acid (TFA) in 1l pure water.
HPLC mobile phase B: 0.1 % TFA in 1l acetonitrile. Keep the mobile phase at 4°C until use.
HPLC-certified non-sterile syringe filter, pore size 0.22 μm.
NovaPak C18 column (3.9 × 150 mm, 5 μm particle size) column.
Refrigerated centrifuge.
HPLC: High-pressure pump, Autosampler, Fluorescence detector.
3. Method
3.1. Isolation of mouse thoracic aorta (~ 2 hours)
Asphyxiate WT mouse or AMPKα2 KO mouse with carbon dioxide (CO2) for 5 min (See Note 2).
Sanitize the chest region of the mouse by spraying with 70% ethanol.
Place and tape the mouse on a surgical board in the supine position.
Remove the superficial skin to expose the superior portion of the peritoneum and inferior portion of the thoracic cavity.
Open abdominal cavity and thoracic cavity to expose the heart and lungs without cutting any blood vessels to avoid bleeding.
Insert 25G needle attached to the 10 ml syringe into the apex of the heart. Perfuse with 5 ml sterile ice-cold 1X PBS over 2–3 min. Cut the right atrium with scissor once perfusion has begun. Remove all fluid in the chest cavity gently with sterile gauze.
Remove the organs, including liver, lung, and stomach, to allow a clear view of the aorta. Grasp the heart gently using forceps and carefully separate the thoracic aorta from dorsal spine using curved dissecting scissors. Place thoracic aorta in tissue culture dish with ice-cold PBS buffer.
Remove the heart and fat tissue around the aortic region without cutting aortic wall under dissecting microscope. (See Note 3)
Cut the aorta into two pieces, one below the arch for measurement of mitochondrial O2•−, the other at the proximity of the diaphragm for measurement O2•−.
3.2. Measurement of O2•− in mouse aorta
3.2.1. Preparing frozen sections of mouse aorta (~ 3 hours)
Cut the cleaned aortic rings into approximate 5 mm long segments using surgical scissors.
Embed aorta vertically into OCT Compound. Immediately place the sample on crushed dry ice to freeze.
Cut frozen section 8 um thick using a cryostat at −20 °C. Thaw mount the sections onto gelatin-coated histological slides. Slides containing cryostat sections are stored at −80 °C. (See Note 4)
3.2.2. DHE Staining (~ 1 hours)
Prepare 50 ml fresh 5 μM DHE staining solution in slide box prior to use. Dissolve 1 mg DHE in 317 ul dimethyl sulfoxide (DMSO) for 10 mM stock solution. Dilute 25 μl DHE stock solution in 50 ml Milli-Q pure H2O for 5 uM staining solution. (See Note 5)
Rinse slides in pure H2O for 30 seconds to wash out OCT Compound.
Immediately place slides in DHE staining solution. Incubate for 5–20 min at room temperature and avoid exposure to light. (See Note 6)
Transfer slides to beaker with deionized H2O for washing for 1 min. Repeat the washing twice and keep slides in deionized H2O. Take fluorescence imaging immediately. (See Note 7)
3.2.3. Fluorescence imaging and analysis fluorescence intensity (~ 1 hours)
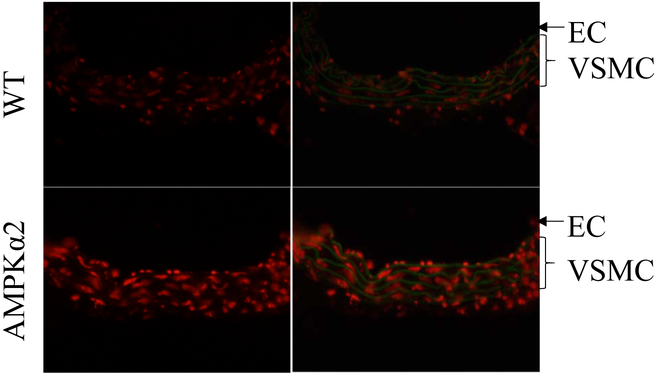
Visualize slides using fluorescence microscope with red excitation filter and green excitation filter. DHE-derived 2-OH-E+ can be visualized with red excitation filter, and autofluorescence of elastin in the internal elastic lamina can be visualized with green excitation filter. 2-OH-E+ in endothelium layer and VSMC can be clearly observed. (See Note 8)
Use 10X magnifications to focus on the sample. Adjust the objective to 40X. Set up exposure time to get optimized imaging and minimal background.
