Abstract
Objective
To determine the outcome of preterm infants whose cystic periventricular leukomalacia “disappeared” on serial screening cranial imaging studies.
Study design
Infants ≤26 weeks of gestation born between 2002 and 2012 who had cranial imaging studies at least twice, the most abnormal study at <28 days of age and another closest to 36 weeks, were reviewed. The outcome of late death (after 36 weeks postmenstrual age) or neurodevelopmental impairment (NDI) in surviving infants at 18–26 months corrected age was compared between the infants with no cystic periventricular leukomalacia on both studies and cystic periventricular leukomalacia that disappeared (cystic periventricular leukomalacia at <28 days but not at 36 weeks), persisted (cystic periventricular leukomalacia on both studies), or appeared late (cystic periventricular leukomalacia only at 36 weeks). Predictors of NDI were evaluated by logistic regression.
Results
Of 7063 eligible infants, 433 (6.1%) had cystic periventricular leukomalacia. Among the 433 infants with cystic periventricular leukomalacia, cystic periventricular leukomalacia disappeared in 76 (18%), persisted in 87 (20%), and 270 (62%) had late cystic periventricular leukomalacia. Loss to follow-up ranged between 3% and 13%. Death or NDI was more common in infants with disappeared cystic periventricular leukomalacia compared with those with no cystic periventricular leukomalacia (38 of 72 [53%] vs 1776 of 6376 [28%]; OR [95% CI] 2.8 [1.8–4.6]). Disappeared, persistent, and late cystic periventricular leukomalacia were all also independently associated with NDI (OR 1.17, 1.21, and 1.16, respectively).
Conclusions
Infants with “disappeared” cystic periventricular leukomalacia are at increased risk of adverse outcome similar to infants with persistent or late cystic periventricular leukomalacia.
Detection of cystic periventricular leukomalacia by cranial ultrasonography screening for high-risk preterm neonates provides important information related to prognosis that can influence strategies for long-term follow-up and care.1 Cystic periventricular leukomalacia on routine screening cranial ultrasonography is typically first seen about 3 weeks after injury.2 However, cystic periventricular leukomalacia may remain apparent for only a few weeks in the periventricular region before the cystic areas coalesce, collapse, and then disappear, often resulting in ventriculomegaly being the visible sequel on cranial ultrasonography.3–5
We previously analyzed dataset from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) Generic Data Base Registry (GDB) and reported that in approximately 1 of 7 (14.3%) preterm infants for whom cystic periventricular leukomalacia was noted on any cranial ultrasound <28 days chronological age (CA), cystic periventricular leukomalacia was not apparent, ie, had disappeared, on the follow up cranial imaging obtained at 36 weeks postmenstrual age (PMA).6 Although detection of cystic periventricular leukomalacia is strongly associated with subsequent neurodevelopmental outcome,1 little is known about the outcome of preterm infants in whom cystic periventricular leukomalacia disappears compared with that of infants with persistent cystic periventricular leukomalacia.5 This prompted us to use our follow-up data registry to determine the outcome for infants who never had cystic periventricular leukomalacia and infants whose cystic periventricular leukomalacia disappeared, persisted, or appeared late.
Our primary hypothesis was that the outcome for preterm infants whose cystic periventricular leukomalacia disappeared would differ from that of infants who never had cystic periventricular leukomalacia, and that the outcome in those whose cystic periventricular leukomalacia disappeared would not differ compared with those in whom it persisted, and those in whom cystic periventricular leukomalacia appeared late on screening imaging. We also hypothesized that cystic periventricular leukomalacia related variables, such as disappeared, persistent, and late cystic periventricular leukomalacia, along with clinical risk factors, could be used in multivariable regression analysis to predict neurodevelopmental impairment (NDI) in preterm infants. Accordingly, the objective was to compare the primary outcome of late death (after 36 weeks PMA) or NDI in infancy for preterm infants who never had cystic periventricular leukomalacia compared with infants whose cystic periventricular leukomalacia disappeared, persisted, or appeared late.
Methods
Prospectively collected data on all infants cared for and/or born at centers of the Eunice Kennedy Shriver NICHD Neonatal Research Network between January 2002 and December 2012 and entered in the NRN’s GDB registry were reviewed retrospectively. The registry contains records of maternal antepartum and intrapartum data, which were collected soon after infant’s birth and, in the case of the infant, collected prospectively from day one until death, or discharge from hospital or 120 days, whichever occurred first. The GDB includes information on NDI in surviving preterm infants who received follow-up assessments at 18–26 months corrected age (infants were seen at 18–22 months corrected age before July 1, 2012, and at 22–26 months corrected age after that). The follow-up evaluations performed also changed over time with the Bayley II examination used before mid-2007 and subsequently the Bayley III examination. Trained research coordinators collected the data using prespecified definitions. Data collection was approved by the local institutional review boards of the NRN centers participating in the GDB registry.
