Abstract
Background
BI 853520 is a potent inhibitor of focal adhesion kinase and is currently under clinical development for the treatment of non-hematological malignancies.
Objective
The objective of this study was to evaluate the effect of food and liquid dispersion on the pharmacokinetics of BI 853520 in two open-label, crossover substudies.
Patients and Methods
Sixteen patients with advanced solid tumors were enrolled in each substudy. The order of administration was randomized, and pharmacokinetic samples were collected for 48 h after administration of a 200 mg dose of BI 853520. Lack of effect would be demonstrated if the 90% confidence interval (CI) of the ratio of the adjusted geometric mean (GMR) of the area under the plasma curve (area under the plasma concentration–time curve from time zero to the last quantifiable concentration at tz [] and observed area under the plasma concentration–time curve extrapolated from time zero to infinity [AUC0–∞,obs]) and maximum plasma concentration (Cmax) did not cross the 80–125% (bioequivalence) boundaries.
Results
Adjusted GMRs (90% CIs) for the fed versus fasted state were 92.46% (74.24–115.16), 98.17% (78.53–122.74), and 87.34% (71.04–107.38) for , AUC0–∞,obs, and Cmax, respectively. Although the 90% CIs were not within bioequivalence limits for the food-effect study, the limited reductions in these pharmacokinetic parameters after administration with a high-fat meal are unlikely to be clinically relevant. Compared with a tablet, administration of BI 853520 as a liquid dispersion did not strongly affect , AUC0–∞,obs, or Cmax, resulting in adjusted GMRs (90% CIs) of 1.00 (0.92–1.09), 0.98 (0.90–1.07), and 0.93 (0.86–1.01), respectively.
Conclusions
These studies demonstrate that BI 853520 can be given with no food restrictions, and as a liquid dispersion, without strongly impacting pharmacokinetics. These pharmacokinetic properties may help make BI 853520 dosing more convenient and flexible, improving treatment compliance.
Clinical trials registration
ClinicalTrials.gov identifier: NCT01335269.
Electronic supplementary material
The online version of this article (10.1007/s11523-018-00618-0) contains supplementary material, which is available to authorized users.
Key Points
| We evaluated the effect of food and liquid dispersion on the pharmacokinetics of BI 853520. |
| Only minimal effects of a high-caloric meal were found, and any effect on pharmacokinetics is unlikely to be clinically relevant; compared with a tablet formulation, administration as a liquid did not strongly affect the pharmacokinetics of BI 853520. |
| These properties may help make BI 853520 dosing more convenient and flexible, thereby improving treatment compliance. |
Introduction
Focal adhesion kinase (FAK), also known as protein tyrosine kinase 2 (PTK2), is a non-receptor cytokine tyrosine kinase that comprises a structural component of focal adhesions. These focal adhesions are protein complexes containing cell surface integrins, which are essential for interaction with the extracellular matrix and transduction of signaling pathways [1]. FAK plays a vital role in proliferation, survival, and migration of tumor cells [2]. In cancer, dysregulation and activation of focal adhesions facilitate cell motility and promote invasive tumor growth [1]. Increased expression of FAK is found in various tumor types, and the extent of expression has been related to the extent of disease progression and metastasis [3]. In particular, FAK overexpression has been implicated in the development of sarcomas, and prostate, colorectal, ovarian, and breast cancer [4–9].
