Subclinical leaflet thrombosis following transcatheter aortic valve (TAV) replacement (TAVR) in native and existing bioprosthetic valves (valve-in-valve “ViV”) has been associated with a greater incidence of valve degeneration and cerebroembolic events1. Native and neo-sinus flow stasis has been implicated in this phenomenon,2, 3 and if this were avoided, it is possible that the risk of developing leaflet thrombus could be avoided. This concern is particularly relevant in ViV cases, where a higher incidence of subclinical leaflet thrombosis is observed4. Recently, several studies have introduced the bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction also known as BASILICA5. Despite being used in the context of mitigating coronary obstruction, BASILICA introduces a laceration to the leaflet that opens a narrow but direct flow pathway between the sinus and the neo-sinus, and thus potentially altering flow dynamics in both sinus and neo-sinus. The objective of this study is to quantify neo-sinus and sinus flow washout with and without leaflet laceration in the context of assessing the potential of laceration as a strategy to reduce the incidence of leaflet thrombosis.
This study adheres to the AHA Journals’ implementation of the Transparency and Openness Promotion (TOP) Guidelines. The data used to generate the findings of this study are available from the corresponding author upon reasonable request. A 23mm Edwards SAPIEN 3 was implanted in a 23mm transparent surgical aortic valve model without and with leaflet laceration (Fig.1a, b). The hemodynamic assessment of the TAV (5L/min cardiac output, 60 beats per minute heart rate and 120/80mmHg pressures) was performed in a pulse duplicator left heart simulator flow loop described previously3. To evaluate the flow field and washout in the sinus and the neo-sinus, particle image velocimetry was performed. Using Lagrangian particle tracking method as previously described3 over eighteen different cardiac cycles, the number of cycles to washout was calculated. Student’s t test was performed to assess the statistical significance of the difference in number of cycles to washout pre and post leaflet laceration after confirming normality using the Shapiro-Wilks test with a p-value of 0.756.
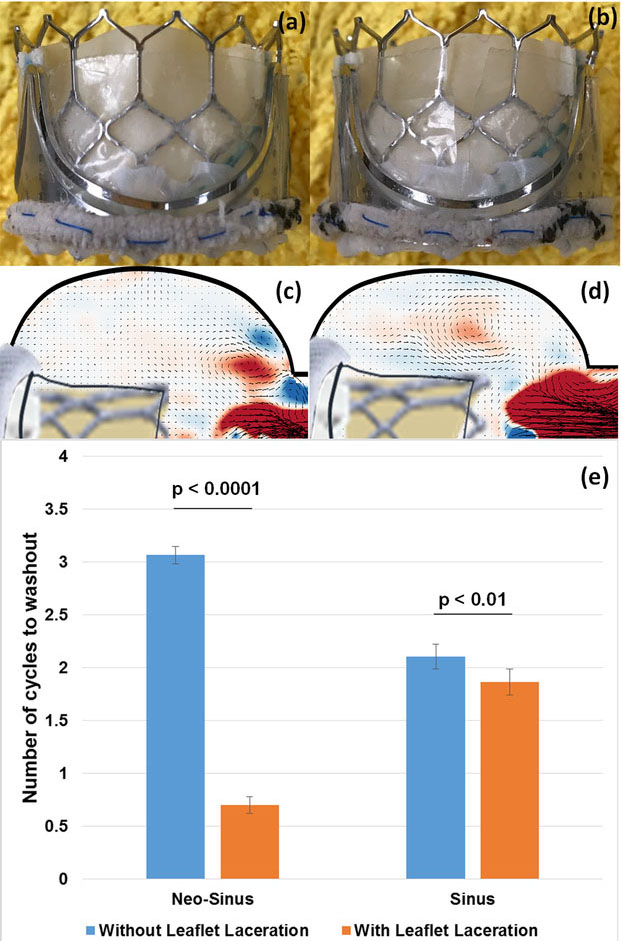
Figure 1:
(a, b) Valve-in-valve setup with SAPIEN 3 TAV without and with leaflet laceration; (c, d) Particle image velocimetry vector field images showing the flow across the leaflet between the neo-sinus and the sinus during leaflet opening; (e) Washout results in the neo-sinus and the sinus with and without leaflet laceration.
