Abstract
Purpose
Deceased donation rates in Canada remain below the predicted potential and lag behind leading countries. Missing a potential donor leads to preventable death and disability of transplant candidates and increased healthcare costs.
Methods
Stakeholders were invited to a national consensus conference on improving deceased organ donor identification and referral (ID&R) and healthcare system accountability. In advance, participants received evidence-based, background documents addressing death audits, clinical triggers, required referral legislation, ethics, clinical pathways, and donation standards. At the conference, expert presentations and summaries of background information prepared by the Steering Committee informed group discussions of the preset questions. The conference’s themes were: 1) expectations of potential donors, recipients and their families; 2) donor ID&R: clinical and legal perspectives; 3) enhancing accountability: gaps and solutions; and 4) enhancing accountability: quality/safety organizations.
Results
Thirty-seven consensus statements were generated. At the healthcare professional (HCP) level, key statements include: 1) donation be consistently addressed as part of end-of-life care but only after a decision to withdraw life-sustaining treatment; 2) HCP know how and when to identify and refer potential donors; and 3) transplant candidates be informed of local allocation guidelines and performance. At the healthcare system level, key statements include: 1) national adoption of clinical criteria to trigger ID&R; 2) dedicated resources to match donation activities, including transfer of a potential donor; 3) performance measurement through death audits; 4) reporting and investigation of missed donation opportunities (MDO); 5) recognition of top performers; and 6) missed donor ID&R be considered a preventable and critical safety incident.
Conclusion
Our consensus statements establish HCP and healthcare system responsibilities regarding potential organ donor ID&R and include the tracking, reviewing and elimination of MDO through system-wide death audits. Once implemented, these consensus statements will help honour patients’ wishes to donate, improve service to potential transplant recipients, and support HCPs in fulfilling their ethical and legal responsibilitites. Next steps include implementation, assessment of their impact on donation rates, and investigation of new evidence-based targets for system improvement.
Résumé
Objectif
Au Canada, les dons des personnes décédées restent inférieurs aux possibilités prédites et loin derrière les pays les plus performants. Le manque de donneurs potentiels aboutit à des décès évitables, à l’invalidité des candidats à la transplantation et à des coûts de soins de santé plus élevés.
Méthodes
Les principaux acteurs ont été invités à une conférence de consensus nationale sur l’amélioration de l’identification et de l’orientation des donneurs d’organes décédés (ID&R —Identification and referral) et sur la responsabilité du système de santé. Les participants ont reçu à l’avance des documents basés sur des données probantes qui abordaient l’audit des décès, les facteurs cliniques identifiants, la législation requise pour l’orientation, l’éthique, les cheminements cliniques et les normes de dons. Au cours de la conférence, les présentations d’experts et des résumés de l’information de fond préparés par le Comité de pilotage ont alimenté les discussions de groupe sur les questions préparées. Les thèmes de la conférence étaient les suivants : 1) attentes des donneurs potentiels, des receveurs et de leurs familles; 2) identification et orientation des donneurs : points de vue cliniques et légaux; 3) amélioration de la responsabilité : lacunes et solutions; et 4) amélioration de la responsabilité : organisations de la qualité/sécurité.
Résultats
Trente-sept énoncés de consensus ont été générés. Au niveau des professionnels de santé, les principaux énoncés sont les suivants : 1) que le don soit constamment abordé dans le cadre des soins de fin de vie, mais seulement après avoir pris la décision d’arrêter les traitements de maintien de vie; 2) les professionnels de santé ont le savoir-faire pour identifier et orienter les donneurs potentiels; et 3) les candidats à la transplantation doivent être informés des lignes directrices locales sur les attributions et sa performance. Au niveau du système de soins de santé, les principaux énoncés sont les suivants : 1) l’adoption au niveau national de critères cliniques déclenchant l’identification et l’orientation des donneurs; 2) des ressources dédiées aux activités d’appariement des dons, y compris au transfert des donneurs potentiels; 3) des mesures de performance par des audits des décès; 4) la déclaration et des investigations sur les opportunités de dons manqués; 5) la reconnaissance des plus performants; et 6) l’identification et l’orientation manquées de donneurs doivent être considérées comme un incident évitable et critique.
Conclusion
Nos énoncés de consensus établissent les responsabilités des professionnels de santé et du système de soins pour ce qui concerne l’identification et l’orientation des donneurs potentiels d’organes; ils incluent le suivi, l’analyse et l’élimination des dons manqués via une vérification des causes de décès dans tout le système. Une fois mis en œuvre, ces énoncés de consensus contribueront à honorer les souhaits des patients en matière de dons, améliorer les services apportés aux receveurs potentiels de greffes et soutenir les professionnels de santé dans l’accomplissement de leurs obligations éthiques et légales. Les étapes suivantes incluront la mise en œuvre, l’évaluation de leur impact sur les taux de dons et la recherche de nouvelles cibles basées sur des données probantes pour améliorer le système.
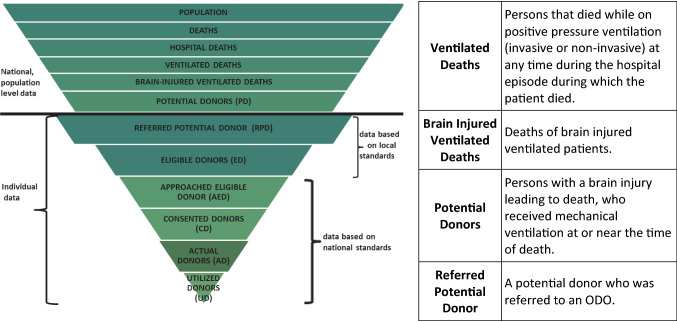
Every year in Canada, there are approximately 250,000 deaths, of which 120,000 occur in hospital.1 A small number of these patients (2,000–4,000) will meet eligibility criteria for donation, and yet only 600 of these individuals will become actual donors (Fig. 1).2 Canada continues to lag behind leading countries in our deceased donation rates, which also remain below the estimated national donor potential.3 Donation and transplant processes are complex and occur during a time of tragedy for families of potential donors. It is during this sensitive time that the first and fundamental step in the donation process must occur—the identification and referral (ID&R) of potential donors.
Table.
