Abstract
Endothelial progenitor cells (EPCs) have been pursued as a potential cellular therapy for stroke and central nervous system injury. However, their underlying mechanisms remain to be fully defined. Recent experimental studies suggest that mitochondria may be released and transferred between cells. In this proof-of-concept study, we asked whether beneficial effects of EPCs may partly involve a mitochondrial phenomenon as well. First, EPC-derived conditioned medium was collected and divided into supernatant and particle fractions after centrifugation. Electron microscopy, western blots and flow cytometry showed that EPCs were able to release mitochondria. ATP and oxygen consumption assays suggested that these extracellular mitochondria may still be functionally viable. Confocal microscopy confirmed that EPC-derived extracellular mitochondria can be incorporated into normal brain endothelial cells. Adding EPC particles to brain endothelial cells promoted angiogenesis and decreased trans-cellular permeability of brain endothelial cells. Next, we asked whether EPC-derived mitochondria may be protective. As expected, oxygen-glucose deprivation increased trans-cellular endothelial permeability. Adding EPC-derived mitochondria particles to the damaged brain endothelium increased levels of mitochondrial protein TOM40, mtDNA copy number, and intracellular ATP. Along with these indirect markers of mitochondrial transfer, endothelial tightness was also restored after oxygen-glucose deprivation. Taken together, these findings suggest that EPCs may support brain endothelial energetics, barrier integrity and angiogenic function partly through extracellular mitochondrial transfer.
Keywords: human endothelial progenitor cells, extracellular mitochondria, brain endothelium, angiogenesis, endothelial tightness
Graphical abstract
Endothelial progenitor cell (EPC)-derived mitochondria particles to damaged brain endothelium after oxygen-glucose deprivation increased levels of mitochondrial protein TOM40, mtDNA copy number, and intracellular ATP along with restoring endothelial tightness after OGD. These findings suggest that EPCs may support brain endothelial energetics, barrier integrity and angiogenic function partly through extracellular mitochondrial transfer.
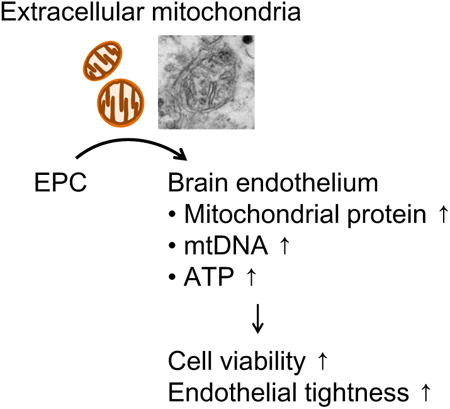
Introduction
Endothelial progenitor cells (EPCs) represent a subset of immature endothelial cells circulating in the bloodstream [1]. EPCs are highly migratory and can home to target areas to regulate angiogenesis and vasculogenesis for tissue homeostasis or repair. In the context of central nervous system (CNS) injury and disease, EPCs may contribute to neurovascular recovery after stroke [2-4]. However, the underlying mechanisms remain to be fully elucidated.
Beneficial effects of any type of cellular therapy should include both direct cell differentiation and replacement as well as indirect pathways via secretion of trophic factors and exchange of help-me signals [5]. Recently, it has been suggested that stem cells may release extracellular mitochondria as part of their potentially beneficial secretome [6]. For example, mesenchymal stromal cells may transfer mitochondria to rescue aerobic respiration in mitochondria-depleted cells [7]. In vivo, bone-marrow-derived mesenchymal stem cells can transfer mitochondria into alveolar epithelium to protect against endotoxin-induced lung injury [8]. The beneficial relevance of mitochondrial transfer in the brain was then supported by a recent study demonstrating that xenogenic transplantation of mitochondria restored respiratory activity and improved long-term outcome in a rat model of focal cerebral ischemia [9]. In this proof-of-concept study, we asked whether human EPCs can produce functional extracellular mitochondria and whether these can be transferred into brain endothelial cells to support their function in both physiological and pathological conditions.
Materials and Methods
Details in Supplementary Material.