In mice, genetic knockout of FAK has been shown to be embryonically lethal, underscoring its role in development, particularly in the formation of blood vessels [10]. Chemical inhibition of FAK has been shown to reduce FAK activity and block tumor growth in a range of xenograft models [11–14]. Moreover, inhibition of FAK on endothelial cells has been shown to improve sensitivity of tumor cells to chemotherapy and immunotherapy in preclinical models [15, 16]. Several inhibitors of FAK have been evaluated in patients with cancer [17–19], both as monotherapy and in combination with chemotherapy, targeted and immune therapies [20]. BI 853520 is a potent inhibitor of FAK, and clinical exploration has shown target engagement and anti-tumor activity in the phase I studies reported by de Jonge et al. [21] and Doi et al. [22] in this issue of Targeted Oncology. In the study by de Jonge et al. [21] involving primarily Caucasian subjects who were dosed in the fasted state, BI 853520 was rapidly absorbed and exhibited at least biphasic disposition kinetics. BI 853520, which is classified as a class 1 drug in the Biopharmaceutics Classification System (BCS), was orally bioavailable, with an observed terminal half-life (t½) of 19 h following administration of a single dose, supporting a once-daily dosing schedule.
A major determinant of drug absorption is the impact of concomitant administration with or without food [23]. Food, among other factors, may influence gastric pH, emptying, and motility. Moreover, the presence of a high-fat meal may improve the solubility of lipophilic drugs, thereby increasing (relative) bioavailability. All of these factors can influence the rate and extent of gastrointestinal absorption and indicate the need to study the effects of food on drug bioavailability during clinical drug development [24, 25]. A marked influence of food on absorption has been reported for several orally dosed anti-cancer drugs [26–29]. In particular, in the case of abiraterone, a 1000% increase in the area under the plasma concentration–time curve (AUC) was demonstrated when the drug was administered with food compared to a fasted state, illustrating a clinically relevant food effect [27].
The requirement to administer drugs in the fasting state can have a major impact on patients’ well-being, especially if the fasting state has to be continued for several hours after drug administration. Further, oral administration of drugs can be problematic for those who cannot swallow whole tablets. This may be particularly relevant in patients with some advanced cancers such as head and neck cancer or esophageal cancer. Therefore, development of an alternative oral formulation could increase the convenience of administration for patients. However, any alternative formulation should be tested clinically first to demonstrate that it achieves appropriate pharmacokinetic exposure.
We report on two randomized, open-label, crossover studies evaluating the effect of administration with or without a high-calorie meal, and the effect of administration as a liquid dispersion on the pharmacokinetics of the novel FAK inhibitor BI 853520. These pharmacokinetic studies were part of a larger phase I dose-finding trial.
Methods
Patients
Patients were eligible for enrollment into the expansion cohorts of the phase I dose-finding study of BI 853520 (ClinicalTrials.gov identifier NCT01335269; see de Jonge et al. [21]) if they had a confirmed diagnosis of advanced, measurable or evaluable, non-resectable and/or metastatic non-hematologic malignancy and disease progression in the last 6 months before study entry as demonstrated by serial imaging. Patients had to have failed conventional treatment or be unamenable to established treatment options, or have no proven therapy available to them. Moreover, patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1, have recovered from reversible toxicities (alopecia excluded) from prior anti-cancer therapies (Common Terminology Criteria for Adverse Events grade < 2), be at least 18 years of age, and have a life expectancy of at least 3 months.
The main exclusion criteria were serious concomitant illness, active infections, pregnancy, breastfeeding, active or symptomatic brain metastases, second malignancies, congestive heart failure of grade III or IV, myocardial infarction within 6 months of inclusion, absolute neutrophil count < 1500/mm3, platelet count < 100,000/mm3, total bilirubin > 1.5 times the upper limit of normal (ULN), and aspartate transferase and/or alanine transferase > 3 times ULN or > 5 times ULN in patients with liver metastases.