Provincial required referral legislation of potential deceased donors to organ donation organizations
| Provincial required referral legislation of potential deceased donor to organ donation agency by hospital/physician | |
|---|---|
| BC |
3(1) A facility must notify BC Transplant Society immediately in the event of death or impending death of a patient 75 yr or younger in its care. 5(1) If the facility has given a notification under section 3 and has not been advised of a determination of the existence of a medical or other condition that will make the tissue of the patient unsuitable for use in another person, the facility must immediately search the registry to determine whether a decision record exists for that patient. |
| AB |
7(1) When a person dies, the medical practitioner who makes the determination of death must consider and document in the patient record the medical suitability of the deceased person’s tissue or organs for transplantation. 7(2) If a medical practitioner determines that a person’s tissue or organs may be suitable for transplantation […], the medical practitioner must notify a donation organization, if any, in a manner satisfactory to the donation organization. |
| SK | Silent |
| MB |
4(1) Subject to the requirements and circumstances established under subsection 4.2(1), a designated facility must notify the required human tissue gift agency when (a) a patient at the facility dies; (b) a physician at the facility advises that the death of a person at the facility is imminent and inevitable; or (c) the facility receives a dead body. |
| ON |
8.1(1) A designated facility shall notify the Network as soon as possible when a patient at the facility has died or a physician is of the opinion that the death of a patient at the facility is imminent by reason of injury or disease. (2) Despite subsection (1), a designated facility is not required to notify the Network if the Network has established requirements that set out circumstances in which notice is not required and those circumstances exist. |
| QC |
204.1 When informed of the imminent or recent death of a potential organ or tissue donor, the director of professional services of an institution operating at a general and specialized hospital shall diligently (1) verify with one of the organizations that coordinate organ or tissue donations and are designated by the Minister under section 2.0.11 of the Act respecting the Régie de l’assurance maladie du Québec (chapter R-5) whether the potential donor’s consent for the post-mortem removal of organs or tissues is recorded in the consent registries established by the Ordre professionnel des notaires du Québec and the Régie de l’assurance maladie du Québec, to determine the donor’s last wishes expressed in this regard in accordance with the Civil Code; and (2) send to such an organization, if consent has been given, any necessary medical information concerning the potential donor and the organs or tissues that may be removed. The director of professional services is informed of the imminent or recent death of a potential organ or tissue donor in accordance with the procedure established by the institution. |
| NS |
Silent 17(1) When an individual dies, or in the opinion of a physician death is imminent, in a hospital, the hospital shall, as soon as possible, provide [prescribed information] to the organ donation program and, where so prescribed, to the tissue bank.* |
N.B. The provinces/territories of PEI, NL, NB, SK, YK, NWT, and NU are silent on the issue of required referral of potential organ or tissue donors. * This text is pending legislation awaiting proclamation.
AB = Alberta; BC = British Columbia; ON = Ontario; MB = Manitoba; NL = Newfoundland and Labrador; NS = Nova Scotia; NWT = Northwest Territories; NU = Nunavut; PEI = Prince Edward Island; QC = Quebec; SK = Saskatchewan; YK = Yukon.
Fig. 1.
Deceased donation information pyramid. Reproduced with permission from: Canadian Blood Services. Deceased Donation Data Working Group1
Despite required referral legislation in many provinces (Table) and an established definition of a potential organ donor,1 failure to refer potential donors is an ongoing problem.4 Given that donation opportunities occur in low numbers, deceased organ donation may not be a high-priority concern for many hospitals and healthcare professionals (HCPs). The Canadian National Transplant Research Program (CNTRP) identified challenges with defining, identifying, and referring potential donors in their study exploring barriers to donation after circulatory death (DCD).5,6 Although virtually all HCPs surveyed by Canadian Blood Services in 2011 believed it was important that patients and families be offered the opportunity to donate organs and/or tissues, only 35% reported that this opportunity was routinely offered at their hospital.7 There are also inconsistencies across the country, as the likelihood of becoming a donor is dependent on the city, hospital, and even department within which a patient dies.4
Missed donor ID&R has life-threatening consequences for patients awaiting transplant because one deceased donor may provide on average two to four transplants each.3 In the case of end-stage renal disease, dialysis is a difficult burden for patients and families, and is expensive to the healthcare system. Compared with dialysis, kidney transplantation is life-preserving and cost effective.8,9 There is also an impact on potential donors and their families who are denied the opportunity to donate. The healthcare system has a responsibility to provide society and patients awaiting transplant with access to a robust organ donation process10 that begins with a standard definition of eligibility, identification based on this definition, and consistent patient referral to organ donation organizations (ODOs) for evaluation.
We describe the proceedings and conclusions of a Canadian consensus conference focused on improving ID&R of potential organ donors (both neurologically determined death [NDD] and DCD) and accountability of the healthcare system in this regard.
Methods
Canadian Blood Services and the CNTRP collaboratively hosted a Potential Organ Donor Identification and System Accountability consensus conference September 20–21, 2016 in Ottawa, Canada.
The conference was designed to generate expert-derived consensus statements for leading practice. Specific objectives included: 1) achieve Canadian agreement on the definition of a potential organ donor and referral criteria (clinical triggers); 2) determine the responsibilities of healthcare professionals (HCP) for donor ID&R; 3) identify barriers and facilitators to donor ID&R; 4) create an implementation plan to operationalize clinical processes around donor ID&R; 5) initiate the development of accountability strategies; and 6) consider whether donor ID&R is a critical healthcare priority with public health concerns.
The conference Steering Committee met over nine months to prepare the agenda, consensus building process, and develop key documents including terms of reference, environmental scan with ODOs,5,6 systematic literature review of criteria to define and identify potential deceased organ donors,6 national assessment of the barriers and enablers to increasing DCD in Canada,5,6 and a terminology guide provided to conference participants. The conference’s 47 participants (Appendix) included pan-Canadian representation from critical care, neurocritical care, emergency medicine, donation, transplantation, research, healthcare administration, health professional education, knowledge translation, ethics, law, patient safety and quality organizations, and family partners. In advance of the conference, participants were invited to review the full breadth of these background documents.
The conference was segmented into four themes: 1) expectations of potential donors, recipients and their families; 2) donor ID&R: clinical and legal perspectives; 3) enhancing accountability: gaps and solutions; and 4) enhancing accountability: quality/safety organizations.
Each theme of the conference was structured around expert presentations and summaries of background documents designed to ensure participants had the same foundational material knowledge to deliberate group participation questions. The background documents used by the Steering Committee to develop the summary documents included published articles, systematic and scoping literature reviews (published and draft), accreditation standards, policies, environmental scan, and professional and public surveys. Discussions were then reported back at the plenary with the goal of reaching consensus. We defined consensus as substantial agreement by all participants such that they could accept a conclusion and support it, both within and outside the conference. All participants had equal input into the statements, which were refined until consensus was achieved by all participants.
During the final theme, following presentations by Accreditation Canada, Health Quality Ontario, Canadian Patient Safety Institute, Public Health Agency of Canada, and the Quality Improvement and Innovation Group under the Centre for Clinical Standards and Quality at the Centers for Medicare & Medicaid Services (USA), participants were asked to recommend potential organ donor ID&R as an issue of healthcare quality and safety. As a last task, participants generated a mission statement to summarize the intended output of the conference.
After the conference, discussions were summarized by the Steering Committee to reflect the participants’ consensus view. A draft of the consensus statements was distributed to all participants to ensure all statements were reflective of the conference and would be supported as written. After editing, the final report of all statements was approved by the Steering Committee.
Assumptions and key considerations
The following assumptions and key considerations were set by the Steering Committee to direct the conference’s process during development of expert consensus statements:
Discussions at the conference will be based on summaries of evidence prepared by the Steering Committee, the experience of practitioners, and Canadian leading practices.
Developing expert consensus statements for potential organ donor ID&R does not dictate organ donation and transplant (ODT) practice, but provides a framework for a more consistent approach that can be adapted to regional/individual applications; individual ODT professionals will continue to make decisions regarding individual patients and families based on their unique circumstances.