Acquire fluorescence images of aortic sections using equal identical laser power, exposure time, sensitivity and resolution. Choose at least 3 different fields for each sample (Figure 2).
Open the red fluorescence imaging with ImageJ software. Measure fluorescence intensity of the 2-OH-E+ signals, and export the value as the relative signal intensity. (See Note 9)
Figure 2.
Detection of O2•− in frozen aortic section from WT and AMPKα2 KO mice using DHE. Fluorescent micrographs of frozen sections of aorta from WT mice and AMPKα2 KO mice are obtained after staining with DHE (5 μM) for 20 min. AMPKα2 deficiency increased O2•− production in both aortic endothelial cells (EC) and vascular smooth muscle cell (VSMC).
3.3. Measurement of mitochondrial O2•− in mouse aorta
3.3.1. Preparing aortic samples for HPLC analysis (~ 3 hours)
Prepare fresh 2 μM MitoSOX staining solution prior to use. Dissolve 50 μg MitoSOX in 33 μl DMSO for 2 mM stock solution. Dilute 5 μl MitoSOX stock solution in 5 ml Krebs buffer. (See Note 10)
Place fresh WT mice and AMPKα2 KO mice aorta in 5 ml Krebs buffer or buffer containing 5 μM mitoTEMPO. (See Note 11)
Incubate at 37 °C for 30 min.
Place aorta to 5 ml 2 μM MitoSOX Red staining solution in tissue culture dish. Perfuse the artery lumen with staining solution using 26G needle attached to the 1 ml syringe. Wrap the dish with aluminum foil to avoid light exposure. Incubate at 37 °C for 20 min. (See Note 12)
Place aorta into 5 ml ice-cold Krebs buffer for washing, and repeat the washing three times (1 minute each).
Transfer aorta to new eppendorf tube with 100 μl ice-cold 50% methanol. Homogenize aorta rings with tissue grinder. (See Note 13)
Transfer 2 μl tissue lysis into an empty eppendorf tube for measurement of protein concentration by BCA.
Transfer 90 μl cell lysate to new eppendorf tube containing 90 μl of 0.2 M HClO4, vortex and keep on ice for 1 hour to allow protein precipitation.
Centrifuge tissue suspension at 4 °C, 12,000 rpm for 15 min.
The tissue supernatant is passed through a 0.22 μm syringe filter.
Transfer the methanol filtrate into HPLC vial with 300 μl conical insert. Seal the vial. Place it in the HPLC refrigerated autosampler at 4 °C.
3.3.2. HPLC operating conditions (~ 4 hours)
Prepare 1L mobile phase A (0.1 % TFA in H2O); and 1L mobile phase B (0.1 % TFA in acetonitrile).
Filter the mobile phase through a 0.22 μm nylon membrane filter under vacuum to remove undissolved solids.
Ultrasound mobile phase A and B for degasification for 30 min.