Results
Human EPCs were identified by representative markers including vWF, lectin-UEA1, CD34, and Flk1 (Figure 1A). EPC-conditioned medium was collected and after centrifugation, supernatant and particle fractions were analyzed (Figure 1B). Western blots demonstrated that the mitochondrial membrane protein TOM40 was enriched in particle fractions (Figure 1C), along with higher ATP levels (Figure 1D). Flow cytometry with MitoTracker Red was consistent with the presence of extracellular mitochondria particles (Figure 1E). Measurements of oxygen consumption suggested that these extracellular mitochondria may still be functional (Figure 1F). Finally, electron microscopy confirmed the presence of extracellular mitochondria particles in EPC-conditioned media (Figure 1G).
Figure 1. Human endothelial progenitor cells (EPC) produced extracellular mitochondria.
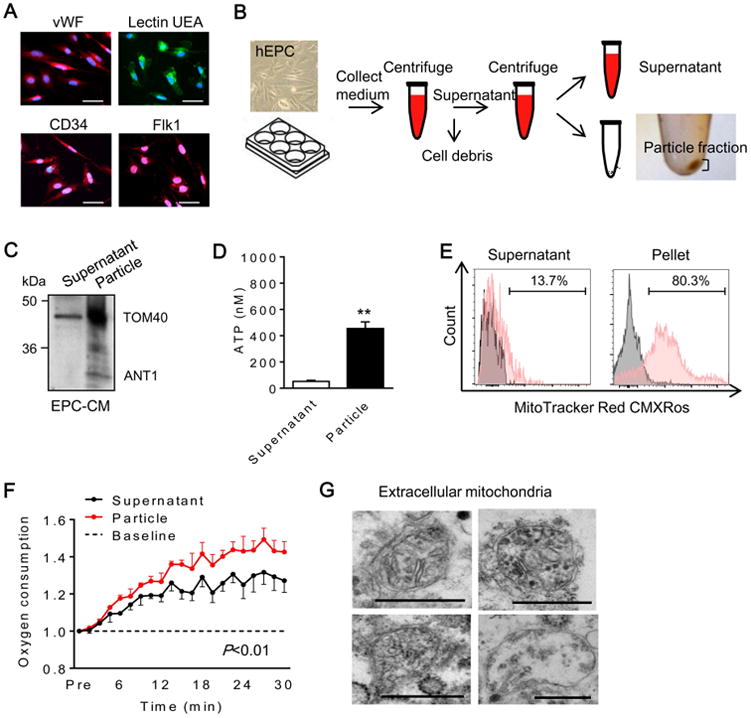
(A) Human EPC were identified by representative markers including vWF, lectin UEA, CD34 and Flk1. Scale: 50 μm. (B) Experimental design to separate fractions from EPC-derived conditioned medium. First centrifuge; 2,000 rpm for 10 min, second centrifuge; 12,000 rpm for 15 min. (C) Western blot confirmed mitochondrial proteins in EPC-derived extracellular fractions. TOM40; mitochondrial outer membrane protein, ANT1; mitochondrial inner membrane protein. (D) ATP measurement showed higher ATP content in pellet compared to supernatant (n=5). **P<0.01. (E) Flow cytometry analysis demonstrated that pellet highly contained MitoTracker positive particles compared to supernatant. (F) Oxygen consumption analysis in EPC-derived pellet and supernatant. EPC-derived pellet had higher oxygen consumption compared to supernatant (n=3). (G) Electron microscopy revealed that EPC-derived pellet contained mitochondria. Scale: 500 nm.
For context, we also examined media derived from human astrocytes, endothelial cells, and pericytes because they are known to release extracellular particles and support vascular function [10-12]. Flow cytometry was performed to examine extracellular particles (Figure 2A, Supplemental Figure 1). Assessment of CD63-positive versus mitochondria-positive subpopulations suggested that EPC-derived extracellular particles may contain comparable fractions of mitochondria as those found in astrocytes, endothelial cells and pericytes (Figure 2B-C). Semi-quantitative flow cytometry data confirmed the ability of EPCs to produce high levels extracellular mitochondria that retained high membrane potentials (Figures 2D-E). Taken together, these findings suggest that cell for cell, human EPCs can be a prolific source of active extracellular mitochondria.
Figure 2. Comparison of extracellular mitochondria among human cells.