All infants born at 220/7 through 266/7 weeks PMA, who were entered in the GDB registry during the study period, survived beyond 36 weeks PMA, and had screening cranial imaging done at both of the 2 time points (ie, within 28 days CA and closest to 36 weeks PMA) were included for this analysis.
We compared the composite outcome of late death (death after 36 weeks PMA) or NDI (in surviving infants) among infants with (1) “no cystic periventricular leukomalacia”—no cystic periventricular leukomalacia on cranial imaging at both 28 days CA and close to 36 weeks PMA; (2) “disappeared” cystic periventricular leukomalacia—cystic periventricular leukomalacia observed only on cranial ultrasonography within the first 28 days CA but not apparent on the cranial imaging close to 36 weeks PMA; (3) “persistent” cystic periventricular leukomalacia- cystic periventricular leukomalacia present on cranial ultrasonography within the first 28 day CA and also on the cranial imaging close to 36 weeks PMA; and (4) “late” cystic periventricular leukomalacia—those infants who had cystic periventricular leukomalacia detected only on the cranial imaging closest to 36 weeks PMA.
Study Definitions
The GDB collects information regarding the presence of parenchymal cystic area(s) including the cystic periventricular leukomalacia and porencephalic cysts, if present on any cranial ultrasonography performed during first 28 days of birth. If more than 1 cranial ultrasonography is done in the first 28 days, results from the cranial ultrasonography with the most severe findings are recorded. Data regarding cystic periventricular leukomalacia and porencephalic cyst are collected and documented separately from imaging studies (ultrasonography, computed tomography [CT] scan, or magnetic resonance imaging [MRI]) done closest to 36 weeks PMA. In infants with more than 1 imaging modality close to 36 weeks PMA, the results to be recorded were MRI, sonogram, and then CT scan. The nature of screening cranial imaging studies (ultrasonography, CT scan, or MRI) performed close to 36 weeks PMA was center dependent. The radiologists at each participating center read the scans, and the findings recorded were based on the radiologists’ reports. Cystic periventricular leukomalacia on cranial ultrasonography was defined as “characteristic lucencies in the periventricular area (most commonly dorsal and lateral to the external angle of the lateral ventricle and may be diffuse or focal in distribution along the front to back axis of the head).”6 “Hyperechogenicity in the periventricular area” or ventriculomegaly was not considered to be cystic periventricular leukomalacia.6 Cystic periventricular leukomalacia diagnosed by MRI on late imaging (closest to 36 weeks PMA) is based on a similar definition as for cranial ultrasonography (ie, presence of cysts). Porencephalic cyst was used to describe all other forms of cystic disease than periventricular leukomalacia.
Gestational age (GA) was based on the obstetrician’s best estimate. Birth weight <10th percentile for GA and sex was small for GA. Antenatal steroids included any corticosteroid given to the mother for fetal indications. Postnatal steroid treatment included use of any corticosteroid for bronchopulmonary dysplasia (BPD).Blood culture positivity after 72 hours of age and antimicrobial therapy for ≥5 days defined late-onset sepsis. Intracranial hemorrhage included blood in the ventricles or in parenchyma. The date of the image with most severe grade of intracranial hemorrhage prior to 28 days, the presence of porencephalic cyst, and ventriculomegaly at 36 weeks PMA screening cranial imaging were also documented. Modified Bell stage IIA or greater necrotizing enterocolitis (NEC) qualified for inclusion as NEC. Supplemental oxygen at 36 weeks PMA was defined as BPD. Stage 3 retinopathy of prematurity or greater with “plus” disease was recorded as severe retinopathy of prematurity. Death after 36 weeks PMA was defined as “late.”
Neurodevelopmental Follow-Up of Infants Surviving to 36 Weeks PMA
The primary outcome variable was a combination of the competing outcomes of late death (death after 36 weeks but before neurodevelopmental assessment) or NDI. NDI was assessed in surviving infants at 18–26 months corrected age by standardized history, neuromotor examinations, and Bayley Scales of Infant Development testing. NDI definition included 1 or more of the following: disabling cerebral palsy with Gross Motor Function Classification System level ≥2; Mental Developmental Index score <70 or Psychomotor Developmental Index score <70 while using Bayley II; Cognitive score <85 while using Bayley III; Bayley III Motor score <85 in infants followed on or after 1/1/2010; bilateral visual impairment (vision <20/200), or bilateral permanent severe hearing loss. Developmental testing and neurologic examination was done by trained and certified examiners.
Statistical Analyses
Demographic characteristics of each cohort were compared using descriptive statistics. Frequencies and percentages, based on nonmissing responses, were reported for categorical variables with differences between the groups tested for using χ2 and Fisher exact tests. The Student t test was used to compare continuous variables. Comparisons were performed for the 4 groups: (1) no cystic periventricular leukomalacia on any screening cranial imaging; (2) “disappeared” cystic periventricular leukomalacia; (3) “persistent” cystic periventricular leukomalacia; and (4) “late” cystic periventricular leukomalacia. A P value of <.05 was considered significant.
