Abstract
Background:
Dental caries is an important public health problem in Mexico, a country also faced with high exposure to toxicants including lead (Pb).
Methods:
Participants were 386 children living in Mexico City. Prenatal (trimester 1–3), early-childhood (12, 24, 36, and 48 months of age) and peri-pubertal (10–18 years of age) blood Pb levels were quantified using graphite-furnace atomic-absorption spectroscopy. Maternal patella and tibia bone Pb at 1 month postpartum were quantified with K X-ray fluorescence instrument. Dental caries presence was evaluated using decayed, missing, and filled teeth (DMFT) scores. Peri-pubertal sugar sweetened beverage (SSB) intake was estimated using a 116-item, interview-administered semi-quantitative food frequency questionnaire (FFQ). Total energy adjusted daily SSB intake was generated using the residual approach. Zero inflated negative binomial (ZINB) Poisson regression models were used to examine the associations between Pb with D1MFT and D4MFT at adolescence.
Results:
Maternal second and third trimester and cumulative early childhood Pb exposure were positively associated with peri-pubertal D1MFT scores in unadjusted ZINB models (2nd trimester: RR=1.17 (1.00, 1.37); 3rd trimester: RR=1.20 (1.03, 1.40); early childhood: RR=1.22 (1.02, 1.48)). These effect sizes were attenuated and no longer statistically significant after adjusting for covariates. When stratified by high/low SSB intake, a one unit increase of log-transformed 2nd trimester Pb exposure was associated with a 1.41 times (1.06, 1.86) higher D1MFT count, and 3rd trimester Pb exposure was associated with a 1.50 times (1.18, 1.90) higher D1MFT count among those with higher than median peri-pubertal SSB. Associations among those with lower SSB intake were roughly half those of the higher group and not statistically significant.
Conclusions:
Pb exposure during sensitive developmental periods was not statistically significantly associated with caries risk after accounting for confounders among our cohort. However, evidence from stratified analysis suggested a Pb-caries association among children with high SSB intake.
Keywords: dental caries, prenatal lead exposure, childhood cumulative lead exposure, sugar sweetened beverage intake, permanent teeth, DMFT score
INTRODUCTION
Dental caries is one of the most prevalent diseases of people worldwide [1]. Despite great achievements in improving oral health, dental caries remain a major health problem in most developing countries, affecting 60–90% of school children [2].
Untreated dental caries among children may cause discomfort and pain and is associated with weight gain and short stature, poor quality of life and cognitive development delay [3]. Fortunately, dental caries is also one of the most preventable childhood afflictions [4, 5]. Potentially modifiable risk factors for caries include diet, inadequate salivary flow, insufficient fluoride exposure, and poor oral hygiene [1]. Among dietary factors, sugar-sweetened beverage (SSB) consumption is most strongly and consistently associated with higher risk of dental caries [6].
Another modifiable exposure that may relate to dental caries risk is lead (Pb) [7, 8, 9]. There are some potential mechanisms to explain a Pb and dental caries relationship. Moss et al. proposed three different mechanisms that linked Pb exposure with dental caries, including salivary gland function, enamel formation, and interference with fluoride in saliva [8]. Known as one of the “bone-seeking” elements, Pb from blood tends to be incorporated into calcified tissues such as bone and teeth, where it can remain for years [10]. From calcified tissue reservoirs, Pb is slowly released, depending on bone turnover rates, and the release rate of Pb from bone varies with age and intensity of exposure [10]. Thus, the detrimental effect of Pb exposure on dental caries could be due to Pb incorporated into the teeth that delay the mineralization of enamel [11]. In animal studies, Pb exposure has been associated with disrupted gut microbiota composition and inflammation status [12, 13, 14].
Several population-based studies [7, 8, 9, 15] suggest an association between Pb levels and dental caries. For example, a cross-sectional epidemiologic study conducted among 1,564 Korean children showed that the prevalence of decayed, missing and filled surfaces (DMFS) in deciduous, but not permanent teeth, increased with each mg/dl of childhood blood Pb exposure [15]. Among 543 urban U.S children 6–10 years old, blood Pb levels in childhood were positively associated with number of caries, after adjustment for demographic and maternal factors and oral care practices [9]. However, these and other studies [7, 8, 9, 15] have several limitations, including cross-sectional study designs [7, 8, 15]; Pb measurements in blood or salivawhich may not represent long-term exposure [8, 9]; and a limited number of tooth samples collected from each individual [7].
In addition, no studies have examined if the timing of Pb exposure changes risk of dental caries. Previous studies have focused on a single exposure period during childhood or adolescence and thus were unable to pinpoint potential sensitive windows of exposure. One potentially sensitive time period which has not been examined previously is prenatal Pb exposure [16]. Although there are no human studies to date, some animal studies suggest an effect of maternal Pb exposure and osteoblast/osteoclast function in the mothers on the offspring [16, 17, 18], which could indirectly be linked to dental caries risk.
To address these research gaps, we conducted a secondary analysis of a cohort study of Mexican children. The primary study aim was to examine the associations between lead exposure at multiple sensitive periods and decayed, missing, filled tooth (DMFT) scores at adolescence. A secondary aim was to evaluate whether there was an interaction between Pb exposure and SSB intake in relation to caries risk in adolescence. We hypothesized that an association between Pb exposure and caries would be more evident among participants with high SSB intake since SSBs are one of the most robust predictors of dental caries among children [6, 19].