Existing legal and ethical frameworks in Canada served as a reference for discussions.
The following points were considered, as they could have impacted the success of the conference:
Leading practice consensus statements pertaining to donor ID&R require thoughtful implementation strategies and must recognize the unique needs of different regions, programs, and HCPs.
A paradigm and culture shift at multiple levels (HCP, intensive care units (ICUs), hospital administrators, professional societies, patients, the public, patient safety representatives, and governments) may be required to agree that missing a potential organ donor should be considered a critical (sentinel) safety event.
Consensus statements
Mission statement
At the conclusion of the conference, participants developed the following mission statement to guide system accountability for potential organ donation ID&R:
An accountable system for potential organ donor ID&R should strive to honour patient and family wishes by ensuring the opportunity to donate. Healthcare professionals should identify potential donors early, and always refer to ODOs, so that no donation opportunities are missed. Potential donor ID&R practices must be coordinated and collaborative. A successful potential donor ID&R system is supported by accurate and timely data, has system and individual accountability, and incentivizes good performance.
Theme 1: Expectations of potential donors, recipients, and their families
Despite a 42% increase in deceased donation rates since 2006,3 Canada still underperforms in comparison with leading countries and the estimated donor potential.2,3,11,12 As a result, patients on transplant waiting lists die each year (260 people in 2016).13 In addition, many others will die having been de-listed or never listed because of advanced disease; statistics are limited on these patients. Kidney transplantation for end-stage renal disease affords significant personal benefits to recipients and, after the first year, yields cost savings (CDN $33,000 to $84,000 annually) to the healthcare system from reduced dialysis costs.3 For non-kidney transplantation, the effects are life-saving but the economic impact is not well studied. Through survey, both HCPs and the public report support for mandatory or physician-led ID&R of potential organ donors,7,14 yet this step of the donation process remains problematic. According to the 2015 Canadian Medical Association policy on organ and tissue donation and transplantation, Canadians are entitled to timely access, on equitable terms, to necessary and effective medical treatment and this includes access to transplantation.15 This responsibility is shared across the entire healthcare system and requires adequate resourcing.
Impact of failing to identify and refer a potential organ donor
Conference participants identified the following risks and consequences of failure to identify and refer potential donors that should be considered in policy and practice:
-
A.
Not respecting the wishes of the potential organ donor who has registered or informed family of their desire to donate.
-
B.
Violation of existing laws in provinces with required referral legislation.
-
C.
Not providing the family with the potential to help others, including missed opportunity for legacy, potential to provide meaning during loss of a loved one, and the positive impact this could have on the grieving process.
-
D.
Preventable death or disability for transplant candidates.
-
E.
Compromised equitable access to transplantation.
-
F.
Ongoing costs of dialysis, which exceed the cost of transplantation for end-stage renal disease.
-
G.
Economic costs of continued care for end-stage (non-renal) organ failure.
-
H.
Loss of economic productivity of those awaiting transplant.
-
I.
Perpetuating failure—the acceptance of failure to identify and refer potential donors by HCP and the healthcare system.
-
J.
Erosion of public and professional trust.
-
K.
Lost opportunity for increasing public and professional education and awareness through long term donor family engagement—the families of donors have stories to tell.
-
L.
Compromising interprofessional trust and accountability among deceased donation and transplant services.
-
A.
Obligations to potential organ donors and their families
Healthcare professionals or ODO representatives should consistently initiate conversations around organ donation as an integrated part of end-of-life (EOL) care.
To avoid any real or perceived conflict of interest, healthcare practitioners should separate the discussions regarding withdrawal of life-sustaining treatment (WLST) from donation discussions.
The healthcare team is properly educated on how and when to identify and refer potential donors, how to effectively and compassionately discuss donation with family members, and how to provide optimal EOL care whether or not consent for donation is given.
It should be assumed and expected that the healthcare team would respect and be accountable to the previously expressed donation wishes made by the dying patient or potential donor.
If a dying patient is not eligible to donate, the family should be informed of the reasons why they have not been approached within the limits of respecting patient privacy and confidentiality.
Considerations
While there is an ethical and legal obligation to offer donation as a standard part of EOL care, emphasis was placed on sensitively transitioning from the topic of EOL to donation, to avoid harm to the family.
The emotional impact of the tragic loss of a loved one should be at the forefront of EOL discussions and requires sensitivity.
During donation discussions, family members expect clear, understandable communication regarding the prognosis of their loved one, the opportunity for donation, and the donation process. These conversations should show compassion and respect for the patient and their family.
When a member of the public registers their wishes, they assume they will be approached at the right time for donation and their registered wishes will be confirmed and acted upon.
-
B.
Obligations to potential transplant recipients and their families
-
6.
The deceased organ donation system must be resourced and organized appropriately to ensure all possible donation opportunities are recognized and maximized.
-
7.
A formal accountability framework should be established to ensure any missed donation opportunities (MDOs) are reported and investigated (“zero missed opportunities”).
-
8.
Mandatory training in donor ID&R be implemented to ensure HCPs who intersect with potential organ donors communicate and work collectively as a well-coordinated multidisciplinary team.
-
9.Standardized information be provided to transplant candidates and their families, and include:
- A description on how the system works, including transplant eligibility criteria.
- Local transplant allocation guidelines.
- Donor ID&R rates, organ donation rates, and waiting times for various organs and regions in Canada.
Considerations
-
e.
Jurisdictional variability in donation performance and geography are acknowledged as obstacles to donation and transplant access, but should not be used as an excuse for poor performance.
-
f.
Transplant candidates lack information on the ODT system. Public action through public interest/advocacy groups should be encouraged and may improve ODT system performance.
-
g.
Public access to donor ID&R rates may influence a culture change where donation is valued by the healthcare system and individual hospitals.
-
h.
Successful organ donation efforts should be celebrated.
-
i.
With future development of the Canadian Blood Services’ Canadian Transplant Registry, it may be possible to collect, monitor, and report on national data related to donor potential and MDOs.
Circumstances where identifying or referring a potential organ donor may be challenging
-
A.
While participants generally felt there were no justifiable circumstances for non-referral, the following circumstances may pose challenges to referral:
-
1.
Conscious patients (e.g., amyotrophic lateral sclerosis, medical assistance in dying).
-
2.
Patients/families with cultural opposition to donation.
-
3.
EOL care situations that are contentious, involve compromised trust, or are medico-legally complicated.
-
4.
Patient or family has expressed prior opposition to donation.
-
5.
Substitute decision maker cannot be identified.
-
6.
Donation is logistically impossible (e.g., resources/infrastructure not available).
-
7.
Healthcare system under substantial strain (e.g., pandemics, mass casualty).
-
B.
In cases where the family/subsitute decision maker or patient have indicated a clear opposition to donation, and the healthcare team decides not to formally approach again, this should not be considered a MDO.
-
C.
Donor ID&R is distinct from consenting a patient for donation. After potential donor ID&R, medical eligibility to donate should be established prior to approaching the family.
-
D.