A logistic regression model identified the variables which were independently associated with the outcome of NDI. The model was created using variables that are known to affect neurodevelopmental outcome as reported by Broitman et al7: antenatal steroids, chorioamnionitis (histologic or clinical), male sex, GA, race, maternal education status, bilateral presence of blood or echodensity in the ventricles or parenchyma (using screening cranial ultrasonography results within the first 28 days), late-onset sepsis, medical or surgical NEC, and BPD. “Disappeared” cystic periventricular leukomalacia, “persistent” cystic periventricular leukomalacia, and “late” cystic periventricular leukomalacia were also included in the logistic regression model. All infants surviving beyond 36 weeks PMA were included in the regression analysis, which was modeled for the outcome of NDI. NRN center was also adjusted for the model. SAS statistical software v 9.4 (SAS Institute, Cary, North Carolina) was used for statistical analyses.
Results
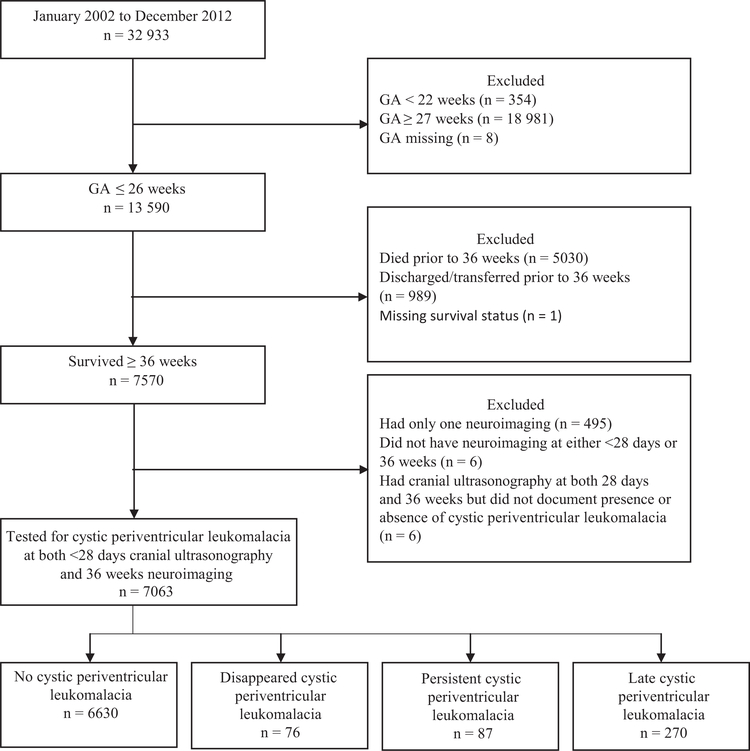
Between January 2002 and December 2012, 32 933 preterm infants were entered in the GDB. As seen in the Figure, 433 (6.1%) of the 7063 infants who met the inclusion criteria were noted to have cystic periventricular leukomalacia on screening cranial imaging; 76 at <28 days CA only, 270 at 36 weeks PMA only, and another 87 at both times. The median (IQR, 25th and 75th percentile) PMA at screening cranial imaging recorded for closet to 36 weeks study was 35 weeks 2 days (31 weeks 3 days, 36 weeks 6 days). The “disappeared” cystic periventricular leukomalacia group included the 76 infants with cystic periventricular leukomalacia visible at <28 days CA (ie, 17.6% of the total 433 cystic periventricular leukomalacia cases) but no longer seen on repeat screening imaging close to 36 weeks PMA. Infants with “disappeared” cystic periventricular leukomalacia were more often preterm; received prenatal steroids less frequently; had an Apgar score of <4 at 5 minutes more often; had higher incidence of blood in ventricles or in parenchyma, BPD, or porencephalic cyst; and received postnatal steroids less frequently compared with the infants with no cystic periventricular leukomalacia (Table I).
Figure.
Study sample.
Table I.