METHODS
3.1. Study Population
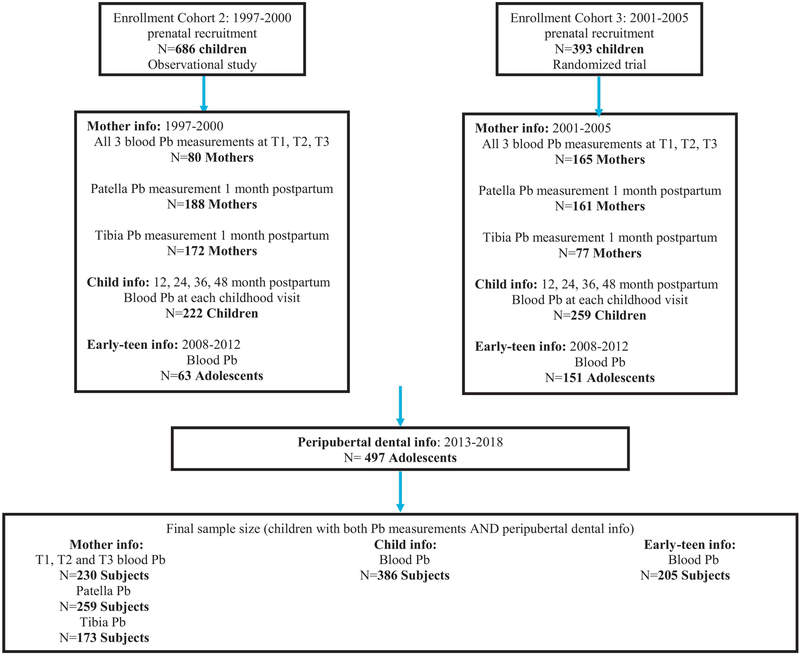
The study population comprises a subset of participants from the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) project, a longitudinal epidemiological study consisting of three sequentially enrolled birth cohorts: enrollment cohort 1, 2, and 3 (Figure 1). A detailed description of the ELEMENT cohort can be found elsewhere [20]. In brief, the mother/child pairs were recruited between 1997 and 2005 at three maternity hospitals representing a low- to moderate-income population in Mexico City. At the baseline clinic visit, mothers provided household and demographic information including age, education, and number of previous pregnancies. Of the initial 1,382 mothers who met eligibility criteria, 617 agreed to participate and continued in the study (Figure 1). Of these, 245 had blood samples collected at all three trimester visits, 349 had patella Pb measured and 249 had tibia Pb measured 1 month postpartum. Their newborns were followed from birth until 4 years of age; blood samples were collected every 12 months. Starting in 2008, we re-contacted a subset of the offspring (n=250; henceforth referred to as the early-teen visit) from enrollment cohorts 2 and 3 based on availability of prenatal and neonatal biospecimens. One more peri-pubertal visit was completed approximately 5 years later (549; henceforth referred to as the peri-pubertal visit), again recruiting children from cohorts 2 and 3, that had prenatal and neonatal biospeciments available. Of those, 497 adolescents had their dental information collected (Figure 1).
Figure 1:
Selection of ELEMENT subjects for the study.
Mothers provided written consent upon enrollment in the study, and children also provided assent at early-teen and peri-pubertal visits. The research protocol was approved by the Human Subjects Committee and participating institutes including the National Institute of Public Health of Mexico, hospitals, and the University of Michigan.
3.2. Laboratory Measurements
Blood Lead (Pb)
Maternal blood (trimester 1–3), and participant blood samples during childhood (12, 24, 36, and 48 months of age) and adolescence (10–18 years of age) were collected and stored in trace-metal–free tubes by trained research assistants using standardized protocols. All samples were measured using graphite-furnace atomic-absorption spectroscopy (model 3000; Perkin-Elmer, Chelmsford, MA, USA) at the research facility of the American British Cowdray Hospital in Mexico City as previously described [21, 22]. All blood Pb levels were above the limit of detection and the precision of this instrument is within 1 μg/dL.
Maternal Postpartum Bone Lead (Pb)
Maternal patella and tibia bone postnatal measurements, which are considered proxies for cumulative prenatal Pb exposure, were obtained using a K X-ray fluorescence instrument [23]. The two estimates for bone lead measurements (one for each leg) were computed, averaged, and weighted by the inverse of the proportion of the measurement error corresponding to each determination [24]. Previous validation test showed K-X-ray fluorescence (K-XRF) instruments measured bone Pb levels correspond to cumulative blood Pb indices [23].
Dental Outcomes
Of the 549 child participants who completed the peri-pubertal visit, 497 had dental information collected, with a total of 13,860 teeth examined. The dental examination was conducted by a trained and calibrated licensed pediatric dentist trained in using the International Caries Detection and Assessment System (ICDAS) [24, 25]. Training was provided by a 2-day in vitro exercise using extracted teeth mounted in dentoform models. In vivo training consisted of a 2-day examination of 30 subjects. Scores were compared with a senior examiner who was previously trained in using the ICDAS.
Prior to the ICDAS exam, the examiner brushed subjects’ teeth using a soft toothbrush. Flossing was not performed. The dental exams were performed with subjects seated in a portable dental chair. Lighting was provided by a portable standard dental light. Cotton rolls were used for isolation, and teeth were dried using compressed air. Examination was done with the aid of a front surface mirror, and a blunt explorer was available to clean the pits and fissures as well as to evaluate cavitations. Standard infection control was followed for each examination.
The examiner evaluated each tooth surface according to the ICDAS index [26]. The index codes classify six stages of caries, from the first white spot lesion in dry enamel to extensive cavitation involving over half the tooth surface. Information on lesion severity and activity and presence of fillings and extracted/exfoliated teeth was also recorded. All extracted/exfoliated teeth were diagnosed and recorded, but only teeth classified as “missing due to caries” were counted in the analysis.
Covariates
Based on a priori knowledge and bivariate analysis, covariates included in final models were sex, cohort, mother’s education and sugar sweetened beverages (SSB) intake during adolescence. Years of mother’s education were reported at the prenatal baseline visit, and categorized into 4 groups: “Did not complete secondary,” “Completed some high school,” “Completed high school,” and “Higher education.” During the early-teen visit (2010), dietary intake over the past week was collected using a 116-item, interview-administered semi-quantitative food frequency questionnaire (FFQ) adapted from the 2006 Mexican Health and Nutrition Survey [27]. The questionnaire asked participants to recall how often they typically consumed one serving of a standard portion size of each food item; response options ranged from never to ≥6 times per day. Children under 12 years of age were assisted by their caregiver in reporting usual food intakes. Total daily energy (kcal) intake was estimated by multiplying frequency values by the kcal in each food serving and then summing over all foods consumed [28]. Total energy adjusted daily SSB intake was generated using the residual approach [28].