Organ donor ID&R is distinct from consent discussions with families. Medical eligibility to donate should be established prior to consent discussion, but are part of the donor ID&R process.
Theme 2: Donor ID&R: clinical and legal perspectives
Identifying and referring a potential donor is challenged by several issues, including inconsistent definition of a potential donor, variability in required referral legislation (Table) that is often not respected and difficult to enforce, and the natural discomfort surrounding the juncture of EOL care and donation. Multiple overlapping criteria are used to define and identify potential donors in Canada and worldwide.5,6 The most common elements include mechanical ventilation, low Glascow Coma Scale, EOL discussions, devastating brain injury, and brain/brainstem death. The acronym GIVE (Glascow Coma Score less than five, Intubated, Ventilated, EOL care)16 has been used as a memory aid for key clinical triggers. Despite common use, consistent national and international criteria for potential donor ID&R are lacking. Currently, five provinces (British Columbia, Manitoba, Ontario, Quebec, and Nova Scotia) have required referral legislation where the ODO must be notified when death is imminent or established, and Alberta requires mandatory consideration of donation after death determination (Table). While organ donation should be embedded as a standard component of EOL care, the duty of care to the dying patient must be of foremost priority. Existing guidelines17,18 recommend safeguards to ensure this duty is met and include the following: ensuring that the dying patient’s interests are the dominant priority; EOL care should address patient’s needs and be applied consistently regardless of the intention or consent to donate; neuroprognostication and EOL decisions should be made prior to and separate from donation considerations, and should not be influenced by donation potential; the procurement/transplant team must not be involved in the decision to WLST; and supporting the family making decisions on behalf of the patient by providing the opportunity and process to actualize donation, if desired by the patient or family.
-
C.
Defining a potential organ donor
-
10.
Patients who meet all the following criteria (clinical triggers) should be considered a potential organ donor and be referred to the ODO:
-
A.
Ventilated (invasive [intubated/tracheotomy] or non-invasive [bilevel positive airway pressure/ continuous positive airway pressure] ventilation);
-
B.
Condition with a grave prognosis in which death is imminent;
-
C.
Decision to WLST has been made (but not yet acted upon).
-
11.
The above definition of a potential organ donor should be adopted in all Canadian jurisdictions to:
-
A.
Support consistency in professional education;
-
B.
Assist HCPs in identifing potential organ donors and optimizing possible opportunities for donation;
-
C.
Minimize loss of potential organ donors due to discretionary clinical judgements by individual HCPs; and
-
D.
Allow for standardized reporting, transparency, and system accountability.
Considerations
-
j.
A clinical trigger is the criteria defining a potential organ donor that will prompt healthcare teams to initiate case referral to the ODO, and is consistent with practice recommendations and provincial required referral legislation.
-
k.
Participants stressed the importance of ensuring that the definition of a potential organ donor is sufficiently permissive and broad to avoid unwarranted exclusions of potential NDD and DCD donors.
-
l.
A permissive definition would likely result in numerous ODO referrals, requiring ODOs to have sufficient capacity with which to respond to the increase in referral requests.
-
m.
Harmonization of commonly used Canadian clinical trigger tools may require provincial support and alignment of provincial protocols.
-
n.
It was acknowledged that there are patients with grave prognosis and imminent death whose lungs are not ventilated as part of their treatment plan. There is diverse opinion as to whether these patients may be considered potential organ donors, as non-therapeutic ventilation of their lungs may be required for organ donation. Guidelines for providing organ donation options to patients seeking medical assistance in dying are currently in development.
-
D.
Potential organ donor ID&R: who and when
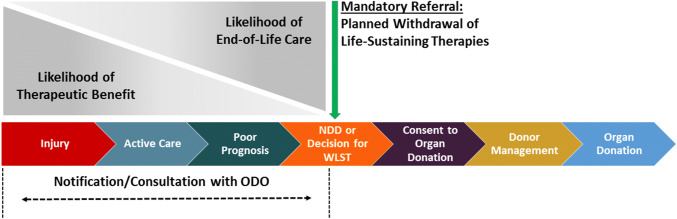
In donation practice and policy, a clear distinction should be made between “referral” and “notification/consultation” to the ODO.
- Referral to ODO:
- defined as the formal process by which the healthcare team seeks to involve the ODO;
- based on fulfilling clinical triggers; and
- should not occur until NDD or after WLST decisions have been made.
- ODO notification/consultation:
- refers to a member of the healthcare team advising the ODO of the presence of a potential donor and may be an option prior to meeting referral criteria; and
- may also be initiated upon family requests for donation information.
-
13.
The most responsible physician, or their designate, is ultimately accountable for ensuring that referral or notification of a potential donor to the ODO has occurred.
-
14.
All HCPs involved in EOL care can and should identify potential donors.
-
15.
The most responsible physician should be consulted on process and timing if another HCP involved in EOL care will be referring a potential organ donor to the ODO.
-
16.
The most responsible physician should be consulted on process and timing if another HCP involved in EOL care will be notifying/consulting the ODO about a potential organ donor.
Considerations
-
o.
These consensus statements aim to clarify the nature of contact between the healthcare team and the ODO.
-
p.
Advantages of early notification/consultation are: it provides specialized knowledge and information, clarifies donor eligibility, initiates early planning and preparations for donation logistics, arranges on-site donor coordinator support when required, provides education, organizes support services, engages staff and families early, and normalizes the integration of donation into EOL care. In some cases, if there is no on-site donor coordinator, early notification allows the coordinator to travel to the hospital.
-
q.
Concerns with early notification/consultation include: a perception of conflict of interest, compromised transparency with families or interprofessional trust, potential for influence on yet-to-be finalized EOL care plans and decisions, and higher ODO workload.
-
r.
The transition between likelihood of therapeutic benefit versus likelihood of EOL care pertaining to donor referral or notification is a similar paradigm to palliative care involvement in injuries or illness with a high risk of mortality (Fig. 2).
-
E.
Early consideration of organ donation: safeguards for patients with devastating injury/illness and their families
Fig. 2.
Sequence of care in deceased donation in relation to notification and referral
-
17.The following previously agreed upon Canadian guidelines should be strictly followed in the process of organ donor referral:
- The decision to WLST should be made prior to any discussion of organ and tissue donation that is initiated by HCPs.
- The surgical retrieval/transplant team must not be involved in the decision to WLST.
-
18.
A second opinion regarding prognostication be obtained before proceeding with DCD.
Considerations
-
s.
While the scope of this conference was limited to potential organ donor ID&R, it is noteworthy to record that participants felt that increased safeguards for prognostication in potential DCD cases are prudent.
-
t.
It is important to communicate existence of safeguards to HCPs and the public, which includes educating ICU staff on donation and safeguards put in place to protect patients.
-
u.
Where donation physicians are available, they may serve as an expert resource to support families and advocate adherence to safeguards.
-
v.
Participants discussed several means of increasing patient/family confidence through: communication, HCPs’ education, accuracy and checks in prognostication, quality assurance audits/tools/processes, and research to show adherence to best practices.