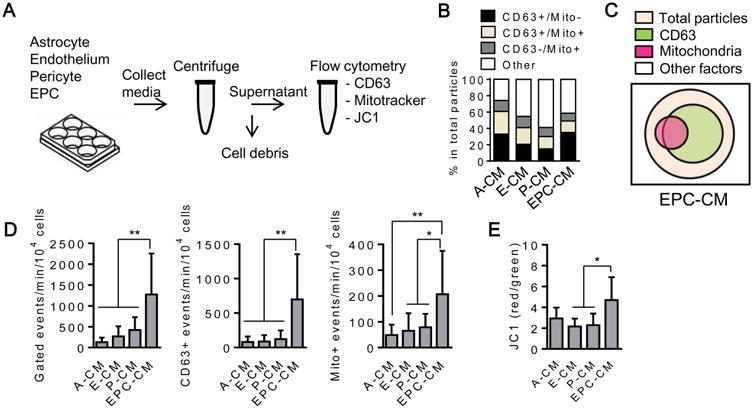
(A) Experimental design for flow cytometry analysis using conditioned media collected from multiple human cell types such as astrocyte, brain endothelium, pericyte, and EPC. Centrifuge; 2,000 rpm for 10 min. (B) Each cell type conditioned medium was incubated with CD63-FITC (1 μg/mL), Mitotracker Deep Red (100 nM), or JC1 (0.5 μM) for 30 min at 37°C, then flow cytometry was performed. EPC-derived extracellular particles may contain comparable profiles of mitochondria as those found in astrocytes, endothelial cells and pericytes. CD63 is used to identify a subset of extracellular particles/vesicles. CD63+/Mito+: sub-population of CD63-positive extracellular particles/vesicles that contain mitochondria, CD63-/Mito+: “naked” mitochondria. (C) Venn diagram schematic to summarize the conceptual distribution of extracellular components in EPC-CM. (D) Normalized quantification of flow cytometry showed that EPCs secreted a high amount of CD63 positive particles and mitochondria per 104 cells compared to other cell types (n=9). *P<0.05, **P<0.01. (E) JC1 analysis by flow cytometry demonstrated that extracellular mitochondria from EPCs had higher membrane potential compared to other cell types (n=6). *P<0.05.
Next, we tested the effects of EPC-conditioned media on assays of endothelial function. Confocal microscopy demonstrated that normal brain endothelial cells were capable of incorporating EPC-derived extracellular mitochondria (Figure 3A). In a matrigel tube formation assay, both supernatant and particle fractions increased angiogenesis in human brain endothelial cells (Figure 3B, Supplemental Figure 2A). No increase in tube formation was observed when ATP-loaded liposome controls were added to brain endothelium (Figure 3C).
Figure 3. EPC-derived particles positively regulate angiogenesis and endothelial tightness.
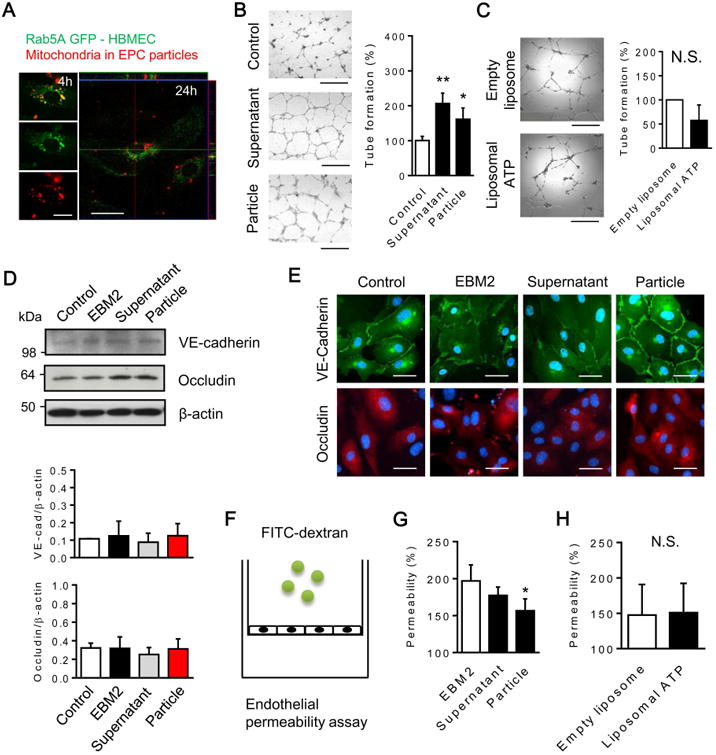
(A) EPCs were pre-labeled by MitoTracker Red CMXRos and EPC-derived particles (fraction of pellet) were isolated after centrifugation. Then, the pellet was added to normal brain endothelial cells. Confocal microscopy confirmed incorporating MitoTracker positive particles in brain endothelial cells. Rab5a-GFP was used to indicate intracellular space. Scale: 20 μm. (B) Both supernatant and pellet isolated from EPC-conditioned media increased the number of rings in in vitro tube formation assay (n=4). Scale: 100 μm. *P<0.05, **P<0.01 vs. control. (C) Liposomal ATP did not increase the number of rings (n=3). Scale: 100 μm. (D) Western blot analysis showed that there were statistically no difference in VE-cadherin and occludin among groups. (E) Immunocytochemistry demonstrated