3.3. Statistical Methods
We constructed a measure of cumulative childhood Pb exposure by calculating the area under each child’s age-by-blood-Pb curve from 12 to 48 months as previously described [29]. A cumulative time-integrated blood Pb index up to the time of the individual’s first blood test was calculated by the trapezoidal rule [23, 30].
DMFT/deft and caries prevalence (DMFT >0) indices were obtained in accordance with WHO assessment criteria. We calculated DMFT for each tooth by adding decayed, missing, and filled surfaces score. Then, the DMFT score for each individual was then generated by aggregating the score from 28 teeth. The difference between D1MFT and D4MFT calculation was D4MFT only counted caries coded as 4 and above, based on ICDAS scale [26].
We conducted bivariate analysis of DMFT outcomes (proportion of participants with any DMFT scores and means ± SD DMFT scores) and categories of maternal and study characteristics, including sex, cohort, mother’s education, socioeconomic status, SSB, zinc, calcium, phosphorus intake, and urinary/water fluoride content according to a priori findings. For ordinal and continuous characteristics (e.g. mother’s education levels, socioeconomic status, SSB, zinc and calcium intake, water and urinary fluoride content), we conducted a test for linear trend by including in the model a continuous variable representing the ordinal levels of the characteristic. For nominal characteristics (e.g. sex, cohort), we utilized a type III Wald test. Using similar methodology, we evaluated Pb exposure at each time period according to categories of maternal and study characteristics. We included sex, cohort, and mother’s education as potential confounders and accounted for sugar sweetened beverages (SSB) intake during adolescence as a source of extraneous variation in final adjusted model based on a priori knowledge and bivariate analysis results.
We used zero inflated negative binomial (ZINB) Poisson regression techniques to evaluate the associations between Pb exposure and dental caries presence (D1MFT and D4MFT scores). This method is appropriate because DMFT scores are count variables and there was evidence of overdispersion and excess zeros. We also evaluated potential effect modification at each life-stage by stratifying by energy-adjusted SSB intake (split at the median) using the same ZINB method. All the analyses were conducted with SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA), with a significance level of p < 0.05.
RESULTS
Among the 386 children with childhood blood lead samples and peri-pubertal DMFT scores, there were 186 males (48.19%) and 200 females (51.81%) aged 10 to 18 (mean = 14, SD=1.96) at follow-up. The decayed-ICDAS lesion score of 1, missing, and filled teeth (D1MFT) score ranged from 0 to 20 with a mean of 5.04; the decayed-ICDAS lesion score of 4, missing, and filled teeth (D4MFT) score ranged from 0 to 12 with a mean of 1.17. The prevalence of zero scores (i.e. no decayed, missing, or filled teeth) were 22.80% for D1MFT and 59.07% for D4MFT, respectively (Table 1). The average prenatal Pb was 6.21 μg/dL (range =0, 35.80 μg/dL) at the 1st trimester, 5.25 μg/dL (range =0, 38.20 μg/dL) at the 2nd trimester and 5.71 μg/dL (range =0, 34.00 μg/dL) at the 3rd trimester. The average cumulative early childhood (age 1–4 years) Pb exposure was 15.33 μg/dL (range =5.19, 76.75 μg/dL), and the average Pb exposure at peri-pubertal period was 3.46 μg/dL (range =0.99, 20.00 μg/dL). In terms of bone Pb exposure, the average Pb content in mother’s patella was 9.16 μg/g (range =−13.57, 47.07 μg/g), and was 7.96 μg/g (range =−15.57, 34.51 μg/g) in the tibia. Individual Pb levels were positively correlated across time periods, with varying strengths (ranging from 0.11 to 0.72). The highest correlations were between close periods. For instance, children of mothers who had high Pb exposure during the 1st trimester tended to have high Pb exposure during the 2nd and the 3rd trimesters. Mothers with high postpartum patella Pb levels tended to have high tibia Pb levels, too. However, there was a weaker positive correlation between Pb exposures in the maternal period and Pb exposures in early childhood or adolescent periods (Supplemental Table 1).
Table 1:
Associations between sociodemographic and lifestyle confounders and peri-pubertal DMFT scores among 386 Mexico City adolescents.
| Table 1: Original to this manuscript. | |||||
|---|---|---|---|---|---|
| Sociodemographic Characteristics | N | % of D1MFT >0 | D1MFT: Mean (±SD) | % of D4MFT>0 | D4MFT: Mean (±SD) |
| 386 | |||||
| Child’s Sex | |||||
| Male | 186 | 74.73 | 4.71±4.47 | 35.48 | 1.05±1.92 |
| Female | 200 | 79.50 | 5.34±4.40 | 46.00 | 1.28±1.84 |
| P value1 | 0.27 | 0.16 | 0.04 | 0.37 | |
| Enrollment Cohort | |||||
| 2 (1997–2000) | 144 | 79.86 | 6.07±4.55 | 52.08 | 1.71±2.34 |
| 3 (2001–2005) | 242 | 75.62 | 4.42±4.26 | 34.30 | 0.85±1.46 |
| P value | 0.34 | <0.001 | <0.001 | <0.001 | |
| Mother’s Education (y) | |||||
| Did not complete secondary (<9) | 48 | 77.08 | 6.00±4.87 | 35.42 | 1.04±1.80 |
| Completed some high school (9 to <12) | 156 | 79.49 | 5.05±4.28 | 48.08 | 1.28±1.97 |
| Completed high school (12) | 129 | 75.97 | 4.91±4.54 | 37.21 | 0.99±1.67 |
| Higher education (>12) | 53 | 73.58 | 4.43±4.21 | 33.96 | 1.38±2.16 |
| P trend2 | 0.46 | 0.10 | 0.26 | 0.87 | |
| Peri-pubertal Sugar Sweetened Beverage (SSB) Intake: Mean (ml) | |||||
| 1stQuartile: 141.52 | 46 | 72.63 | 4.89±4.60 | 44.21 | 1.25±1.77 |
| 2nd Quartile: 399.74 | 59 | 82.69 | 5.18±4.13 | 40.38 | 1.35±2.21 |
| 3rd Quartile: 663.00 | 48 | 78.72 | 5.38±4.87 | 45.74 | 1.17±1.70 |
| 4th Quartile: 1097.33 | 50 | 74.19 | 4.67±4.17 | 33.33 | 0.88±1.76 |
| P trend | 0.98 | 0.81 | 0.23 | 0.13 | |
P value from 2-sample t test.