Theme 3: Enhancing accountability: gaps and solutions
There are several system-level gaps to be addressed to improve donor ID&R: incomplete data on MDOs, HCP knowledge of the process, and unequal access to donation services and ICU beds. Organ donation programs must perform routine, system-wide audits of all hospital deaths, known as death audits, to determine performance and identify opportunities for improvement. In Canada, most ODOs perform death audits, but the methodology and scope vary.19 Currently, there is a lack of standardized data concerning MDOs in Canada. There are also gaps in HCPs’ knowledge, with donor ID&R identified as a top priority for organ donation education.20 Lastly, across Canada, access to donation services, including ICU care, operating rooms, and surgical retrieve teams varies by location. Many ICUs operate at, or near, capacity, compromising the potential donors’ access to an ICU bed.7
-
F.
Measurement and reporting
A national minimum data set and standards should be developed and implemented for death audits and MDOs should be reported consistently across Canada.
Standardizing death audit methodology and donor referral criteria will improve data quality, allow for comparative measurements, and improve system performance.
A single, electronic, standardized national database and reporting system should be used for all potential donors.
Considerations
-
w.
Initiatives to improve data accessibility should be mindful of cost, and wherever feasible, align with existing IT infrastructure (e.g., electronic health records, province specific ODO databases).
-
x.In the absence of a single electronic national database of all potential organ donors, options to consider may include:
- Further development of Canadian Blood Services’ Canadian Transplant Registry, which is standardized, centralized, and automated.
- A donor management system (implemented or being considered in some provinces)
would require modifications to incorporate death audit data.
Canadian Blood Services may have a role in facilitating electronic reporting and data collection from centres who do not use iTransplant.
-
y.
Distinguish between nationally collated and reported data (aggregate) as opposed to locally collected and reported data.
-
G.
Implementation strategies and professional education
Provinces and territories that currently do not have required referral legislation should consider implementing such legal change.
Initiatives to ensure compliance with existing required referral legislation and policy for donor ID&R should include:
Local champions (donor coordinators, donation physicians) to ensure implementation of best practices, measurements, advocacy, and education;
Embedding donation into EOL care/WLST protocols and checklists that include all professionals involved in EOL care (e.g., respiratory therapists, neuroscience consultants);
Compliance measurement through chart reviews or death audits;
Elevating adherence to policy and law within hospital or regional accountability structures; and
Public reporting of donor ID&R compliance rates.
Donation activity-based funding that is directed to the unit where donation services are provided.
Professional education initiatives that include:
National education toolkit of donor ID&R and clinical trigger strategies for HCPs.
May include clinical trigger cards, posters, simplified messaging (e.g., “Donation Before Extubation”, “Pause Before Withdraws”).
Certification for critical care and emergency medicine staff in partnership with professional associations.
Considers donation as part of hospital or specialty credentialing.
Considers Royal College or provincial medical college licensure requirements.
Donor ID&R should be covered in medical and nursing school curriculums.
Considerations
Participants emphasized a need for clarification of who would hold HCPs accountable for compliance to donor ID&R policies.
Building measurement and accountability into the system will be critical to increasing equity, reducing MDOs, ensuring compliance with provincial laws and policy, and compiling data on performance and areas for improvement.
Because deceased donation is an infrequent event with high impact, it is important to develop methods to enhance and maintain HCPs’ competencies.
If a province does not have required referral legislation, clarification of provincial laws may be required. Specifically, privacy rules in relation to sharing potential donor information with ODOs need review to ensure disclosure of information is permissible.
A common understanding of the term “imminent death”, as articulated in provincial donor referral legislation, may need to be established.
Patients on transplant waiting lists should be engaged as advocates of donor ID&R because patient stories emphasize and humanize the importance of ID&R.
-
H.
Access of potential organ donors to hospitals with donation services and ICU beds
Donor services should be patient/family centric, not hospital centric. While the type of deceased donation (NDD, DCD, or tissue) may have logistic differences, donation services should be offered regardless.
Dedicated donor resources may be justified with the understanding that caring for a donor is caring for multiple living recipients.
Agreements and collaboration between the emergency room and ICU be established to allow for transfer of potential donors to preserve the opportunity to donate.
Transfer of potential donors to hospitals with donation, procurement, and surgical retrieval capacity:
Criteria for transfer be clear and transparent to HCPs and families;
In cases of DCD potential, priorities of patient care and donor care should be reconciled;
Any decisions regarding relocating potential donors require engagement and discussion with corresponding transplant teams;
Families may suffer stress and hardship if their loved one requires transfer to actualize donation services. Services should be offered to help avoid undue stress, financial (e.g., travel costs for family members between home and the procurement centre, costs to transport the body for burial) and otherwise, on the families of potential donors.
Considerations
Intensive care unit/operating room capacity and access of potential donors to ICU beds remains a challenging problem and options for managing access include:
Hospitals instituting dedicated ICU donor beds and agreements around the use of operating rooms for the retrieval of donated organs.
The Ontario model of Criticall was supported as one mechanism to assign ICU beds for province-wide triage and access for potential donors.
On-call management and retrieval teams represent one method of serving rural and remote regions. Consideration should be given to regional and interprovincial agreements such that larger, better-resourced provinces could support remote regions of smaller provinces.
Resistance may be encountered from critical care staff who may oppose using resources preferentially for donors.
In hospital emergency departments and ICUs operating at capacity, there may be natural disincentives to refer potential donors. Managing the donation process increases ICU workload and length of stay of potential donors. In transplant hospitals and ICUs, performing more transplants increases resource consumption, workload, and occupancy.
Theme 4: Enhancing accountability: quality/safety organizations
Individual and system accountability for donor ID&R is currently challenged by the following realities: fragmentation and lack of harmonization of definitions and measurements; lack of clarity or consistency in current accountability structures, roles and responsibility for deceased donation; enforceability of accreditation standards; and significant practice and measurement variability between hospitals.
-
I.
Accountability for potential organ donor identification and referral
Potential organ donor ID&R should be considered a Required Organizational Practice, as per Accreditation Canada guidelines.
Organ donation should be established as a Program of Distinction, as per Accreditation Canada guidelines.
Programs of Patient Engagement should be implemented to provide a voice to donor families and patients on transplant waiting lists.
Developing a clear accountability structure at the regional, institutional, and individual level would facilitate measurement and improvement, and include:
Harmonization of clinical definitions, roles, and responsibilities.
Each hospital having designated/assigned responsibility for ID&R.
Data-driven assessments with public reporting on deceased donation based on death audits will recognize high performance and drive motivation for improvement. Systems should be developed where potential organ donor ID&R can be accurately tracked and used as an important quality measure and indicator of hospital, ODO, and provincial performance.
Deceased donation balanced scorecards should be part of emergency department and ICU standard reporting to hospital administration, ODOs, and be available to the general public.
Donor ID&R should be considered an issue of preventable harm to potential organ donors and transplant candidates.
Donor ID&R should be considered an “Always Event” and missed potential organ donor ID&R be considered a “Never Event”.
Missing a potential donor referral should be reported as a “Sentinel Event”, such that the risk of adverse outcomes due to recurrence be recognized as calls for immediate investigation and response.