that membrane localization of VE-cadherin was recovered by treatment with the pellet. Scale: 50 μm. (F) Scheme for our dextran permeability assay. (G) Compared to EBM-2 (basal medium), EPC-derived pellet significantly improved endothelial tightness in the dextran permeability assay during serum starvation (n=4). *P<0.05 vs. EBM-2. (H) Liposomal ATP did not improve endothelial permeability during serum starvation (n=3). Note: Centrifugation cannot perfectly separate these mixed and overlapping populations of EPC components. Please see Suppl Fig 2A for Venn diagram schematic that summarizes the conceptual distribution of EPC-CM components isolated in this experiment.
Besides angiogenesis, barrier function is a key aspect of brain endothelium. VE-cadherin and occludin were examined as representative adherens junction and tight junction proteins respectively. Western blots did not show any difference in protein expression (Figure 3D, Supplemental Figure 2A). However, immunocytochemistry suggested that the particle fraction of EPC media enhanced VE-cadherin membrane localization (Figure 3E, Supplemental Figure 2A), and decreased trans-endothelial permeability (Figure 3F-G, Supplemental Figure 2A). ATP-loaded liposome controls had no effects (Figure 3H).
Finally, we asked whether EPC-derived mitochondria might also protect against injury. Brain endothelial cells were subjected to oxygen-glucose deprivation for 3 hours, then treated with EPC-derived particles or equivalent levels of empty media transfer (Figure 4A). Western blots confirmed that mitochondrial protein TOM40 was increased in the damaged endothelium after treatment with EPC particles (Figure 4B, Supplemental Figure 2B). Intracellular mtDNA and ATP levels were also partially restored (Figure 4C-D, Supplemental Figure 2B). These indirect markers of mitochondria transfer were accompanied by an improvement in endothelial tightness after oxygen-glucose deprivation (Figure 4E, Supplemental Figure 2B). To further support this idea, we used a different method to isolate extracellular mitochondria from EPCs. Extracellular mitochondria were isolated from EPC conditioned media by FACS and then key endpoints were re-assessed. Following nucleotide staining by PicoGreen, we confirmed that mtDNA was sufficiently labeled as previously described [13] (Figure 4F-G). Thereafter, treatment with purified extracellular mitochondria (5×105 per well) restored trans-endothelial permeability and improved cell viability in brain endothelial cultures following OGD (Figure 4H, Supplemental Figure 2C).
Figure 4. EPC-derived particles restored brain endothelial energetics and integrity after oxygen-glucose deprivation (OGD).