P value from linear regression analysis.
In bivariate analyses, cohort was associated with prenatal, early childhood and bone Pb concentrations, and DMFT scores. Mother’s education was negatively associated with early childhood Pb exposure (Table 1 & Table 2). Sociodemographic variables were not statistically significantly associated with blood and bone Pb.
Table 2:
Associations between sociodemographic and lifestyle confounders and lead exposures at different life stages.
| Table 2: Original to this manuscript. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Pb (μg/dL) : Mean (±SD) | Bone Pb1 (μg/g): Mean (±SD) | |||||||||||
| N | 1st Tri | 2nd Tri | 3rd Tri | N | Childhood | N | Peripuberty | N | Patella | N | Tibia | |
| 230 | 386 | 205 | 259 | 173 | ||||||||
| Child’s Sex | ||||||||||||
| Male | 112 | 6.06±3.84 | 5.24±4.06 | 5.67±3.48 | 186 | 15.48±7.29 | 98 | 3.60±3.22 | 130 | 8.64±10.11 | 81 | 7.18±10.31 |
| Female | 118 | 6.36±5.08 | 5.25±4.67 | 5.73±4.46 | 200 | 15.18±6.94 | 107 | 3.34±2.68 | 129 | 9.68±11.05 | 92 | 8.64±9.65 |
| P value2 | 0.61 | 0.98 | 0.91 | 0.68 | 0.53 | 0.43 | 0.34 | |||||
| Enrollment Cohort | ||||||||||||
| 2 (1997–2000) | 73 | 8.68±5.83 | 7.29±4.98 | 7.47±4.84 | 144 | 17.61±8.41 | 59 | 3.15±2.35 | 113 | 11.70±11.45 | 103 | 11.70±11.45 |
| 3 (2001–2005) | 157 | 5.07±3.17 | 4.29±3.70 | 4.89±3.25 | 242 | 13.97±5.80 | 146 | 3.59±3.15 | 146 | 7.20±9.45 | 70 | 7.20±9.45 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | 0.33 | <0.001 | 0.004 | |||||
| Mother’s Education (y) | ||||||||||||
| Did not complete secondary (<9) | 29 | 6.10±4.61 | 5.30±3.12 | 5.49±3.21 | 48 | 16.14±6.84 | 22 | 3.26±1.41 | 32 | 7.13±9.26 | 27 | 5.69±10.70 |
| Completed somehigh school (9 to <12) | 94 | 6.45±4.62 | 5.36±4.85 | 6.13±5.00 | 156 | 16.12±8.20 | 79 | 3.50±2.92 | 101 | 11.05±9.91 | 63 | 9.85±9.29 |
| Completed high school (12) | 75 | 6.01±4.65 | 5.28±4.64 | 5.45±3.09 | 129 | 15.26±6.49 | 75 | 3.66±3.33 | 89 | 6.46±9.96 | 59 | 6.66±9.65 |
| Higher education (>12) | 32 | 6.10±3.92 | 4.78±3.19 | 5.23±3.20 | 53 | 12.42±3.99 | 29 | 3.03±2.85 | 37 | 12.27±13.11 | 24 | 8.75±11.18 |
| P trend3 | 0.75 | 0.63 | 0.42 | 0.004 | 0.87 | 0.72 | 0.85 | |||||
| Peri-pubertal SSB Intake: Mean (ml) | ||||||||||||
| 1st Quartile: 141.52 | 53 | 6.22±4.44 | 6.26±6.63 | 6.29±5.21 | 95 | 15.26±6.71 | 46 | 3.50±2.31 | 67 | 9.14±11.26 | 49 | 7.47±10.04 |
| 2nd Quartile: 399.74 | 62 | 6.35±4.32 | 4.78±3.43 | 5.62±3.51 | 104 | 14.87±6.67 | 59 | 3.25±2.93 | 70 | 8.16±11.27 | 50 | 8.13±9.21 |
| 3rd Quartile: 663.00 | 52 | 6.18±3.66 | 5.43±3.08 | 6.35±3.77 | 94 | 15.54±5.85 | 48 | 3.44±2.88 | 57 | 10.92±10.74 | 37 | 8.35±10.50 |
| 4th Quartile: 1097.33 | 63 | 6.10±5.40 | 4.69±3.58 | 4.77±3.31 | 93 | 15.69±8.95 | 52 | 3.69±3.53 | 65 | 8.72±8.88 | 37 | 8.00±10.67 |
| P trend | 0.83 | 0.12 | 0.09 | 0.55 | 0.66 | 0.82 | 0.77 | |||||
Maternal bone samples from postnatal measurements.
P value from 2-sample t test.
P value from linear regression analysis.
In unadjusted ZINB Poisson regression models, we found statistically significant, positive associations between maternal blood Pb levels during the 2nd and 3rd trimesters and early childhood blood Pb levels and D1MFT scores. This association was of greatest magnitude in early childhood, although point estimates were all comparable (2nd trimester: rate ratio=1.17 (CI: (1.00, 1.37), P=0.046); 3rd trimester: rate ratio=1.20 (CI: (1.03, 1.40), P=0.019); early childhood: rate ratio=1.22 (CI: (1.02, 1.48), P=0.030)). All three effect sizes were attenuated and no longer statistically significant after adjusting for sex, cohort, maternal education, and peri-pubertal SSB intake (Table 3). No statistically significant association was observed between Pb measured at any time point and D4MFT.