A formal accountability framework should be established to track the utilization and reasons for non-use of all potential organs and organ donors identified, so that any missed opportunities for use of transplantable organs can be investigated and reported upon.
Transplant program organ utilization scorecards should be part of standard reporting to hospital administration, ODOs, and be available to the general public.
Considerations
System failure for donor ID&R is not clearly defined, identified, or measured.
The degree to which missed donor ID&R contributes to provincial variation in donation performance and consequently, access to transplantation, is not well studied nor reported.
Jurisdictions with low referral rates have the most room for improvement, but have the least data on which to base improvement strategies and inform policy.
In the absence of a donation conversation, the death of a potential donor may pass without any recognition by the family, the healthcare team or the public. The consequences of missing a potential organ donor includes failure to respect the wishes of the dying patient, as well as the consequences to those waiting for transplant and the healthcare system. Missed donors and the loss of available organs for transplant increases mortality, morbidity, and healthcare costs.
Missed donation opportunities in any jurisdiction contribute to inequities in access to transplantation. For patients on transplant waiting lists, MDOs represent a significant but often hidden concern. There may be reluctance to communicate the nature and magnitude of this problem to the public or transplant candidates.
While MDOs are not routinely measured or publicly reported throughout Canada, Ontario through Trillium Gift of Life Network has initiated public reporting of hospital ID&R rates with a focus on celebrating high performance.
There was some disagreement by participants on whether failing to identify and refer a potential donor constituted a public health concern. Yet participants agreed that: missing a potential donor has an impact on public health and waitlisted transplant candidates; the public should be aware of missed opportunities; and the healthcare system needs to be accountable to the public.
Emphasis should be placed on sharing patient stories and highlighting benefits to transplant recipients, which may be obscured from those working in critical care, particularly in centres that do not offer transplants.
Frame arguments around MDOs as patient-related consequences and preventable harm. MDOs result in lost organs and lives.
While measuring and reporting, performance may foster “friendly competition” between units, hospitals, and provinces to improve performance, some participants did not favor public reporting, questioning the effectiveness, and advocated for continuous quality improvement initiatives.
Practitioners may respond better to local peer and regional accountability.
Consideration should be given around financial incentives for good performance and/or penalties for poor performance related to MDOs. Should there be a greater penalty for missing a donor who had registered their intention to donate?
Change behaviour and the culture change follows.
Discussion and conclusion
This report provides the first expert consensus statement on ID&R of potential deceased organ donors in Canada. It establishes expectations and accountability for HCPs and the healthcare system of meeting the needs of potential transplant receipients and providing potential organ donors and their families with the opportunity to donate without compromising the duty of care to the dying patient. Consensus statements focus on ensuring an optimal donor ID&R process through professional education, system accountability, and the identification, tracking, and elimination of MDOs through system-wide death audits. Moreover, MDOs are to be viewed as events of preventable harm requiring appropriate investigation and follow up.
The strengths of this work are that the consensus statements were derived by pan-Canadian experts representing all aspects of the donor ID&R process, such as emergency medicine, critical care, neurocritical care, donation, transplantation, research, healthcare administration, patient safety and quality organizations, and family partners. The conference was organized into four themes that provided a 360° view of donor ID&R. Expert presentions and evidence-based summaries of background information provided participants with varying expertise and perspectives, and required fundamental/foundational knowledge to ensure complete and equal contribution by all participants during discussions and deliberations. The conference respected a strict consensus process, where all statements were fully supported by participants.
There are limitations to this consensus-based process, as the consensus statements were not based on formal systematic reviews or meta-analysis, nor were they drafted based on the GRADE process. Although evidence-based literature and presentations informed participants discussions, there was inadequate evidence for a more formal process on donor ID&R to generate recommendations.
We recognize that there will be challenges with the implementation of these consensus statements, which reflect the unique needs and resources of different jurisdictions, hospitals, ODOs, and HCPs. Nonetheless, establishing leading practice through these consensus statements is an essential step towards system-wide improvements. It will also be important, moving forward, that these consensus statements continue to align and evolve with ongoing work by Canadian Blood Services and key stakeholders in the organ donation community related to defining minimum datasets and data collection processes for deceased donation in general and death audits specifically. This will be key to the successful implementation of these consensus statements.
If the Canadian healthcare system continues to accept MDOs, we risk endorsing a culture of low expectation with little incentive to improve. Once implemented, these consensus statements will help honour patients’ wishes to donate, improve service to potential transplant recipients, and support HCPs in performing their ethical and legal responsibilitites. Next steps include implementation, assessing impact on donation rates, and examining new evidence-based targets for system improvement. A robust implementation strategy will include collaboration among key stakeholders and a multi-streamed approach focused on national death audits, professional education, public awareness, and system accountability.
Acknowledgements
We would like to acknowledge the family partners who participated and greatly inspired us during the Potential Organ Donor Identification and System Accountability conference. In additon, we recognize, with great respect, all patients and their families who have generously consented to organ donation. Finally, we acknowledge patients and their families who have suffered disability and death due to the shortage of organs for transplantation.
Disclosures
The work was financially supported by Canadian Blood Services and the CNTRP. Canadian Blood Services receives funding from the provincial and territorial Ministries of Health and the federal government, through Health Canada. The views expressed herein do not necessarily represent the views of the federal, provincial, or territorial governments. Canadian Blood Services is a national, not‐for-profit charitable organization that manages the supply of blood and blood products in all provinces and territories in Canada (with the exception of Quebec) and oversees the OneMatch Stem Cell and Marrow Network. Canadian Blood Services also received a mandate in 2008 for national activities related to organ and tissue donation and transplantation (OTDT), which includes: development of leading practices, public awareness and education, system performance measurement, and establishing transplant patient registries. Canadian Blood Services is not responsible for the management or funding of any Canadian ODO or Transplant Program. Learn more at https://professionaleducation.blood.ca/en/organs-tissues.
This work was also funded by the Canadian National Transplant Research Program (CNTRP) through a strategic program grant from the Canadian Institutes of Health Research and partners (Grant Number TFU 127880). The CNTRP is a national research initiative designed to increase organ and tissue donation in Canada and enhance the survival and quality of life of Canadians living with a transplant. The CNTRP brings together basic and clinical scientists in organ/tissue donation, solid organ transplantation and hematopoietic cell transplantation together with health economics, legal and ethics researchers, policy experts, and knowledge users to overcome challenges to optimal use of donation and transplantation. Learn more at www.cntrp.ca.
Authors received funding to defray travel expenses for conference planning and attendance. Participants received funding for travel expenses to attend the conference.
Dr. Zavalkoff disclosed association with the Canadian Patient Safety Institute. Dr. Shemie disclosed that he is a medical advisor for deceased organ donation at Canadian Blood Services, and he disclosed government work. Dr. Grimshaw disclosed associations with Abbott Diagnostics, the Canadian Patient Safety Institute and NHS Education Scotland.
The remaining authors have disclosed no potential conflicts of interest.
Editorial responsibility
This submission was handled by Dr. Sangeeta Mehta, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
All authors contributed substantially to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data and drafting the article.