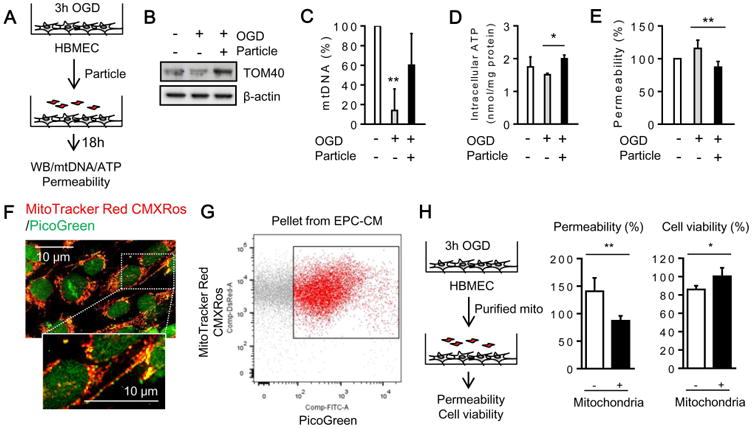
(A) Scheme of the experimental design. Human brain endothelial cells subjected to OGD for 3 hours were co-incubated with EPC-derived particles for 18 hours. (B) Western blot confirmed that particles increased mitochondrial protein TOM40 in brain endothelial cells. (C) MtDNA content was analyzed by Real-time PCR. MtDNA in brain endothelial cells was significantly decreased after OGD, but EPC-derived particles restored mtDNA content (n=3). **P<0.01 vs. control mtDNA. (D) Intracellular ATP was also restored by the treatment with EPC-derived particles (n=3). *P<0.05. (E) Endothelial permeability assay demonstrated that EPC-derived particles restored endothelial tightness after OGD (n=4). **P<0.01. (F) PicoGreen staining (3 μL/mL) successfully labeled mtDNA in EPCs. Scale: 10 μm. (G) FACS was performed to isolate PicoGreen-positive extracellular mitochondria in pellets collected from EPC-CM. (H) Particles (5×105 per well) restored endothelial tightness and cell viability after OGD/reoxygenation (n=4). *P<0.05, **P<0.01. Note: Please see Suppl Fig 2B-C for Venn diagram schematic that summarizes the conceptual distribution of EPC-CM components isolated in this experiment.
Discussion
Interactions between brain and circulating blood cells profoundly influence mechanisms of injury and repair in the CNS [14]. Peripheral administration of CD34+ cells promoted angiogenesis and enhanced endogenous neurogenesis after focal cerebral ischemia [15]. In humans, circulating EPC levels track the temporal profile of stroke recovery [16]. However, the underlying mechanisms of EPC-brain interactions are complex and remain poorly understood. In this study, we showed that (i) human EPCs can produce extracellular functional mitochondria, and (ii) transfer of EPC-derived mitochondria into brain endothelial cells promote angiogenesis, support barrier function and ameliorate injury after oxygen-glucose deprivation.
Mitochondria function is critical to maintain neurovascular function under the physiological and pathophysiological condition in the CNS [17, 18]. Recently, intercellular mitochondrial transfer has been proposed as a new paradigm for cell-cell signaling [19, 20]. Mesenchymal stem cells can transfer mitochondria to rescue metabolism in target cells and protect against tissue injury [8]. In the heart, mitochondrial transplantation can protect myocytes against ischemia-reperfusion injury [21]. In the CNS, astrocytes and microglia may exchange mitochondria with adjacent neurons for neuroprotection [22, 23]. Our findings suggest that EPCs may also utilize mitochondria transfer for protecting brain endothelium. Furthermore, although the majority of data on the protective vascular effects of EPCs have been collected in experimental animal models [24], our data suggest that EPC-mediated mitochondrial transfer and endothelial protection may also occur in human cells.
If EPCs can indeed transfer mitochondria into brain endothelium, what intracellular signals link upstream mitochondrial incorporation to downstream regulation of endothelial function? mtDNA can increase components of the respiratory chain and ATP synthase, so it is possible that protective effects are mediated via a general rescue of metabolic integrity. Additionally, mtDNA may upregulate Fgf2 [25], and FGF-2 is known to protect endothelium [26]. In our model system, a preliminary assessment was performed using a vascular proteome array. Brain endothelial cells were deprived of oxygen and glucose for 3 hours, then EPC-derived extracellular particles containing Mitotracker-labeled mitochondria were added, and flow cytometry was used to collect brain endothelium that contained EPC-derived mitochondria (Supplemental Figure 3A). Overall, many angiogenesis and blood-brain barrier-related proteins were upregulated, including bFGF (FGF-2), FGF-4, plasminogen, and serpin E1 (Supplemental Figure 3B-C). Future studies are required to map the effects of EPC-derived mitochondria on endothelial genes and proteins.