Table 3:
Associations between log-transformed lead exposure at specific life stage and D1MFT score, in unadjusted and adjusted zero-inflated negative binomial Poisson regression model.
| Table 3: Original to this manuscript. | ||||
|---|---|---|---|---|
| Blood Pb | Unadjusted | Adjusted1 | Unadjusted | Adjusted |
| 1st Trimester (N=230) | Rate Ratio (95% CI) | Rate Ratio (95% CI) | Probability of being DMFT score = 0 | Probability of being DMFT score = 0 |
| Log (Pb) | 1.12 (0.95, 1.31) | 1.07 (0.90, 1.27) | 1.00 (0.59, 1.68) | 1.22 (0.68, 2.21) |
| P value2 | 0.174 | 0.444 | 0.985 | 0.506 |
| 2nd Trimester (N=230) | ||||
| Log (Pb) | 1.17 (1.00, 1.37) | 1.12 (0.94, 1.32) | 1.20 (0.70, 2.03) | 1.47 (0.82, 2.62) |
| P value | 0.046 | 0.202 | 0.507 | 0.194 |
| 3rd Trimester (N=230) | ||||
| Log (Pb) | 1.20 (1.03, 1.40) | 1.17 (0.99, 1.37) | 0.90 (0.52, 1.53) | 1.02 (0.56, 1.86) |
| P value | 0.019 | 0.066 | 0.689 | 0.949 |
| Early Childhood (N=386) | ||||
| Log (Pb) | 1.22 (1.02, 1.48) | 1.14 (0.94, 1.38) | 0.74 (0.38, 1.46) | 0.81 (0.39, 1.65) |
| P value | 0.030 | 0.181 | 0.385 | 0.557 |
| Peri-puberty (N=205) | ||||
| Log (Pb) | 0.92 (0.77, 1.11) | 0.97 (0.81, 1.16) | 1.13 (0.61, 2.08) | 1.10 (0.59, 2.08) |
| P value | 0.388 | 0.751 | 0.695 | 0.761 |
| Bone Pb3 | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Patella (N=259) | Rate Ratio (95% CI) | Rate Ratio (95% CI) | Probability of being DMFT score = 0 | Probability of being DMFT score = 0 |
| Log (Pb) | 0.97 (0.89, 1.05) | 0.95 (0.88, 1.03) | 1.05 (0.78, 1.41) | 1.10 (0.81, 1.49) |
| P value | 0.417 | 0.233 | 0.733 | 0.542 |
| Tibia (N=173) | ||||
| Log (Pb) | 1.01 (0.91, 1.12) | 0.98 (0.88, 1.08) | 1.21 (0.77, 1.89) | 1.41 (0.82, 2.43) |
| P value | 0.899 | 0.677 | 0.410 | 0.209 |
Adjusted for sex, cohort, mother’s education, sugar sweetened beverages intake. Female, cohort 2 and “did not complete secondary” subjects were used as reference population.
P=0.05 was the cutoff point in order to determine significance.
Maternal bone samples from postnatal measurements.
To evaluate the effects of interactions between Pb exposure and SSB intake on D1MFT, we stratified study subjects at each life period into ≤ SSB median intake (low) and > SSB median intake (high) groups (Table 4). The rate ratio for the association between Pb exposure and D1MFT was greater among those in the high SSB than low SSB intake group for blood Pb levels measured at each time period but not for maternal bone Pb measures (Table 4). However, this was not true for the associations with D4MFT.
Table 4:
Associations between log-transformed blood Pb exposure and D1MFT and D4MFT scores at adolescence, in unadjusted and adjusted zero-inflated negative binomial Poisson regression model, stratified by above vs. below median sugar sweetened beverages intake levels during adolescence.
| Table 4: Original to this manuscript. | ||||
|---|---|---|---|---|
| Prenatal Blood Pb | ||||
| D1MFT | D4MFT | |||
| Unadjusted | Adjusted1 | Unadjusted | Adjusted | |
| Rate Ratio (95% CI) | Rate Ratio (95% CI) | Rate Ratio (95% CI) | Rate Ratio (95% CI) | |
| 1st Trimester: | ||||
| Sugar Sweetened Beverages Intake ≤ Median (519.43 ml): N=115 | ||||
| Log (Pb) | 1.09 (0.89, 1.34) | 1.02 (0.83, 1.25) | 0.85 (0.41, 1.76) | 1.15 (0.49, 2.69) |
| P value2 | 0.406 | 0.882 | 0.656 | 0.752 |
| Sugar Sweetened Beverages Intake > Median (519.43 ml): N=115 | ||||
| Log (Pb) | 1.18 (0.91, 1.51) | 1.25 (0.93, 1.67) | 1.19 (0.57, 2.51) | 1.53 (0.63, 3.71) |
| P value | 0.207 | 0.137 | 0.641 | 0.346 |
| 2st Trimester: | ||||
| Sugar Sweetened Beverages Intake ≤ Median (519.43 ml): N=115 | ||||
| Log (Pb) | 1.10 (0.90, 1.36) | 1.00 (0.82, 1.23) | 1.24 (0.59, 2.60) | 1.52 (0.71, 3.29) |
| P value | 0.348 | 0.985 | 0.570 | 0.283 |
| Sugar Sweetened Beverages Intake > Median (519.43 ml): N=115 | ||||
| Log (Pb) | 1.26 (1.00, 1.60) | 1.41 (1.06, 1.86) | 1.15 (0.54, 2.47) | 1.59 (0.65, 3.87) |
| P value | 0.053 | 0.017 | 0.720 | 0.307 |
| 3st Trimester: | ||||
| Sugar Sweetened Beverages Intake ≤ Median (519.43 ml): N=115 | ||||
| Log (Pb) | 1.10 (0.89, 1.36) | 0.98 (0.79, 1.22) | 0.93 (0.43, 1.98) | 1.09 (0.46, 2.54) |
| P value | 0.368 | 0.876 | 0.844 | 0.849 |
| Sugar Sweetened Beverages Intake > Median (519.43 ml): N=115 | ||||
| Log (Pb) | 1.32 (1.06, 1.65) | 1.50 (1.18, 1.90) | 0.87 (0.40, 1.87) | 1.10 (0.45, 2.71) |
| P value | 0.012 | 0.001 | 0.719 | 0.836 |
| Early Childhood Blood Pb | ||||
| Sugar Sweetened Beverages Intake ≤ Median (504.39 ml): N=193 | ||||
| Log (Pb) | 1.24 (0.94, 1.62) | 1.09 (0.84, 1.43) | 0.83 (0.31, 2.18) | 0.75 (0.26, 2.14) |
| P value | 0.125 | 0.507 | 0.701 | 0.589 |
| Sugar Sweetened Beverages Intake > Median (504.39 ml): N=193 | ||||
| Log (Pb) | 1.22 (0.94, 1.57) | 1.20 (0.91, 1.57) | 0.67 (0.26, 1.72) | 0.84 (0.31, 2.28) |
| P value | 0.128 | 0.194 | 0.399 | 0.733 |
| Peri-pubertal Blood Pb | ||||
| Sugar Sweetened Beverages Intake ≤ Median (512.21 ml): N=102 | ||||