Appendix: Steering committee and conference participant affiliations
Steering Committee
Dr. Sam Shemie, Conference co-chair
Division of Pediatric Critical Care, Montreal Children’s Hospital, McGill University Health Centre and Research Institute
Professor of Pediatrics, McGill University
Medical Advisor, Deceased Donation, Canadian Blood Services
Ms. Amber Appleby
Associate Director, Deceased Donation and Transplantation, Canadian Blood Services
Dr. Michaël Chassé
Scientist, Centre Hospitalier de l’Université de Montréal Research Center
Clinical Assistant Professor, Department of Medicine, University of Montreal
Division of Critical Care, Department of Medicine, Centre Hospitalier de l’Université de Montréal (CHUM)
Dr. Jeremy Grimshaw, Conference co-chair
Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute
Full Professor, Department of Medicine, University of Ottawa
Canada Research Chair in Health Knowledge Uptake and Transfer
Mr. David Hartell
Executive Director, Canadian National Transplant Research Program
Dr. Greg Knoll
Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute
Full Professor, Department of Medicine, Division of Nephrology, University of Ottawa
Ms. Jehan Lalani
Program Manager, Deceased Donation and Transplantation, Canadian Blood Services
Ms. Stefanie Linklater
Research Coordinator, Clinical Epidemiology Program, Ottawa Hospital Research Institute
Mr. Ken Lotherington
Senior Program Manager, Deceased Donation and Transplantation, Canadian Blood Services
Dr. Janet E. Squires
Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute
Dr. Samara Zavalkoff
Assistant Professor of Pediatrics, McGill University Division of Pediatric Critical Care, Montreal Children’s Hospital
Medical Officer (PICU), Patient Safety and Quality Improvement, McGill University Health Centre
Medical Director, Extracorporeal Life Support Program, Montreal Children’s Hospital
Conference Participants
Ms. Amber Appleby; Associate Director Deceased Donation and Transplantation, Canadian Blood Services, Vancouver, BC
Ms. Janice Beitel (expert presenter); Director, Hospital Programs, Education and Professional Practice, Trillium Gift of Life Network, Toronto, ON
Dr. Desmond Bohn (expert presenter); Provincial Medical Director, CritiCall Ontario, Toronto, ON
Dr. Michaël Chassé (expert presenter); Intensivist, Division of Critical Care, Department of Medicine, Centre Hospitalier de l’Université de Montréal (CHUM); Scientist, CHUM Research Center; Clinical Assistant Professor, Department of Medicine, University of Montreal, Montreal, QC
Ms. Rosanne Dawson (expert presenter); Legal Counsel, Canadian Blood Services, Ottawa, ON
Dr. Sonny Dhanani; Intensivist, Pediatric Critical Care Medicine, Children’s Hospital of Eastern Ontario; Chief Medical Officer, Trillium Gift of Life Network, Ottawa, ON
Ms. Karen Dryden-Palmer; Clinical Nurse Specialist, Paediatric Intensive Care Unit, Child Health Evaluative Sciences, The Hospital for Sick Children; Past President - Canadian Association of Critical Care Nurses, Toronto, ON
Dr. Shane English; Neurocritical Care, Associate Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON
Mr. Clay Gillrie; Program Manager Deceased Donation, Canadian Blood Services, Vancouver, BC
Dr. Robert Green; Emergency and Critical Care Medicine, QEII Health Science Centre Provincial Medical Director, Nova Scotia Trauma Program, Halifax, NS
Dr. Jeremy Grimshaw (conference co-chair, expert presenter); Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON
Dr. Jennifer Hancock (expert presenter); Associate Professor, Critical Care Medicine; Dalhousie University; QEII Health Science Centre, Halifax, NS
Mr. David Hartell; Executive Director, Canadian National Transplant Research Program, Ottawa, ON
Dr. Michael K. Hartwick; Intensivist and Palliative Care Physician, The Ottawa Hospital; Assistant Professor, Divisions of Critical Care Medicine and Palliative Medicine, University of Ottawa; Regional Medical Lead, Trillium Gift of Life Network, Toronto, ON
Ms. Karen Hornby (expert presenter); Senior Program Manager, Data and Analytics, Canadian Blood Services, Montréal, QC
Ms. Cindy Hyson (expert presenter); Associate Director, Surveillance and Epidemiology, Infectious Disease Prevention Control Branch, Public Health Agency of Canada, Ottawa, ON
Dr. George Isac; Medical Director, Critical Care, Vancouver General Hospital; Medical Lead, Donation, Vancouver General Hospital; Anaesthesiologist, Vancouver Coastal Health, Vancouver, BC
Dr. Sean Keenan; Provincial Medical Director, Donation Services, BC Transplant; Intensivist, Royal Columbian Hospital, New Westminster, BC; Representative for the Canadian Critical Care Society; Clinical Associate Professor of Medicine, University of British Columbia, Vancouver, BC
Ms. Karen Kieley (expert presenter); Accreditation Product Development Specialist, Accreditation Canada, Ottawa, ON
Dr. Greg Knoll (expert presenter); Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute; Full Professor, Department of Medicine, Division of Nephrology, University of Ottawa
Dr. Andreas Kramer; Intensive Care Services and Clinical Neurosciences, Foothills Medical Centre; Medical Director, Southern Alberta Organ & Tissue Donation Program, Calgary, AB
Ms. Jehan Lalani; Program Manager Deceased Donation and Transplantation, Canadian Blood Services, Calgary, AB
Ms. Lori Lamont; Interim President & CEO, Winnipeg Regional Health Authority, Winnipeg, MB
Ms. Stefanie Linklater; Research Coordinator, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON
Mr. Ken Lotherington; Senior Program Manager, Deceased Donation and Transplantation, Canadian Blood Services, Dartmouth, NS
Dr. Paul McGann (expert presenter); Centers for Medicare & Medicaid Services (CMS) Chief Medical Officer for Quality Improvement, US
Dr. Lisa Mielniczuk; Department of Medicine, Divisions of Cardiology and Cellular and Molecular Medicine; Director, Heart Failure Program; Medical Director, Heart Transplant Program; Medical Director, Pulmonary Hypertension Clinic, Ottawa, ON
Ms. Debbie Neville (expert presenter); Family Representative, RN and Manager Surgical Services and Acute Pain Services, Cape Breton Regional Hospital, Sydney, NS
Dr. Damon Scales (expert presenter); Scientist, Evaluative Clinical Sciences, Trauma, Emergency & Critical Care Research Program, Sunnybrook Research Institute; Staff Physician, Department of Critical Care Medicine, Sunnybrook Health Sciences Centre; Adjunct Scientist, Institute for Clinical Evaluative Sciences; Associate Faculty, Institute for Health Policy, Management and Evaluation, Toronto, ON
Dr. Sam Shemie (conference co-chair, expert presenter); Division of Pediatric Critical Care, Montreal Children’s Hospital, McGill University Health Centre and Research Institute; Professor of Pediatrics, McGill University; Medical Advisor, Deceased Donation, Canadian Blood Services, Montreal, QC