Characteristics of the study sample: no cystic periventricular leukomalacia vs disappeared cystic periventricular leukomalacia vs persistent cystic periventricular leukomalacia vs late cystic periventricular leukomalacia
| Variables/ characteristics | No cystic periventricular leukomalacia (n = 6630) | Disappeared cystic periventricular leukomalacia (n = 76) | Persistent cystic periventricular leukomalacia (n = 87) | Late cystic periventricular leukomalacia (n = 270) | Disappeared cystic periventricular leukomalacia vs no cystic periventricular leukomalacia P value, OR (95% CI) |
|---|---|---|---|---|---|
| Maternal | |||||
| Antenatal steroids | 5609 (85) | 56 (74) | 69 (80) | 206 (76) | .01, 0.50 (0.30, 0.83) |
| Multiple birth | 1519 (23) | 14 (18) | 22 (25) | 53 (20) | .35, 0.76 (0.42, 1.36) |
| Chorioamnionitis | 1988 (50) | 31 (53) | 37 (60) | 78 (47) | .70, 1.11 (0.66, 1.85) |
| Education | |||||
| <high school | 1299 (20) | 19 (25) | 12 (14) | 47 (18) | .24, 1.37 (0.81, 2.31) |
| ≥high school | 3689 (56) | 39 (52) | 53 (61) | 133 (50) | .44, 0.84 (0.53, 1.32) |
| Unknown | 1552 (24) | 17 (23) | 22 (25) | 84 (32) | .83, 0.94 (0.55, 1.62) |
| Neonatal | |||||
| Male | 3409 (51) | 38 (50) | 43 (49) | 129 (48) | .80, 0.94 (0.60, 1.48) |
| Birth weight, g (mean ± SD) | 751.2 ± 151.8 | 762.4 ± 146.8 | 787.1 ± 141.9* | 742.7 ± 160.3* | .52, 1.00 (1.00, 1.00) |
| GA, wk (mean ± SD) | 25.4 ± 1.0 | 24.9 ± 0.9† | 25.3 ± 0.9† | 25.1 ± 0.9 | <.01, 0.67 (0.54, 0.83) |
| Small for GA | 386 (6) | 0 | 2(2) | 14(5) | Not applicable |
| Race/ethnicity | |||||
| White | 3457 (53) | 37 (49) | 53 (62) | 139 (52) | .55, 0.87 (0.55, 1.37) |
| Black | 2744 (42) | 34 (45) | 29 (34) | 121 (46) | .55, 1.15 (0.73, 1.82) |
| Other | 347 (5) | 4 (5) | 3 (4) | 5(2) | .99, 1.01 (0.37, 2.77) |
| 5-min Apgar <4 | 737 (11) | 15 (20) | 13 (15) | 40 (15) | .02, 1.98 (1.12, 3.51) |
| ICH | 1535 (61) | 67 (88)‡ | 66 (76)‡ | 178 (84) | <.01, 4.68 (2.32, 9.43) |
| PDA | 3912 (59) | 53 (70) | 52 (60) | 153 (57) | .06, 1.60 (0.98, 2.62) |
| Late onset sepsis | 2832 (43) | 36 (47) | 38 (44) | 130 (48) | .41, 1.21 (0.77, 1.90) |
| NEC | 707 (11) | 12 (16) | 16 (18) | 46 (17) | .15, 1.57 (0.84, 2.92) |
| BPD | 3956 (60) | 56 (74) | 67 (77) | 183 (68) | .01, 1.87 (1.12, 3.13) |
| ROP ≥stage 3 | 1837 (28) | 27 (36) | 31 (36) | 89 (33) | .13, 1.44 (0.90, 2.31) |
| Postnatal steroids | 1466 (22) | 8 (11)§ | 21 (24)§ | 69 (26) | .02, 0.42 (0.20, 0.87) |
| Porencephalic cyst cranial | 135 (7) | 22 (33) | 21 (24) | 42 (16) | <.01, 6.98 (4.07, 11.96) |
ICH, intracranial hemorrhage; PDA, patent ductus arteriosus; ROP, retinopathy of prematurity.
Porencephalic cyst—on closest to 36 weeks PMA cranial imaging, ICH—blood in ventricles or in parenchyma.
Percentages based on nonmissing responses; P values between the 2 groups denoted with
are 0.02, 0.03, 0.04, and 0.03, respectively.
are 0.02, 0.03, 0.04, and 0.03, respectively.
are 0.02, 0.03, 0.04, and 0.03, respectively.
are 0.02, 0.03, 0.04, and 0.03, respectively.
The demographic, perinatal, and clinical characteristics were similar between the “disappeared” and “persistent” cystic periventricular leukomalacia groups except that the infants with “disappeared” cystic periventricular leukomalacia were more preterm (P = .03, OR 0.69, 95% CI 0.50–0.96), had blood in ventricles or in parenchyma more often (P = .04, OR 2.37, 95% CI 1.01–5.55), and received postnatal steroid less often (P = .03, OR 0.38, 95% CI 0.16–0.91). However, as seen in Table I, all the characteristics except the birth weight were similar between the “persistent” and “late” cystic periventricular leukomalacia groups.
Loss to follow-up was 8%, 3%, 13%, and 8%, respectively, in the no cystic periventricular leukomalacia, disappeared, persisted, and late cystic periventricular leukomalacia groups. As seen in Table II, the primary composite outcome of late death or NDI was more common in infants with “disappeared” cystic periventricular leukomalacia compared with infants with no cystic periventricular leukomalacia (38 of 72 or 53% vs 1776 of 6376 or 28%; P < .01, OR 2.89, 95% CI 1.82–4.61); however, among all infants with any cystic periventricular leukomalacia, the incidence of the composite primary outcome did not differ between the infants with “disappeared” cystic periventricular leukomalacia and “persistent” cystic periventricular leukomalacia (P = .74), or between the infants with “disappeared” cystic periventricular leukomalacia and those infants who had cystic periventricular leukomalacia detected only on the 36 week cranial imaging (Table II). Detailed description of components of NDI are also shown in Table II.