| Log (Pb) | 0.87 (0.65, 1.15) | 0.92 (0.71, 1.20) | 1.43 (0.60, 3.41) | 1.45 (0.59, 3.55) |
| P value | 0.312 | 0.546 | 0.424 | 0.418 |
| Sugar Sweetened Beverages Intake > Median (512.21 ml): N=103 | ||||
| Log (Pb) | 0.96 (0.75, 1.22) | 1.02 (0.80, 1.30) | 0.90 (0.37, 2.18) | 0.87 (0.33, 2.31) |
| P value | 0.749 | 0.877 | 0.818 | 0.779 |
| Maternal Postpartum Patella Pb | ||||
| Sugar Sweetened Beverages Intake ≤ Median (497.05 ml): N=130 | ||||
| Log (Pb) | 0.97 (0.86, 1.08) | 0.96 (0.86, 1.07) | 1.00 (0.67, 1.50) | 1.04 (0.68, 1.59) |
| P value | 0.550 | 0.424 | 0.989 | 0.856 |
| Sugar Sweetened Beverages Intake > Median (497.05 ml): N=129 | ||||
| Log (Pb) | 0.97 (0.86, 1.09) | 0.96 (0.86, 1.08) | 1.11 (0.71, 1.74) | 1.16 (0.73, 1.86) |
| P value | 0.575 | 0.496 | 0.642 | 0.529 |
| Maternal Postpartum Tibia Pb | ||||
| Sugar Sweetened Beverages Intake ≤ Median (440.42 ml): N=86 | ||||
| Log (Pb) | 1.01 (0.86, 1.18) | 0.94 (0.81, 1.09) | 1.19 (0.60, 2.38) | 1.30 (0.56, 3.02) |
| P value | 0.928 | 0.410 | 0.619 | 0.536 |
| Sugar Sweetened Beverages Intake > Median (440.42 ml): N=87 | ||||
| Log (Pb) | 1.00 (0.87, 1.16) | 0.98 (0.84, 1.14) | 1.22 (0.67, 2.22) | 1.33 (0.65, 2.68) |
| P value | 0.965 | 0.777 | 0.522 | 0.434 |
Adjusted for sex, cohort, mother’s education. Female, cohort 2 and “did not complete secondary” subjects were used as reference population.
P=0.05 was the cutoff point in order to determine significance.
DISCUSSION
A limited number of population-based studies have examined the potential associations between Pb exposure and dental caries risks in adolescence, and to our knowledge, none have assessed the associations of Pb exposure at multiple sensitive life periods. In this secondary analysis of data from a Mexico cohort, we found signals suggesting potential associations between pre-natal (maternal) and childhood Pb lead levels and D1MFT but after adjusting for sex, cohort effect, mother’s education level and SSB intake, effect sizes were attenuated and no longer statistically significant. We found no associations with D4MFT, which measures more severe dental caries. There were no associations with post-partum maternal bone Pb levels with either D1MFT or D4MFT. Overall, we did not find strong evidence that Pb exposure was related to worse dental caries outcomes in permanent teeth. However, a stratified analysis suggested that high SSB intake during adolescence might act as a “second hit”, interacting with prenatal Pb exposure and lead to worse peri-pubertal dental caries outcomes.
Contrary to previous cross-sectional studies with Pb exposure information from one life period only [7, 8, 9, 15], we did not observe similar positive, statistically significant associations between Pb concentrations and dental caries presence among Mexico City adolescents, after adjusting for confounders. One potential reason for discrepancies include the cross-sectional nature; it could be that children with caries are also more susceptible to Pb deposition [32]. Moreover, previous studies have used logistic or linear regression, which do not consider the count nature of the outcome data and may over-inflate effect estimates. In contrast, we used a more conservative approach to modeling [33]. In addition, we used a longitudinal study design, and measured blood and bone Pb at multiple time points. However, our sample size was smaller than some of the earlier studies, which decreased our power to detect statistically significant associations.
Although not statistically significant, the largest effect estimates we found were from Pb measured during prenatal life and early childhood, suggesting there may be sensitive windows for effects of Pb on caries formation. Pb exposure during these early life timepoints likely would affect caries of primary teeth more directly than caries of permanent teeth [34]. Although we did not have information on primary teeth, other studies have reported correlations between caries of primary teeth and permanent teeth [35, 36, 37]; thus for our study, permanent caries may be a reasonable proxy for primary caries.
We observed a greater association with Pb levels among those consuming high (above the median in the population) levels of sugar sweetened beverages (SSB). This is in line with other evidence showing sugar or SSB exposure can increase risks of dental decay in individuals with higher susceptibility due to other conditions, including hyposalivation, amelogenesis imperfecta (AI), dentinogenesis imperfecta (DI) and drug use [38]. For instance, individuals most prone to caries development typically have low salivary buffering capacity and a high sucrose diet with frequent carbohydrate exposure [39]. Other intervention papers suggested that limiting sugar intake was fundamental to reducing further problems in teeth affected by AI, a hereditary oral condition that affect enamel formation [40, 41]. Further mechanistic studies are needed to evaluate whether the effects of Pb on caries formation are exacerbated in the presence of sugar.