The results revealed that introducing a laceration to the leaflet allows flow to cross laterally from the neo-sinus to the sinus compared to cases where no-lacerations were modeled (Fig.1c, d). This resulted in an enhancement of washout in the neo-sinus and in “pushing” the existing sinus blood volume into exiting distally in parallel with aortic blood flow. The velocity at the proximity of the leaflet between the neo-sinus and the sinus regions at peak systole without laceration was 0.017±0.002m/s, while after laceration was 0.029±0.006m/s. Quantitatively, as shown in Fig.1e, after lacerating the leaflet, the number of cycles to washout in the neo-sinus decreased from 3.07±0.08 cycles to 0.70±0.11 cycles (p-value < 0.0001). Similarly, relatively marginal improvement in washout was also found in the sinus after leaflet laceration, requiring only 1.87±0.13 cycles compared to that without laceration 2.11±0.08 cycles (p < 0.01).
Many factors are implicated in the likelihood of leaflet thrombosis, and the incidence of leaflet thrombus formation after ViV with balloon-expandable TAVs is more common compared to other procedures and other valve types4. Lacerating the leaflet may actually provide a promising solution to avoid this complication or as an additional intervention to prevent re-occurrence of leaflet thrombosis.
In summary, this study examined the potential benefits that leaflet laceration may offer in the context of improving neo-sinus and sinus flow, mitigating relative hemostasis in these areas and likely decreasing the possibility of thrombus formation. These experiments showed that introducing a laceration that allows direct flow between the neo-sinus and the sinus improves sinus washout making it a potentially promising technique to prevent leaflet thrombus. Although this study simulated ViV conditions, it is foreseeable that the same mechanisms would apply to TAVR in native aortic valves, where reduced sinus flow has also been implicated in subclinical leaflet thrombosis and adverse clinical outcomes.
Sources of Funding
The research done was partly supported by National Institutes of Health (NIH) under Award Number R01HL119824.
Footnotes
Disclosures
Dr. Dasi reports having patent applications filed on novel polymeric valves, vortex generators and superhydrophobic/omniphobic surfaces. No other conflicts were reported.
References
- 1.Makki N, Shreenivas S, Kereiakes D and Lilly S. A Meta-Analysis of Reduced Leaflet Motion for Surgical and Transcatheter Aortic Valves: Relationship to Cerebrovascular Events and Valve Degeneration. Cardiovascular Revascularization Medicine. 2018;19:868–873. [DOI] [PubMed] [Google Scholar]
- 2.Midha PA, Raghav V, Sharma R, Condado JF, Okafor IU, Rami T, Kumar G, Thourani VH, Jilaihawi H, Babaliaros V, Makkar RR and Yoganathan A. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neo-sinus. Circulation. 2017;136:CIRCULATIONAHA. 117.029479 1598–1609. [DOI] [PubMed] [Google Scholar]
- 3.Hatoum H, Dollery J, Lilly SM, Crestanello JA and Dasi LP. Implantation Depth and Rotational Orientation Effect on Valve-in-Valve Hemodynamics and Sinus Flow. The Annals of Thoracic Surgery. 2018;106:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jose J, Sulimov DS, El-Mawardy M, Sato T, Allali A, Holy EW, Becker B, Landt M, Kebernik J and Schwarz B. Clinical bioprosthetic heart valve thrombosis after transcatheter aortic valve replacement: incidence, characteristics, and treatment outcomes. JACC Cardiovascular Interventions. 2017;10:686–697. [DOI] [PubMed] [Google Scholar]
- 5.Dvir D, Khan J, Kornowski R, Komatsu I, Chatriwalla A, Mackenson G, Simonato M, Ribeiro H, Wood D and Leipsic J. Novel strategies in aortic valve-in-valve therapy including bioprosthetic valve fracture and BASILICA. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2018;14:AB74–AB82. [DOI] [PubMed] [Google Scholar]