Table II.
Outcomes of the study sample: no cystic periventricular leukomalacia vs disappeared cystic periventricular leukomalacia vs persistent cystic periventricular leukomalacia vs late cystic periventricular leukomalacia
| Outcomes | No cystic periventricular leukomalacia (A) | Disappeared Cystic periventricular leukomalacia (B) | Persistent cystic periventricular leukomalacia (C) | Late cystic periventricular leukomalacia (D) | A vs B P value, OR (95% CI) | B vs C P value | B vs D P value | C vs D P value |
|---|---|---|---|---|---|---|---|---|
| Children at follow-up | N = 6376 | N = 72 | N = 76 | N = 257 | ||||
| Late death (death after 36 wk) or 22–26 mo corrected age follow-up | 370 (6) | 5 (7) | 7 (9) | 40 (16) | .68, 1.21 (0.49, 3.02) | .61 | .06 | .16 |
| Late death or NDI on 18–22 mo | 1776 (28) | 38 (53) | 38 (50) | 146 (57) | <.01, 2.89 (1.82, 4.61) | .74 | .54 | .29 |
| Infants surviving to 18–22 mo or 22–26 mo corrected age follow-up | N = 5240 | N = 63 | N = 57 | N = 190 | ||||
| NDI | 1406 (27) | 33 (52) | 31 (54) | 106 (56) | <.01, 3.00 (1.82, 4.94) | .83 | .64 | .85 |
| Unimpaired | 4004 (76) | 27 (43) | 26 (46) | 76 (40) | <.01, 0.23 (0.14, 0.38) | .76 | .69 | .45 |
| Moderate/severe CP | 355 (7) | 22 (35) | 23 (40) | 72 (38) | <.01, 7.37 (4.34, 12.51) | .54 | .67 | .74 |
| Bayley II Mental Development Index <70 | 746 (39) | 10 (71) | 13 (87) | 36 (58) | .01, 3.93 (1.23, 12.59) | .31 | .36 | .04 |
| Psychomotor Development Index <70 | 551 (29) | 9 (64) | 11 (73) | 42 (69) | <.01, 4.45 (1.48, 13.34) | .60 | .74 | .74 |
| Bayley III Cognitive <70 | 316 (10) | 16 (33) | 11 (27) | 43 (35) | <.01, 4.70 (2.55, 8.67) | .51 | .87 | .35 |
| Bayley III Cognitive <85 | 943 (29) | 24 (50) | 20 (49) | 66 (53) | <.01, 2.49 (1.41, 4.40) | .91 | .70 | .62 |
| Bayley III Motor score <70 | 302 (13) | 13 (37) | 8 (30) | 36 (45) | <.01, 3.98 (1.98, 7.98) | .54 | .43 | .16 |
| Bayley III Motor score <85 | 772 (33) | 21 (60) | 15 (56) | 56 (70) | <.01, 3.04 (1.54, 6.01) | .73 | .29 | .17 |
| Bayley III Language <70 | 611 (19) | 18 (38) | 13 (32) | 42 (35) | <.01, 2.57 (1.43, 4.65) | .57 | .73 | .73 |
| Bayley III Language <85 | 1653 (51) | 28 (58) | 21 (51) | 79 (65) | .32, 1.34 (0.75, 2.39) | .50 | .40 | .11 |
| Bayley III scores (mean ± SD) | ||||||||
| Cognitive composite score | 51.9 ± 8.0 | 47.2 ± 12.5 | 50.5 ± 11.0 | 46.7 ± 9.8 | <.01, 0.94 (0.92, 0.97) | .23 | .79 | .06 |
| Language composite score | 83.6 ± 16.7 | 78.9 ± 21.9 | 79.2 ± 21.5 | 74.9 ± 18.5 | .06, 0.98 (0.97, 1.00) | .94 | .23 | .21 |
CP, cerebral palsy.
Percentages based on nonmissing responses.
Logistic regression analyses identified all the cystic periventricular leukomalacia subtypes (disappeared, persistent, or late) on screening cranial imaging were each independently associated with an increased risk of NDI (Table III).
Table III.
Logistic regression model for NDI
| Characteristics | OR (95% CI) | P value |
|---|---|---|
| Antenatal steroids | 0.97 (0.91,1.04) | .38 |
| Chorioamnionitis* | 0.99 (0.94,1.03) | .59 |
| Less than high school† | 1.07 (1.01,1.13) | .02 |
| Male | 1.11 (1.06,1.16) | <.01 |
| Black | 0.96(0.87,1.07) | .49 |
| White | 1.00(0.91,1.10) | .98 |
| GA | 0.95‡ (0.93,0.98) | <.01 |
| ICH§ | 1.15 (1.09,1.20) | <.01 |
| Late onset sepsis | 1.05 (1.00,1.10) | .05 |
| NEC | 1.13 (1.05,1.21) | <.01 |
| BPD | 1.16(1.10,1.22) | <.01 |
| Disappearing cystic periventricular leukomalacia | 1.17 (1.04,1.31) | .01 |
| Persistent cystic periventricular leukomalacia | 1.21 (1.06,1.37) | <.01 |
| Late cystic periventricular leukomalacia | 1.16 (1.06,1.27) | <.01 |
Model adjusted for center.