Study Design
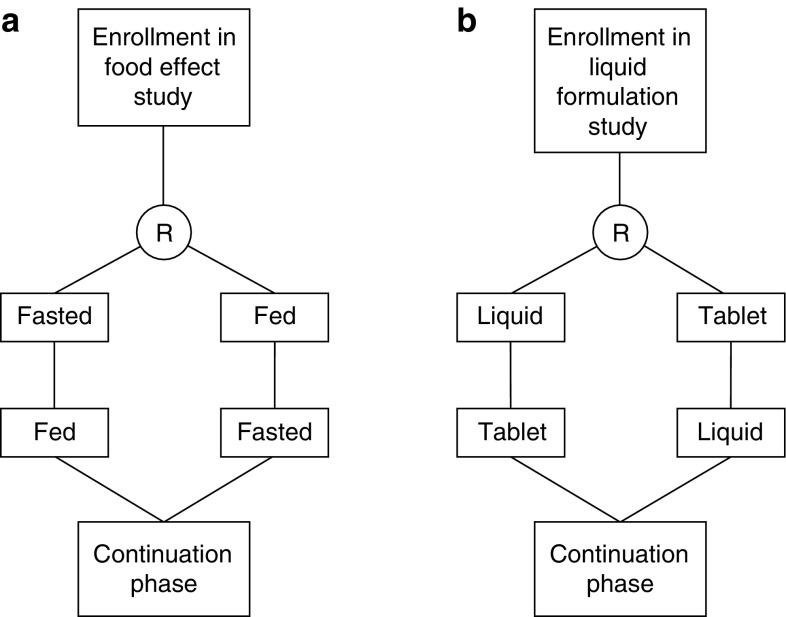
An overview of the design of both studies is provided in Fig. 1. The effect of food on the pharmacokinetics of BI 853520 was investigated in a randomized, open-label, crossover, single-dose study in patients with advanced solid tumors. Patients received a single 200 mg tablet of BI 853520 either in a fed or fasted state (see details of the conditions of drug administration in Sect. 2.3) with a washout period of 7 days between each administration. The order of fasted–fed or fed–fasted was established through randomization. Further information on the determination of the sample size for each substudy is provided in the Electronic Supplementary Material.
Fig. 1.
Schematic of randomized, open-label, crossover trials to evaluate the effect of food and formulation on the pharmacokinetics of a 200 mg dose of the focal adhesion kinase (FAK) inhibitor BI 853520. The order of administration [fasted–fed vs. fed–fasted (a) or tablet–liquid vs. liquid–tablet (b)] was randomized (R), and a washout period of 1 week applied between the two treatments. After the pharmacokinetic studies, patients continued on a daily dose of 200 mg of BI 853520 (as a tablet) until disease progression, intolerability of the study medication, or withdrawal of consent
The pharmacokinetics of a single 200 mg dose of BI 853520 in a liquid dispersion were evaluated in a separate study with the same randomized, open-label, crossover design using the 200 mg tablet as reference. The order of administration (liquid–tablet or tablet–liquid) was randomized and a 7-day washout period applied as already described.
After the last pharmacokinetic sample of each pharmacokinetic study, patients continued treatment with a daily dose of 200 mg of BI 853520 until disease progression, intolerability of the study medication, or withdrawal of consent.
Drug Administration
In the food-effect study, BI 853520 was administered after an overnight fast, either with approximately 240 mL of water or with a standardized high-calorie meal. No food was allowed for 4 h after intake of the drug. Water was allowed 1 h after taking the drug. The high-calorie meal was a high-fat breakfast containing approximately 950 kcal (at least half of which were from fat) and was ingested within no more than 30 min. Directly after the meal, the single 200 mg tablet of BI 853520 was administered.
In the tablet-versus-liquid formulation study, patients received BI 853520 in a fasted state, as described earlier. Patients remained fasted for 4 h after intake of the drug. The liquid formulation was prepared by dissolution of the tablet in 20 mL of a reconstitution solution containing sucralose (4 mg/mL), menthol (2 mg/mL), and benzoic acid (1 mg/mL). The tablet was submersed in the solution in a child-resistant screw-cap bottle, without being crushed. The bottle was then closed and shaken thoroughly for 30 s. After shaking, the bottle was set aside for 10 min. If the tablet was not dispersed completely, the bottle would be shaken for another 30 s and set aside for 5 min. This procedure was repeated until the tablet was dispersed completely into a homogeneous dispersion without noticeable lumps. No further dilution of the dispersion was allowed.