In addition to our longitudinal study design as well as blood and bone Pb measurements at multiple time points, our study had several other strengths. In order to understand the process of caries manifestation, we examined the potential associations between macro-, micro-nutrients intake, urinary/water fluoride content and DMFT scores in bivariate analyses before conducting adjusted analysis. In order to better model the DMFT count data with over-dispersion and excess zeros, we went through a rigorous model selection process, and found ZINB was the best model with the smallest AIC value [42, 43].
Dental caries is a complex biofilm dependent disease induced by multiple internal and external factors. A larger sample size and data elucidating biological mechanisms should be taken into account in future studies examining an association between Pb exposure and dental caries. To our knowledge, this is the first study that applied longitudinal study design to examine the association between Pb exposure and dental caries presence at multiple sensitive life periods. We were however, unable to reject the hypothesis that an elevated Pb level in early life are involved in cariogenic process.
Supplementary Material
Highlights:
Blood and bone lead measurements from multiple sensitive life periods.
Associations between lead exposure and dental caries presence in permanent teeth.
Sugar sweetened beverages intake can modify the associations between lead exposure and dental caries risks.
Acknowledgments
FUNDING DETAILS
We thank the study team in Mexico for collecting the data for this study. The study was supported by the grants R01ES021446, R01ES007821, P42-ES05947 and P30ES017885 from the U.S. National Institute of Environmental Health Sciences (NIEHS), the grant P01ES022844/RD83543601 from NIEHS/U.S. Environmental Protection Agency, by the National Institute of Public Health/Ministry of Health of Mexico, and by a grant from the Binational/Cross-cultural Health Enhancement Center at IUPUI. The American British Cowdray Hospital provided facilities used for this research.
Abbreviations:
- Pb
Lead
- DMFT
Decayed, missing, and filled teeth
- ZINB
Zero inflated negative binomial
- SSB
Sugar-sweetened beverage
- ICDAS
International Caries Detection and Assessment System
- FFQ
Food frequency questionnaire
Footnotes
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.“What Is the Burden of Oral Disease?” World Health Organization, World Health Organization, 8 December 2010, www.who.int/oral_health/disease_burden/global/en/. [Google Scholar]
- 3.Sheiham A Dental caries affects body weight, growth and quality of life in pre-school children. Br Dent J. 2006;201(10):625–626. doi: 10.1038/sj.bdj.4814259. [DOI] [PubMed] [Google Scholar]
- 4.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131(7):887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 5.Pitts NB. Are we ready to move from operative to non-operative/preventive treatment of dental caries in clinical practice? In: Caries Research. Vol 38; 2004:294–304. doi: 10.1159/000077769. [DOI] [PubMed] [Google Scholar]
- 6.Marshall TA, Levy SM, Broffitt B, Warren JJ, Eichenberger-Gilmore JM, Burns TL, Stumbo PJ. Dental Caries and Beverage Consumption in Young Children. Pediatrics. 2003;112(3):184–191. doi: 10.1542/peds.112.3.e184. [DOI] [PubMed] [Google Scholar]
- 7.Gil F, Facio A, Villanueva E, Pérez ML, Tojo R, Gil A. The association of tooth lead content with dental health factors. Sci Total Environ. 1996;192(2):183–191. doi: 10.1016/S00489697(96)05313-2. [DOI] [PubMed] [Google Scholar]
- 8.Moss ME. Association of Dental Caries and Blood Lead Levels. JAMA. 1999;281(24):2294. doi: 10.1001/jama.281.24.2294. [DOI] [PubMed] [Google Scholar]
- 9.Gemmel A, Tavares M, Alperin S, Soncini J, Daniel D, Dunn J, Crawford S, Braveman N, Clarkson TW, McKinlay S, et al. Blood lead level and dental caries in school-age children. Environ Health Perspect. 2002;110(10). doi: 10.1289/ehp.021100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa F Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Env Heal Perspect. 2005;113(12):1669–1674. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brito J a a, McNeill FE, Webber CE, Chettle DR. Grid search: an innovative method for the estimation of the rates of lead exchange between body compartments. J Environ Monit. 2005;7:241247. doi: 10.1039/b416054a. [DOI] [PubMed] [Google Scholar]
- 12.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodríguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293(2):F616–F623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Wen XW, Faulk C, Boehnke K, Zhang H, Dolinoy DC, Xi C. Perinatal lead exposure alters gut microbiota composition and results in sex-specific bodyweight increases in adult mice. Toxicol Sci. 2016;151(2):324–333. doi: 10.1093/toxsci/kfw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B, Chi L, Mahbub R, Bian X, Tu P, Ru H, Lu K. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem Res Toxicol. 2017;30(4):996–1005. doi: 10.1021/acs.chemrestox.6b00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y-S, Ha M, Kwon H-J, Kim H-Y, Choi Y-H. Association between Low blood lead levels and increased risk of dental caries in children: a cross-sectional study. BMC Oral Health. 2017;17(1):42. doi: 10.1186/s12903-017-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Linden EJ, Burdi AR, de Jongh HJ. Critical periods in the prenatal morphogenesis of the human lateral pterygoid muscle, the mandibular condyle, the articular disk, and medial articular capsule. Am J Orthod Dentofac Orthop. 1987. doi: 10.1016/0889-5406(87)90205-8. [DOI] [PubMed] [Google Scholar]