Chorioamnionitis defined as clinical or histologic.
Less than high school- defined as anyone who had education up to 12th grade but did not graduate.
OR for each additional week of GA.
Blood in ventricles or in parenchyma.
Discussion
The neuroimaging technique of choice for routine screening of cystic periventricular leukomalacia in preterm extremely low birth weight newborns is still cranial ultrasonography.1 Detection of cystic periventricular leukomalacia on cranial ultrasonography correlates well with neuropathology8,9 and is the best ultrasonography marker for predicting cerebral palsy among preterm extremely low birth weight infants.10 Such cerebral palsy is related to the position of cystic periventricular leukomalacia (periventricular regions dorsal and lateral to the external angle of the lateral ventricle) in the regions that are occupied by motor, somatosensory, callosal, and associative fiber tracts.11 Prior investigations have examined the neurodevelopment of preterm infants based on characteristics of cystic periventricular leukomalacia such as location of the lesion, number of lesions, and size of the lesion.12 This study, which is a follow-up analysis to a prior work,6 addressed a unique feature, the transient nature of some of the cystic periventricular leukomalacia lesions and the impact on outcome. In a large cohort of preterm infants ≤26 weeks PMA with cystic periventricular leukomalacia, we noted that almost 1 in 6 cases of cystic periventricular leukomalacia seen on an earlier cranial ultrasonography disappeared by the time the later screening cranial imaging study was obtained. We also report that these lesions, although transient, are harbingers of adverse outcome including neurodevelopmental deficits. In this study, consistent with our hypothesis, death or NDI was more frequent among infants whose cystic periventricular leukomalacia disappeared compared with those who never had cystic periventricular leukomalacia. Furthermore, cystic periventricular leukomalacia detected at any time was independently associated with NDI, regardless of whether cysts disappeared, persisted, or appeared late.
This study has a potential policy implication for schedules of surveillance cranial imaging given the prognostic importance of disappearing cystic periventricular leukomalacia for NDI. The American Academy of Neurology and the Child Neurology Society recommend that late screening cranial ultrasonography should be “optimally” done “at 36 to 40 weeks’ postmenstrual age.”1 This recommendation is “designed to detect evidence for periventricular leukomalacia and/or ventriculomegaly, which are useful for prognosis and best seen when the infants are examined at term.” Contrary to the recommendation by the American Academy of Neurology and the Child Neurology Society, the finding of the present study and other previous reports5,6,10 demonstrating some cases of cystic periventricular leukomalacia disappearing on repeat screening imaging close to 36 weeks stresses once more the importance of sequential late screening neuroimaging to capture additional cases of cystic periventricular leukomalacia. Similar observations have been made recently by Martinez-Biarge et al based on sequential MRI in infants with nonhemorrhagic white matter injury.13
Although the overall incidence of cystic periventricular leukomalacia was low, access to the large NICHD NRN database to test our hypothesis was a strength for this study. The absence of a central cranial ultrasonography reader and lack of reporting of the neuroimaging findings in a standardized format across centers were potential limitations. However, a previous report from the NRN comparing local and central cranial ultrasonography interpretations indicated that the diagnosis of major cranial ultrasonography findings, especially cystic periventricular leukomalacia, is reliable (kappa for grade 3/4 intraventricular hemorrhage or periventricular leukomalacia was 0.81).14
We acknowledge that NRN sites perform multiple cranial ultrasonography as part of usual care and different centers have varying schedules of cranial ultrasonography surveillance, which may contribute to center differences in cranial ultrasonography ascertainment. However, this should have limited impact on the results because our analysis was adjusted for NRN center. Our results also included only the infants who survived beyond 36 weeks PMA to have cranial imaging at both of the 2 time points. Furthermore, we compared cranial ultrasonography at <28 days to any late cranial imaging modality (although cranial ultrasonography constituted majority of the late images done close to 36 weeks PMA) despite the fact that cranial ultrasonography, MRI, and CT scan have different diagnostic accuracy for white matter abnormalities. The transition from the Bayley II to III for follow-up evaluations was also a concern because Bayley Scales of Infant Development III Motor Composite threshold of 85 likely overstates disability.15 Similarly, the transition from 18–22 months to 22–26 months corrected age follow-up assessments during the study period could impact neurodevelopmental testing results. Nevertheless, this study extends the observations from our previous report demonstrating that infants in whom cystic periventricular leukomalacia is no longer apparent around 36 weeks PMA share the same level of neurodevelopmental risks as infants whose cystic periventricular leukomalacia is still readily apparent at 36 weeks PMA. ■
Acknowledgments
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies through cooperative agreements. The authors declare no conflicts of interest.