HPLC separations are performed by NovaPak C18 column and monitored with a fluorescence detector (Ex/Em = 510/580 nm). Gradient HPLC method is used for the analysis of oxidized mitoSOX products. The initial mobile phase composition is maintained at 100% mobile phase A, changed linearly to 100% mobile phase B (0–20 min), then followed by a return to the initial conditions within 5 min (20–25 min) and kept 5 min for the chromatograph column equilibrium. Flow rate is maintained at 1.0 ml/min, and injection volume of 10 μl. (See Note 14)
3.3.3. Analysis HPLC results (~ 1 hours)
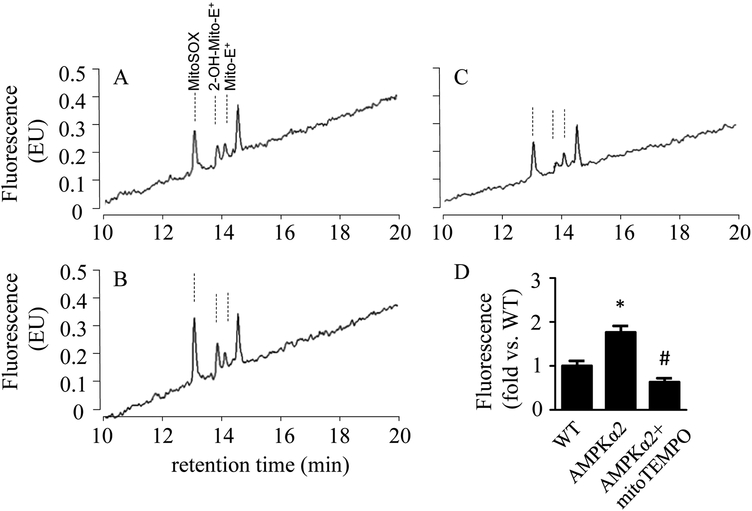
Comparing the HPLC trace of AMPKα2 KO mice aorta in Fig 3B and 3C, the HPLC peak with a retention time of 13.8 minutes is reduced after mitoTEMPO treatment. Thus, this peak is identified as 2-OH-mito-E+. (See Note 15)
Measure the area of the 2-OH-mito-E+ peak using the software provided with the HPLC system. (See Note 16)
Normalize the 2-OH-mito-E+ peak areas with the protein concentration of aorta tissue lysates.
Statistical analysis of 2-OH-mito-E+ level in WT mice aorta and AMPKα2 KO mice aorta is performed with One-Way ANOVA. P < 0.05 is considered statistically significant. (See Note 17)
Figure 3.
Detection of mitochondrial O2•− in WT and AMPKα2 KO mice aorta using HPLC. Fresh WT mice aorta (A), AMPKα2 KO mice aorta pretreatment without (B) or with (C) MitoTEMPO (5 μM) are incubated with mitoSOX (2 μM) for 20 minutes. Typical HPLC chromatograms of aorta extracts are present (A-C). Quantification of 2-OH-mito-E+ peak area in mice aorta (D). n = 3. * p < 0.05 vs. WT, # p < 0.05 vs. no mitoTEMPO treatment.
4. Notes
4.1 MitoSOX™ Red mitochondrial superoxide indicator, which is novel fluorogenic dye specifically targeted to mitochondria, is purchased from ThermoFisher Scientific,
4.2 CO2 asphyxiation is a safe and humane method of euthanasia for mouse. It causes less oxidative stress, no observable histological changes in tissue. All animal procedures are in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
4.3 Caution should be applied in the removal of adhering perivascular tissue using surgical forceps. Do not clamp the vessel which could result in damaged endothelial layer.
4.4 Cryo-sectioning and DHE staining could be performed on other mouse arteries, including extrapulmonary artery, carotid artery, mesentery artery or abdominal aorta.
4.5 Both H2O and PBS could be used to dilute DHE staining solution. We observed low-background and high-contrast fluorescence imaging using Milli-Q pure H2O-diluted DHE solution.
4.6 Time-course imaging studies, e.g. 5, 10, 15, 20 minutes, need to be performed to optimize incubation time for DHE staining.
4.7 The DHE-derived fluorescence intensity is stable over the first 30 minutes. Thus, all fluorescence images should be taken within 30 minutes.
4.8 E+ intercalates with DNA becoming highly fluorescent in nucleus. 2-OH-E+ is majorly localized in cytosol. Therefore, 2-OH-E+ and E+ could be distinguished according to their localization.
4.9 Sacrificing mice, preparing frozen aortic section, and DHE staining should be performed same day. If not, do not store frozen sections at −80 °C more than 3 days.
4.10 Krebs buffer is more suitable than cell culture medium for dissolving MitoSOX. Because cell culture medium may include less amount of superoxide radical scavengers which remove superoxide, resulting in underestimation mitochondrial O2•−.
4.11 MitoTEMPO is a specific mitochondrial O2•− scavenger. AMPKα2 KO mice aorta was treated with mitoTEMPO as a negative control.