Dr. Janet E. Squires; Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON
Mr. Angus Steele (expert presenter); Senior Specialist; Health Quality Ontario, Toronto, ON
Dr. Joshua Tepper (expert presenter); President and CEO; Health Quality Ontario, Toronto, ON
Mr. Emile Therien (expert presenter); Donor Family Member, Ottawa, ON
Ms. Maeghan Toews; Research Associate, Health Law Institute, University of Alberta, Edmonton, AB
Mr. David Unger; Ethicist, Providence Health Care, Vancouver, BC
Mr. Hugues Villeneuve; Chef du service de l’enseignement et du développement hospitalier; Transplant Québec, Montréal, QC
Mr. Dennis Wagner (expert presenter); Acting Director for the Centers for Medicare & Medicaid Services (CMS) for Clinical Standards and Quality, Quality Improvement Group, US
Dr. Matthew Weiss; Pediatric Intensivist; Centre Mère-Enfant Soleil; Quebec City, QC
Ms. Kim Werestiuk (expert presenter); Manager, Transplant Manitoba - Gift of Life, Winnipeg, MB
Dr. Lori West (expert presenter); Director, Canadian National Transplant Research Program, Edmonton, AB
Ms. Carla Williams (expert presenter); Patient Safety Improvement Lead, Safety Improvement and Capability Building, Canadian Safety Patient Institute, St. John’s, NL
Ms. Kim Worton; Unit Manager, Transplant Services, Organ and Tissue Donation Programs, Alberta Health Services, Edmonton, AB
Ms. Linda Wright; Former University Health Network Director of Bioethics, Toronto, ON
Ms. Juliana Wu; Manager Decision Support CORR and Trauma Registries; Canadian Institute for Health Information, Toronto, ON
Ms. Kimberly Young (expert presenter); Director Donation and Transplantation, Canadian Blood Services, Edmonton, AB
Dr. Samara Zavalkoff; Assistant Professor of Pediatrics, McGill University Division of Pediatric Critical Care, Montreal Children’s Hospital; Medical Officer (PICU), Patient Safety and Quality Improvement, McGill University Health Centre; Medical Director, Extracorporeal Life Support Program, Montreal Children’s Hospital, Montréal, QC
Footnotes
This article is accompanied by an editorial. Please see Can J Anesth 2019; 66: this issue
References
- 1.Canadian Blood Services. Deceased Donation Data Working Group. Ottawa - 2016. Available from URL: https://professionaleducation.blood.ca/sites/msi/files/DDDWG_report_FINAL%202016-06-30.pdf (accessed September 2018).
- 2.Canadian Institute for Health Information. Deceased Organ Donor Potential in Canada. Ottawa, Canadian Institute for Health Information, 2014. Available from URL: https://www.cihi.ca/sites/default/files/organdonorpotential_2014_en_0.pdf (accessed September 2018).
- 3.Canadian Blood Services. Organ Donation and Transplantation in Canada: System Progress Report 2006 - 2015. Ottawa, Canadian Blood Services, 2016. Available from URL: https://blood.ca/sites/default/files/ODT_Report.pdf (accessed September 2018).
- 4.Redelmeier DA, Markel F, Scales DC. Organ donation after death in Ontario: a population-based cohort study. CMAJ. 2013;185:E337–E344. doi: 10.1503/cmaj.122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squires JE, Graham N, Coughlin M, et al. Barriers and enablers to organ donation after circulatory determination of death: a qualitative study exploring the beliefs of frontline intensive care unit professionals and organ donor coordinators. Transplant Direct. 2018;4:e368. doi: 10.1097/TXD.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squires JE, Coughlin M, Dorrance K, et al. Criteria to Identify a potential deceased organ donor: a systematic review. Crit Care Med. 2018;46:1318–1327. doi: 10.1097/CCM.0000000000003200. [DOI] [PubMed] [Google Scholar]
- 7.Ipsos Reid. OTDT Professional Survey, February 7th, 2011 - Final Report. Prepared for: Canadian Blood Services. Available from URL: https://professionaleducation.blood.ca/sites/msi/files/OTDT-Professional-Survey-Final-Report.pdf (accessed September 2018).
- 8.Klarenbach SW, Tonelli M, Chui B, Manns BJ. Economic evaluation of dialysis therapies. Nat Rev Nephrol. 2014;10:644–652. doi: 10.1038/nrneph.2014.145. [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 10.Delmonico FL, Dominguez-Gil B, Matesanz R, Noel L. A call for government accountability to achieve national self-sufficiency in organ donation and transplantation. Lancet. 2011;378:1414–1418. doi: 10.1016/S0140-6736(11)61486-4. [DOI] [PubMed] [Google Scholar]
- 11.Collège des Médecins du Québec. Comité de transplantation. Les donneurs potentiels d’organes dans les hospitaux du Quebec - Années 2000 à 2010. Montréal, 2015. Available from URL: http://www.cmq.org/publications-pdf/p-1-2015-03-01-fr-donneurs-potentiels-organes-hopitaux-du-quebec.pdf (accessed September 2018).
- 12.Trifunov R. Deceased Organ Donor Potential in Canada - a Review and Estimate from Past Studies and Sources. Canadian Blood Services: Ottawa; 2010. [Google Scholar]
- 13.Canadian Blood Services. Organ Donation and Transplantation in Canada: 2016 System Progress Report Update. Ottawa, 2017. Available from URL: https://professionaleducation.blood.ca/sites/msi/files/odt_report-2017-final.pdf (accessed September 2018).
- 14.Canadian Blood Services; Ipsos Reid. Organ and Tissue Donation General Public Survey. Ottawa, 2017.
- 15.Canadian Medical Association. Organ and Tissue Donation and Transplantation (Update 2015). Ottawa, 2015. Available from URL: https://www.cma.ca/Assets/assets-library/document/en/advocacy/cma_policy_organ_and_tissue_donation_and_transplantation_update-2015_pd16-02-e.pdf (accessed October 2018).
- 16.Neate S, Marck CH, Weiland TJ, et al. Australian emergency clinicians’ perceptions and use of the GIVE clinical trigger for identification of potential organ and tissue donors. Emerg Med Australas. 2012;24:501–509. doi: 10.1111/j.1742-6723.2012.01598.x. [DOI] [PubMed] [Google Scholar]
- 17.Shemie SD, Simpson C, Blackmer J, et al. Ethics guide recommendations for organ-donation-focused physicians: endorsed by the Canadian Medical Association. Transplantation. 2017;101:S41–S47. doi: 10.1097/TP.0000000000001694. [DOI] [PubMed] [Google Scholar]
- 18.Shemie SD, Baker AJ, Knoll G, et al. National Recommendations for donation after cardiocirculatory death in Canada: donation after cardiocirculatory death in Canada. CMAJ. 2006;175:S1. doi: 10.1503/cmaj.060895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canadian Blood Services. Analysis of Deceased Organ Donation Medical Records Review. Ottawa, 2015.
- 20.Hancock J, Shemie SD, Lotherington K, Appleby A, Hall R. Development of a Canadian deceased donation education program for health professionals: a needs assessment survey. Can J Anesth. 2017;64:1037–1047. doi: 10.1007/s12630-017-0882-4. [DOI] [PubMed] [Google Scholar]