Glossary
- BPD
Bronchopulmonary dysplasia
- CA
Chronological age
- CT
Computed tomography
- GA
Gestational age
- GDB
Generic Data Base Registry
- MRI
Magnetic resonance imaging
- NDI
Neurodevelopmental impairment
- NEC
Necrotizing enterocolitis
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- PMA
Postmenstrual age
Appendix
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies through cooperative agreements. While NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2007); Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Martin Keszler, MD; William Oh, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS RNC-NIC; Barbara Alksninis, PNP; Kristin M. Basso, BSN MaT; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd CAES; Emilee Little, RN BSN; Elisabeth C. McGowan, MD; Elisa Vieira, RN BSN; Victoria E. Watson, MS CAS; Suzy Ventura.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Anna Marie Hibbs, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA RN; Allison H. Payne, MD MS; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA. Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (U10 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Knutson, BSN RNC-NIC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Cathy Grisby, BSN CCRC; Kate Bridges, MD; Barbara Alexander, RN; Estelle E. Fischer, MHSA MBA; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Lenora Jackson, CRC; Kristin Kirker, CRC; Greg Muthig, BS; Jean J. Steichen, MD; Stacey Tepe, BS; Marcia Worley Mersmann, RN CCRC; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, UL1 TR1117, M01 RR30, UL1 TR1111) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Patricia L. Ashley, MD PhD; William F. Malcolm, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNPBC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; Joanne Finkle, RN JD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Diane Warner, MD MPH; Janice Wereszczak, NNP.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – David P. Carlton, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN BSN; Ann M. Blackwelder, RNC BS MS; Diane I. Bottcher, RN MSN; Colleen Mackie, BS RRT.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Gregory M. Sokol, MD; Brenda B. Poindexter, MD MS; James A. Lemons, MD; Anna M. Dusick, MD (deceased); Lu-Ann Papile, MD; Carolyn Lytle, MD MPH; Abbey C. Hines, PsyD; Heike M. Minnich, PsyD HSPP; Dianne E. Herron, RN CCRC; Lucy Smiley, CCRC; Susan Gunn, NNP CCRC; Leslie Dawn Wilson, BSN CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Georgia E. McDavid, RN; Esther G. Akpa, RN BSN; Julie Arldt-McAlister, RN BSN;; Nora I. Alaniz, BS; Katrina Burson, RN BSNPamela J. Bradt, MD MPH; Susan Dieterich, PhD; Allison Dempsey, PhD; Andrea F. Duncan, MD;; Patricia W. Evans, MD; Claudia I. Franco, RNC MSN; Carmen Garcia, RN CCRP; Charles Green, PhD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD; Layne M. Lillie, RN BSN; Anna E. Lis, RN BSN; Terri Major-Kincade, MD MPH Karen Martin, RN; Sara C. Martin, RN BSN; Brenda H. Morris, MD; Patricia Ann Orekoya, RN BSN; Stacey Reddoch, BA; Shawna Rodgers, RN; Saba Siddiki, MD; Maegan C. Simmons, RN; Daniel Sperry, RN; Patti L. Pierce Tate, RCP; Laura L. Whitely, MD; Sharon L. Wright, MT(ASCP).
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278) – Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner, MD; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – Dennis Wallace, PhD; Marie G. Gantz, PhD; W. Kenneth Poole, PhD (deceased); Margaret M. Crawford, BS CCRP; Jenna Gabrio, BS CCRP; Betty K. Hastings; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70) – David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Marian M. Adams, MD; Alexis S. Davis, MD MS Epi; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Barbara Bentley, PsychD MSEd; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Beth Earhart, PhD; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Casey Krueger, PhD; Andrew W. Palmquist, RN; Hali E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Furey, MPH; Ellen Nylen, RN BSN; Elisabeth C. McGowan, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; David Kaegi, MD; Maynard R. Rasmussen, MD; Paul R.Wozniak, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT; Yvonne E. Vaucher, MD MPH.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59) – Tarah T. Colaizy, MD MPH; Michael J. Acarregui, MD; Jane E. Brumbaugh, MD; Dan L. Ellsbury, MD; John A. Widness, MD; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NC; Diane L. Eastman, RN CPNP MA; Nancy J. Krutzfield, RN MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; Alexandra Stoerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Lu-Ann Papile, MD; Jean R. Lowe, PhD; Tara Dupont, MD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Andrea F. Duncan, MD MScr; Rebecca Montman, BSN.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD; Noah Cook, MD.