- 17.Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. In: Environmental Health Perspectives. Vol 91; 1991:17–32. doi: 10.1289/ehp.919117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silbergeld EK. Lead in bone: Implications for toxicology during pregnancy and lactation. In: Environmental Health Perspectives.; 1991. doi: 10.1289/ehp.919163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall TA. Preventing dental caries associated with sugar-sweetened beverages. J Am Dent Assoc. 2013;144(10):1148–1152. doi: 10.14219/jada.archive.2013.0033. [DOI] [PubMed] [Google Scholar]
- 20.Cantoral A, Téllez-Rojo MM, Levy TS, Hernández-Ávila M, Schnaas L, Hu H, Peterson KE, Ettinger AS. Differential association of lead on length by zinc status in two-year old Mexican children. Environ Heal A Glob Access Sci Source. 2015;14(1). doi: 10.1186/s12940-015-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afeiche M, Peterson KE, Sánchez BN, Cantonwine D, Lamadrid-Figueroa H, Schnaas L, Ettinger AS, Hernández-Avila M, Hu H, Téllez-Rojo MM. Prenatal lead exposure and weight of 0- to 5year-old children in Mexico city. Environ Health Perspect. 2011;119(10):1436–1441. doi: 10.1289/ehp.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Avila M, Gonzalez-Cossio T, Hernandez-Avila JE, Romieu I, Peterson KE, Aro A, Palazuelos E, Hu H. Dietary calcium supplements to lower blood lead levels in lactating women: a randomized placebo-controlled trial. Epidemiology. 2003;14(2):206–212. doi: 10.1097/01.EDE.0000038520.66094.34. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Pepper L, Goldman R. Effect of repeated occupational exposure to lead, cessation of exposure, and chelation on levels of lead in bone. Am J Ind Med. 1991;20(6):723–735. doi: 10.1002/ajim.4700200603. [DOI] [PubMed] [Google Scholar]
- 24.Téllez-Rojo MM, Hernández-Avila M, González-Cossío T, Romieu I, Aro A, Palazuelos E, Schwartz J, Hu H. Impact of breastfeeding on the mobilization of lead from bone. Am J Epidemiol. 2002;155(5):420–428. doi: 10.1093/aje/155.5.420. [DOI] [PubMed] [Google Scholar]
- 25.Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, Pitts NB. The International Caries Detection and Assessment System (ICDAS): An integrated system for measuring dental caries: Methods. Community Dent Oral Epidemiol. 2007. doi: 10.1111/j.1600-0528.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 26.Ormond C, Douglas G, Pitts N. The use of the International Caries Detection and Assessment System (ICDAS) in a National Health Service general dental practice as part of an oral health assessment. Prim Dent Care. 2010. doi: 10.1308/135576110792936177. [DOI] [PubMed] [Google Scholar]
- 27.Shivakumar K, Prasad S, Chandu G. International Caries Detection and Assessment System: A new paradigm in detection of dental caries. J Conserv Dent. 2009;12(1):10–16. doi: 10.4103/09720707.53335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villalpando S, Shamah-Levy T, Ramírez-Silva CI, Mejía-Rodríguez F, Rivera J a. Prevalence of anemia in children 1 to 12 years of age: results from a nationwide probabilistic survey in Mexico. Salud Publica Mex. 2003;45(1):490–498. doi: 10.1590/S0036-36342003001000005. [DOI] [PubMed] [Google Scholar]
- 29.Willett W Nutritional Epidemiology; 2013. doi: 10.1093/acprof:oso/9780199754038.001.0001. [DOI] [Google Scholar]
- 30.Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 mu g/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116(2):243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: Measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115(3):455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina GF, Costa De Almeida GR, De Souza Guerra C, Cury JA, De Almeida AP, Barroso RC, Gerlach RF. Lead deposition in bovine enamel during a pH-cycling regimen simulating the caries process. Caries Res. 2011;45(5):469–474. doi: 10.1159/000330602. [DOI] [PubMed] [Google Scholar]
- 33.Preisser JS, Stamm JW, Long DL, Kincade ME. Review and recommendations for zero-inflated count regression modeling of dental caries indices in epidemiological studies. Caries Res. 2012;46(4):413–423. doi: 10.1159/000338992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson GE, Davis BA, Raubertas RF, Pearson SK, Bowen WH. Influence of maternal lead ingestion on caries in rat pups. Nat Med. 1997. doi: 10.1038/nm0997-1024. [DOI] [PubMed] [Google Scholar]
- 35.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baginska J, Rodakowska E, Milewski R, Kierklo A. Dental caries in primary and permanent molars in 7–8-year-old schoolchildren evaluated with Caries Assessment Spectrum and Treatment (CAST) index. BMC Oral Health. 2014. doi: 10.1186/1472-6831-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farooqi FA, Khabeer A, Moheet IA, Khan SQ, Farooq I, Arrejaie AS. Prevalence of dental caries in primary and permanent teeth and its relation with tooth brushing habits among schoolchildren in Eastern Saudi Arabia. Saudi Med J. 2015. doi: 10.15537/smj.2015.6.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy DA, Harrell L, Fintzy R, Vitero S, Gutierrez A, Shetty V. Soda Consumption Among Methamphetamine Users in the USA: Impact on Oral Health. Oral Health Prev Dent. 2016;14(3):227–234. doi: 10.3290/j.ohpd.a35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3). J Clin Pediatr Dent. 2004;28(August 2015):203–214. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 40.McDonald S, Arkutu N, Malik K, Gadhia K, McKaig S. Managing the paediatric patient with amelogenesis imperfecta. In: British Dental Journal. Vol 212; 2012:425–428. doi: 10.1038/sj.bdj.2012.366. [DOI] [PubMed] [Google Scholar]
- 41.Ayers KM, Drummond BK, Harding WJ, Salis SG, Liston PN. Amelogenesis imperfecta--multi-disciplinary management from eruption to adulthood. Review and case report. N Z Dent J. 2004;100(4):101–104. [PubMed] [Google Scholar]
- 42.Cox DR. Some remarks on overdispersion. Biometrika. 1983;70(1):269–274. doi: 10.1093/biomet/70.1.269. [DOI] [Google Scholar]
- 43.Perumean-Chaney SE, Morgan C, McDowall D, Aban I. Zero-inflated and overdispersed: What’s one to do? J Stat Comput Simul. 2013;83(9):1671–1683. doi: 10.1080/00949655.2012.668550. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.