4.12 MitoSOX staining must be performed in fresh aorta, but not frozen sections. In fresh tissue, mitoSOX translocates into the mitochondria and reacts with mitochondrial superoxide. But in frozen aortic section, MitoSOX reacts with both cytoplasmic O2•− and mitochondrial O2•− to form oxidative products.
4.13 During preparing HPLC samples, aortic sample should always be kept on ice under reduced light in avoiding to fluorescence quenching.
4.14 If 2-mito-OH-E+ peak is becoming wide and difficult to separate with mito-E+, check column pressure. If column pressure increase significantly, please stop running samples and start to rinse column using 50 ml water, methanol, and acetonitrile respectively.
4.15 To identify MitoSOX, mito-E+, and 2-OH-mito-E+ in HPLC, we compare HPLC trace from aorta treatment with or without mitoTEMPO and identify reduced peak as 2-OH-mito-E+. In addition, Jacek Zielonka et al synthesize 2-OH-mito-E+ and mito-E+ are by reacting mitoSOX with nitrosodisulfonate or chloranil, then use them as standard to quantify mitochondrial O2•− in cells and tissue [10,12].
4.16 If fluorescence signaling of 2-mito-OH-E+ is undetectable, collect at least 2–3 mice aortas and homogenize together as a group for HPLC assay.
4.17 Besides O2•− and mitochondrial O2•−, some stable by-products modified under conditions of oxidative stress are measured as biomarkers of oxidative stress, e.g. nitrotyrosine-, 4-hydroxynonenal- or S-glutathionylation-modified protein, oxidized low-density lipoprotein and oxidized phospholipids [17]. Additionally, ROS-producing enzymes (e.g. NADPH oxidase and myeloperoxidase) and anti-oxidative enzymes (e.g. MnSOD, glutathione peroxidase) are measured in tissues to evaluate oxidative stress using immunohistochemistry and western blot [1,18].
References
- 1.Song P, Zou MH (2014) Redox regulation of endothelial cell fate. Cell Mol Life Sci 71 (17):3219–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dikalov S, Griendling KK, Harrison DG (2007) Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49 (4):717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oak JH, Cai H (2007) Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes 56 (1):118–126. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q, Zhang J, Wang H (2015) PGC-1alpha overexpression suppresses blood pressure elevation in DOCA-salt hypertensive mice. Biosci Rep 35 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD (2008) Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52 (10):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercer JR, Yu E, Figg N, Cheng KK, Prime TA, Griffin JL, Masoodi M, Vidal-Puig A, Murphy MP, Bennett MR (2012) The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free Radic Biol Med 52 (5):841–849. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, Zou MH (2017) Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes 66 (1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quijano C, Castro L, Peluffo G, Valez V, Radi R (2007) Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol 293 (6):H3404–3414. [DOI] [PubMed] [Google Scholar]
- 9.Madesh M, Zong WX, Hawkins BJ, Ramasamy S, Venkatachalam T, Mukhopadhyay P, Doonan PJ, Irrinki KM, Rajesh M, Pacher P, Thompson CB (2009) Execution of superoxide-induced cell death by the proapoptotic Bcl-2-related proteins Bid and Bak. Mol Cell Biol 29 (11):3099–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI (2010) Therapeutic targeting of mitochondrial superoxide in hypertension. Circulation research 107 (1):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI (2016) Mitochondrial Cyclophilin D in Vascular Oxidative Stress and Hypertension. Hypertension 67 (6):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B (2008) Cytochrome c-mediated oxidation of hydroethidine and mitohydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med 44 (5):835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song P, Zou MH (2012) Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med 52 (9):1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M (2007) Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med 42 (1):64–78. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Foretz M, Xie Z, Zhang M, Zhu Z, Xing J, Leclerc J, Gaudry M, Viollet B, Zou MH (2014) PRKAA1/AMPKalpha1 is required for autophagy-dependent mitochondrial clearance during erythrocyte maturation. Autophagy 10 (9):1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH (2010) AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circulation research 106 (6):1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov SI, Harrison DG (2014) Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal 20 (2):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panth N, Paudel KR, Parajuli K (2016) Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv Med 2016:9152732. [DOI] [PMC free article] [PubMed] [Google Scholar]