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, U10 HD40521, M01 RR44, UL1 TR42) – Carl T. D’Angio, MD; Dale L. Phelps, MD; Ronnie Guillet, MD PhD; Satyan Lakshminrusimha, MD; Julie Babish Johnson, MSW; Erica Burnell, RN; Linda J. Reubens, RN CCRC; Cassandra A. Horihan, MS; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Gary J. Myers, MD; Mary Rowan, RN; Holly I.M. Wadkins, MA; Anne Marie Scorsone, MS, CCRC; Melissa Bowman, RN, MSN, NP; Julianne Hunn, BS; Stephanie Guilford, BS; Deanna Maffett, RN; Osman Farooq MD; Diane Prinzing; Anne Marie Reynolds, MD, MPH; Michael G. Sacilowski, MAT, CCRC; Ashley Williams, MSEd; Karen Wynn, RN; Kelley Yost, PhD; William Zorn, MD; Lauren Zwetsch, RN MS PNP.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Luc P. Brion, MD; R. Sue Broyles, MD; Roy J. Heyne, MD; Merle Ipson, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Diana M. Vasil, MSN BSN RNC-NIC; Lijun Chen, PhD RN; Alicia Guzman; Gaynelle Hensley, RN; Jackie F. Hickman, RN; Melissa H. Leps, RN; Susie Madison, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Lara Pavageau, MD; Sally S. Adams, MS RN CPNP; Cristin Dooley, PhD LSSP; Elizabeth T. Heyne, MS MA PA-C PsyD; Lizette E. Lee, RN; Linda A. Madden, BSN RN CPNP; Catherine Twell Boatman, MS CIMI.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64, UL1 RR25764) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC BSN; Kimberlee Weaver-Lewis, RN MS; Shawna Baker, RN; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Michael Steffen, MS CPM; Jennifer J. Jensen, RN BSN; Sarah Winter, MD; Karen Zanetti, RN.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy J. Peters, RN CCRP; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Athina Pappas, MD; Rebecca Bara, RN BSN; Laura A. Goldston, MA; Geraldine Muran, RN BSN; Girija Natarajan, MD; Mary Christensen, RT; Stephanie A. Wiggins, MS; Diane White RT.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, ULTR142, M01 RR125) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Patricia Gettner, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Elaine Romano, MSN; Janet Taft, RN BSN; Joanne Williams, RN BSN.
References
- 1.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2002;58:1726–38. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa F, Okumura A, Kato T, Kuno K, Watanabe K. Determination of the timing of brain injury in preterm infants with periventricular leukomalacia with serial neonatal electroencephalography. Pediatrics 1999;104:1077–81. [DOI] [PubMed] [Google Scholar]
- 3.Dubowitz LMS, Bydder GM, Mushin J. Developmental sequence of periventricular leukomalacia. Arch Dis Child 1985;60:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdjalov V, Srinivasan P, Baumgart S, Spitzer AR. Handheld, portable ultrasound in the neonatal intensive care nursery: a new, inexpensive tool for the rapid diagnosis of common neonatal problems. J Perinatol 2002;22:478–83. [DOI] [PubMed] [Google Scholar]
- 5.Pierrat V, Duquennoy C, van Haastert IC, Ernst M, Guilley N, de Vries LS. Ultrasound diagnosis and neurodevelopmental outcome of localised and extensive cystic periventricular leucomalacia. Arch Dis Child Fetal Neonatal Ed 2001;84:F151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar S, Shankaran S, Laptook AR, Sood BG, Do B, Stoll BJ, et al. for the Generic Database Subcommittee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Screening cranial imaging at multiple time points improves cystic periventricular leukomalacia detection. Am J Perinatol 2015;32:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broitman E, Ambalavanan N, Higgins RD, Vohr BR, Das A, Bhaskar B, et al. for the National Institute of Child Health and Human Development Neonatal Research Network. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr 2007;151:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trounce JQ, Fagan D, Levene MI. Intraventricular hemorrhage and periventricular leukomalacia: ultrasound and autopsy correlation. Arch Dis Child 1986;61:1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bejar R, Coen RW, Merritt TA, Vaucher Y, Trice J, Centeno R, et al. Focal necrosis of the white matter (periventricular leukomalacia): sonographic, pathologic, and electroencephalographic features. AJNR Am J Neuroradiol 1986;7:1073–80. [PMC free article] [PubMed] [Google Scholar]
- 10.De Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr 2004;144:815–20. [DOI] [PubMed] [Google Scholar]
- 11.Judas M, Rados M, Jovanov-Milosevic N, Hrabac P, Stern-Padovan R, Kostovic I. Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol 2005;26:2671–84. [PMC free article] [PubMed] [Google Scholar]
- 12.Bass WT. Periventricular Leukomalacia. Neoreviews 2011;12:e76–84. [Google Scholar]
- 13.Martinez-Biarge M, Groenendaal F, Kersbergen KJ, Benders MJ, Foti F, Cowan FM, et al. MRI based preterm white matter injury classification: the importance of sequential imaging in determining severity of injury. PLoS ONE 2016;11:e0156245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hintz SR, Slovis T, Bulas D, Van Meurs KP, Perritt R, Stevenson DK, et al. for the NICHD Neonatal Research Network. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr 2007;150:592–6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan AF, Bann C, Boatman C, Hintz SR, Vaucher YE, Vohr BR, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Do currently recommended Bayley-III cutoffs overestimate motor impairment in infants born 27 weeks’ gestation? J Perinatol 2015;35:516–21 [DOI] [PMC free article] [PubMed] [Google Scholar]