The present proof-of-concept findings are observational, and there are several caveats. First, the molecular mechanisms of EPC mitochondrial release-uptake remain unknown. In terms of mitochondrial uptake, our experiments are based on media transfer, and so macropinocytosis [27] and integrin-mediated endocytosis [22] may potentially be involved in internalization of mitochondria. But other modes such as tunneling nanotubes [28] may also play a role. Second, the EPC secretome is complex. In general, stem cells release a complex mix of exosomes and microvesicles. How mitochondria are distributed within this spectrum of extracellular particles require careful study. For example, EPC-derived microvesicles containing mRNA and miRNA may induce angiogenesis [29, 30]. How extracellular mitochondria may interact with other EPC signals should be further examined. Finally, other CNS cells will be involved. For example, pericytes are a rich source of pro-angiogenic exosomes [31], and astrocytes produce extracellular mitochondria and exosomes carrying mtDNA [22, 32]. Further studies are warranted to explore whether and how extracellular mitochondria from the neurovascular unit may contribute to EPC-endothelium interactions.
EPCs may serve as adult stem cells for endothelial repair and tissue vascularization. Here, we provided proof-of-concept that human EPCs secrete mitochondrial particles that may support and protect brain endothelial function. It is important to emphasize that these findings do not mean that mitochondrial transfer is the most important “mode of action” for EPCs. The EPC secretome comprises a rich complex repertoire of soluble factors and extracellular particles/vesicles that contain other material such as miRNA, mRNA, peptides and proteins. The present study suggests that mitochondrial exchange between EPCs and brain endothelium may be another relevant “mode of action”, and so this phenomenon should be further explored as part of an overall cellular strategy for therapies against CNS injury and disease.
Supplementary Material
Supplemental Figure 1. (A) Flow cytometry analysis in each conditioned medium (CM) collected from human EPC (E-CM), human astrocyte (A-CM), human brain endothelial cell (E-CM), and human pericyte (P-CM). (B) JC1 assessment in flow cytometry.
Supplemental Figure 2. (A) EPC-CM is composed of particles (including CD63+ particles and mitochondria) and other soluble factors. After centrifugation, EPC-CM is separated to mitochondria-enriched pellet (particle) and supernatant. Each fraction was added to normal endothelial cells in order to test angiogenic response and cell permeability in Figure 3. (B) Mitochondria-enriched pellet (particle) was treated in brain endothelium after oxygen-glucose deprivation (OGD) in Figure 4A to 4E. (C) Extracellular mitochondria were purified by FACS and treated in OGD-injured brain endothelium in Figure 4F to 4H.
Supplemental Figure 3. (A) Brain endothelial cells were subjected to oxygen-glucose deprivation (OGD), then EPC-derived particles were added to brain endothelium culture. FACS was performed at 18 hours after incubation showing EPC-mitochondria transferred into endothelial cells. (B) Representative blot-array and angiogenic proteome analysis. (C) Proteome analysis showed that many angiogenesis and blood-brain barrier-related proteins were upregulated, including bFGF, FGF-4, plasminogen, and serpin E1 in EPC-derived mitochondria positive endothelial cells compared with other cells which did not incorporate extracellular mitochondria (n=3).
Acknowledgments
This research was supported in part by the National Institutes of Health, the Miguel Servet program (CPII15/00003), and the Rappaport Foundation.
Footnotes
Author's contributions: KH: Conception and design, financial support, administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.\SJC, ETM, JHP, MB: Collection and assembly of data, data analysis and interpretation.\KA: Conception and design, financial support.\JM, AR: Conception and design, financial support, provision of study patients, manuscript writing, final approval of manuscript.\EHL: Conception and design, financial support, administrative support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest: There is no conflict of interest.