Pharmacokinetic Sampling
In both studies, blood samples were collected before and at 0.5, 1, 2, 3, 4, 6, 8, 10, 24, and 48 h after drug administration. Plasma concentrations of BI 853520 were measured by validated assays based on liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS). The lower limit of detection for the assay was 1 nM for plasma; at BI 853520 plasma concentrations ranging from 2.5 to 800 ng/mL, precision ranged from − 1.6 to − 5.1% and accuracy ranged from 5.9 to 11.3%.
Data Analysis
Based on the plasma concentration–time curves, pharmacokinetic parameters were calculated using non-compartmental analysis. Parameters of interest were maximum plasma concentration (Cmax), time to Cmax (tmax), AUC calculated from time zero to 48 h, from time zero to the last quantifiable concentration at tz, and extrapolated from time zero to infinity (AUC0–48, , and AUC0–∞,obs, respectively) and plasma t½ (calculation of t½ is described in the Electronic Supplementary Material).
The 90% confidence interval (CI) was calculated for the ratio of the adjusted geometric mean (adjusted GMR) Cmax, AUC0–∞,obs, and for a 200 mg dose under fed and fasted conditions, and for the 200 mg tablet and liquid formulation. Calculation of an adjusted GMR accounted for sources of variation, such as patients without valid data for both treatment states (fasted and fed) or both formulations (liquid and tablet). Lack of difference was demonstrated if the 90% CI of the adjusted GMRs of Cmax, AUC0–∞,obs, and were within the 80–125% limits, in accordance with US Food and Drug Administration (FDA) guidelines for food effect and bioequivalence studies [30, 31].
Reasons for exclusion from the pharmacokinetic analysis included vomiting within 4 h after ingestion, failure to take the full BI 853520 dose, and expired sample stability.
Trial Conduct and Registry
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients provided written informed consent before enrollment, in accordance with the International Conference on Harmonization Good Clinical Practice and local legislation. The competent authority that approved the trial was the Centrale Commissie Mensgebonden Onderzoek, Den Haag, The Netherlands. This trial was registered in the United States National Institutes of Health clinical trial registry under the ClinicalTrials.gov identifier NCT01335269.
Results
In total, 16 patients were enrolled in each study; patient characteristics are presented in Table 1. In the food-effect study, 15 patients were evaluable for treatment in at least one state (fed or fasted), and one plasma concentration–time profile was excluded for one patient due to vomiting after drug administration. In the liquid–tablet study, all 16 patients were evaluable for treatment with at least one dose (liquid or tablet) of BI 853520, and one plasma concentration–time profile was excluded for one patient due to incomplete drug administration.
Table 1.
Characteristics of evaluable patients in both studies
| Characteristic | Food effect study | Liquid formulation study |
|---|---|---|
| Patients, n | 15 | 16 |
| Gender, n (%) | ||
| Male | 5 (33.3) | 8 (50.0) |
| Female | 10 (66.6) | 8 (50.0) |
| Mean age, years [range] | 56 [25–72] | 60 [55–89] |
| Mean weight, kg (CV) | 70 (24.5) | 71 (15.3) |
| Mean height, cm (CV) | 169 (6.6) | 172 (5.9) |
| Tumor type, n (%) | ||
| Soft-tissue sarcoma | 11 (73.3) | 0 |
| Esophageal carcinoma | 0 | 6 (37.5) |
| Pancreatic adenocarcinoma | 2 (13.3) | 4 (25.0) |
| Ovarian carcinoma | 1 (6.7) | 6 (37.5) |
| Other | 1 (6.7) | 0 |
CV coefficient of variation
Food Effect
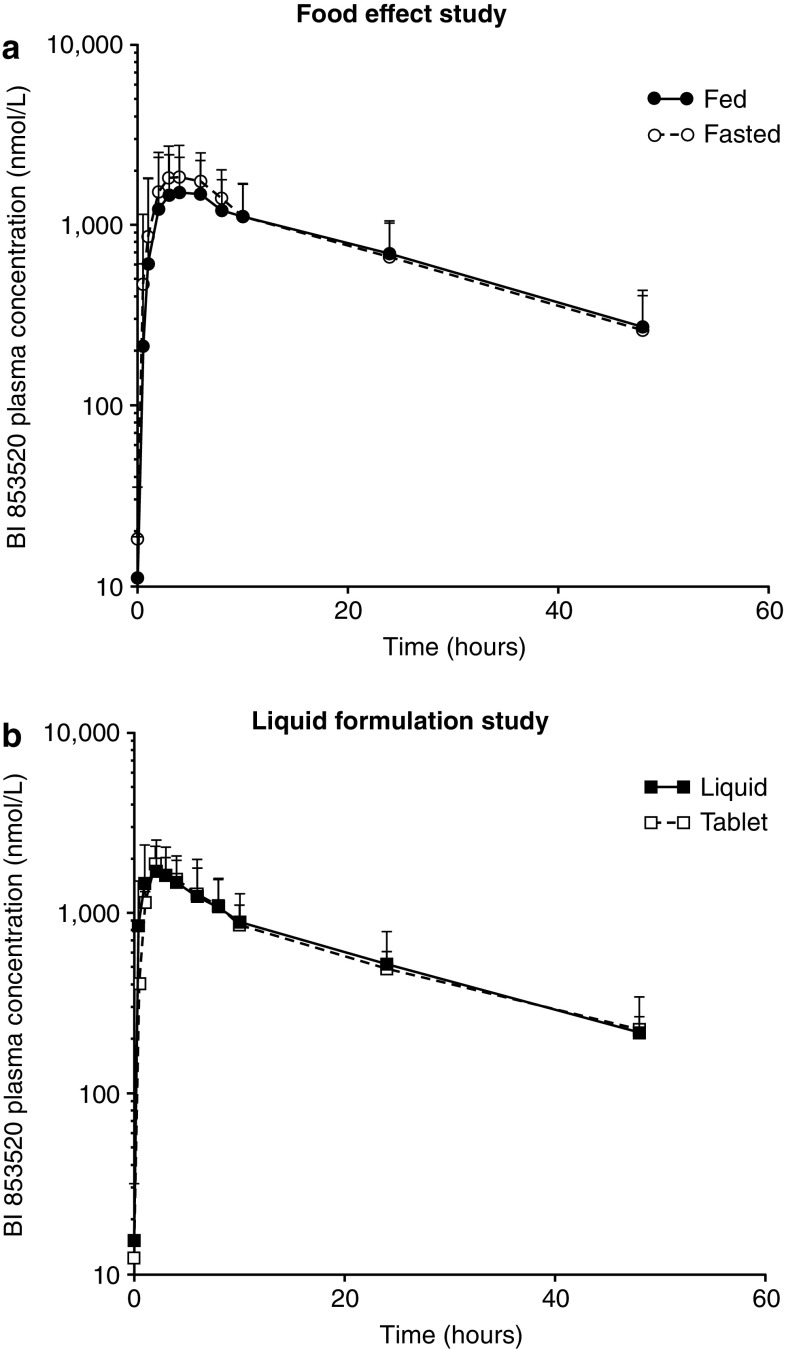
Plasma concentration–time curves of patients receiving 200 mg of BI 853520 under fed and fasted conditions are presented in Fig. 2. The plasma profile of BI 853520 was not markedly influenced by concomitant administration of the high-calorie meal. A summary of the pharmacokinetic parameters of interest is provided in Table 2. The adjusted GMRs (90% CIs) for the fed versus fasted state were 92.46% (74.24–115.16), 98.17% (78.53–122.74), and 87.34% (71.04–107.38) for , AUC0–∞,obs, and Cmax, respectively. All 90% CIs crossed the lower of the 80–125% boundaries. The tmax and t½ of BI 853520 administered after a high-calorie meal were not different to those in fasted patients.