References
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko Y, Tajiri N, Shinozuka K, et al. Cell therapy for stroke: emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr Pharm Des. 2012;18:3731–3734. doi: 10.2174/138161212802002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morancho A, Ma F, Barcelo V, et al. Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:1547–1551. doi: 10.1038/jcbfm.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht N, Schneider UC, Czabanka M, et al. Endothelial progenitor cells augment collateralization and hemodynamic rescue in a model of chronic cerebral ischemia. J Cereb Blood Flow Metab. 2014;34:1297–1305. doi: 10.1038/jcbfm.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing C, Lo EH. Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery. Prog Neurobiol. 2017;152:181–199. doi: 10.1016/j.pneurobio.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz FF, Rocco PRM. Stem-cell extracellular vesicles and lung repair. Stem Cell Investig. 2017;4:78. doi: 10.21037/sci.2017.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinclair KA, Yerkovich ST, Hopkins PM, et al. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther. 2016;7:91. doi: 10.1186/s13287-016-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang PJ, Kuo CC, Lee HC, et al. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transplant. 2016;25:913–927. doi: 10.3727/096368915X689785. [DOI] [PubMed] [Google Scholar]
- 10.Todorova D, Simoncini S, Lacroix R, et al. Extracellular Vesicles in Angiogenesis. Circ Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villasenor R, Kuennecke B, Ozmen L, et al. Region-specific permeability of the blood-brain barrier upon pericyte loss. J Cereb Blood Flow Metab. 2017;37:3683–3694. doi: 10.1177/0271678X17697340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms HC, Aldana BI, Groth S, et al. Characterization of the L-glutamate clearance pathways across the blood-brain barrier and the effect of astrocytes in an in vitro blood-brain barrier model. J Cereb Blood Flow Metab. 2017;37:3744–3758. doi: 10.1177/0271678X17690760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley N, Harris D, Poulton J. Detection of mitochondrial DNA depletion in living human cells using PicoGreen staining. Exp Cell Res. 2005;303:432–446. doi: 10.1016/j.yexcr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Kannurpatti SS. Mitochondrial calcium homeostasis: Implications for neurovascular and neurometabolic coupling. J Cereb Blood Flow Metab. 2017;37:381–395. doi: 10.1177/0271678X16680637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutkai I, Merdzo I, Wunnava SV, et al. Cerebrovascular function and mitochondrial bioenergetics after ischemia-reperfusion in male rats. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17745028. 271678X17745028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa K, Bruzzese M, Chou SH, et al. Extracellular Mitochondria for Therapy and Diagnosis in Acute Central Nervous System Injury. JAMA Neurol. 2018;75:119–122. doi: 10.1001/jamaneurol.2017.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gollihue JL, Rabchevsky AG. Prospects for therapeutic mitochondrial transplantation. Mitochondrion. 2017;35:70–79. doi: 10.1016/j.mito.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaza AK, Wamala I, Friehs I, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153:934–943. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocca CJ, Goodman SM, Dulin JN, et al. Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich's ataxia. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaj2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, et al. Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke. 2008;39:2158–2165. doi: 10.1161/STROKEAHA.107.507251. [DOI] [PubMed] [Google Scholar]
- 25.Bueno M, Lai YC, Romero Y, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendfeldt K, Radojevic V, Kapfhammer J, et al. Basic fibroblast growth factor modulates density of blood vessels and preserves tight junctions in organotypic cortical cultures of mice: a new in vitro model of the blood-brain barrier. J Neurosci. 2007;27:3260–3267. doi: 10.1523/JNEUROSCI.4033-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitani T, Kami D, Matoba S, et al. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J Cell Mol Med. 2014;18:1694–1703. doi: 10.1111/jcmm.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 30.Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 31.Gaceb A, Ozen I, Padel T, et al. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab. 2018;38:45–57. doi: 10.1177/0271678X17719645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guescini M, Genedani S, Stocchi V, et al. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A) Flow cytometry analysis in each conditioned medium (CM) collected from human EPC (E-CM), human astrocyte (A-CM), human brain endothelial cell (E-CM), and human pericyte (P-CM). (B) JC1 assessment in flow cytometry.
Supplemental Figure 2. (A) EPC-CM is composed of particles (including CD63+ particles and mitochondria) and other soluble factors. After centrifugation, EPC-CM is separated to mitochondria-enriched pellet (particle) and supernatant. Each fraction was added to normal endothelial cells in order to test angiogenic response and cell permeability in Figure 3. (B) Mitochondria-enriched pellet (particle) was treated in brain endothelium after oxygen-glucose deprivation (OGD) in Figure 4A to 4E. (C) Extracellular mitochondria were purified by FACS and treated in OGD-injured brain endothelium in Figure 4F to 4H.
Supplemental Figure 3. (A) Brain endothelial cells were subjected to oxygen-glucose deprivation (OGD), then EPC-derived particles were added to brain endothelium culture. FACS was performed at 18 hours after incubation showing EPC-mitochondria transferred into endothelial cells. (B) Representative blot-array and angiogenic proteome analysis. (C) Proteome analysis showed that many angiogenesis and blood-brain barrier-related proteins were upregulated, including bFGF, FGF-4, plasminogen, and serpin E1 in EPC-derived mitochondria positive endothelial cells compared with other cells which did not incorporate extracellular mitochondria (n=3).