Fig. 2.
Plasma concentration–time curves for BI 853520 (200 mg) in the food-effect and liquid formulation studies. Mean plus standard deviation of the plasma concentration–time curves for a 200 mg BI 853520 tablet administered to patients in a fed and fasted state (a) and a 200 mg dose of BI 853520 administered as a liquid dispersion and tablet (b)
Table 2.
Pharmacokinetic parameters for a 200 mg tablet of BI 853520 administered under fed and fasted conditions
| Parameter | Fed | Fasted | Adjusted GMR, % (90% CI) [fed/fasted] |
|---|---|---|---|
| Patients, n | 15 | 15 | – |
| t max a | 4 [1–24] | 3 [1–6] | – |
| , nM·h | 30,992c | 33,518 | 92.46 (74.24–115.16) |
| AUC0–∞,obs, nM·h | 39,219 | 39,949 | 98.17 (78.53–122.74) |
| Cmax, nM | 1636 | 1873 | 87.34 (71.04–107.38) |
| t½, hb | 18.0 (16.1)c | 18.0 (22.6) | – |
Unless otherwise specified, data are adjusted geometric mean
AUC0–∞,obs observed area under the plasma concentration–time curve extrapolated from time zero to infinity, area under the plasma concentration–time curve from time zero to the last quantifiable concentration at tz, Cmax maximum plasma concentration, t½ terminal half-life, tmax time to maximum plasma concentration, – not calculated
aMedian [range]
bGeometric mean [coefficient of variation (%)]
cn = 14
Liquid Formulation
Plasma concentration–time curves for the liquid formulation study are provided in Fig. 2. Calculated parameters for the pharmacokinetics of the liquid dispersion and tablet are presented in Table 3. The tmax and t½ were not affected by dispersing BI 853520 in a liquid. Adjusted GMRs (90% CIs) of , AUC0–∞,obs, and Cmax for the liquid versus tablet formulation were 99.98% (92.28–108.33), 101.65% (93.57–110.42), and 107.15% (98.79–116.22), respectively. All 90% CIs were within the 80–125% limits, indicating no important impact on pharmacokinetic exposure.
Table 3.
Pharmacokinetic parameters of 200 mg BI 853520 administered as a tablet or liquid formulation
| Parameter | Tablet | Liquid | Adjusted GMR, % (90% CI) [tablet/liquid] |
|---|---|---|---|
| Patients, n | 16 | 14 | – |
| t max a | 2 [1–6] | 2 [1–6] | – |
| , nM·h | 26,376 | 26,381 | 99.98 (92.28–108.33) |
| AUC0–∞,obs, nM·h | 31,978 | 31,460 | 101.65 (93.57–110.42) |
| Cmax, nM | 1721 | 1606 | 107.15 (98.79–116.22) |
| t½, hb | 19.5 (16.4) | 18.4 (22.7) | – |
Unless otherwise specified, data are adjusted geometric mean
AUC0–∞,obs observed area under the plasma concentration–time curve extrapolated from time zero to infinity, area under the plasma concentration–time curve from time zero to the last quantifiable concentration at tz, Cmax maximum plasma concentration, t½ terminal half-life, tmax time to maximum plasma concentration, - indicates not calculated
aMedian [range]
bGeometric mean [coefficient of variation (%)]
Discussion
The possible effects of food and formulation (liquid dispersion vs. tablet) on pharmacokinetic parameters of BI 853520 were assessed in two randomized, open-label, crossover pharmacokinetic studies. A total of 16 patients were planned for enrollment in each study. This planned sample size was not based on a power calculation, but was judged to be appropriate to achieve the aims of this exploratory substudy, and as being adequate to provide a minimum of 12 evaluable patients for the analysis, as required by FDA guidance.
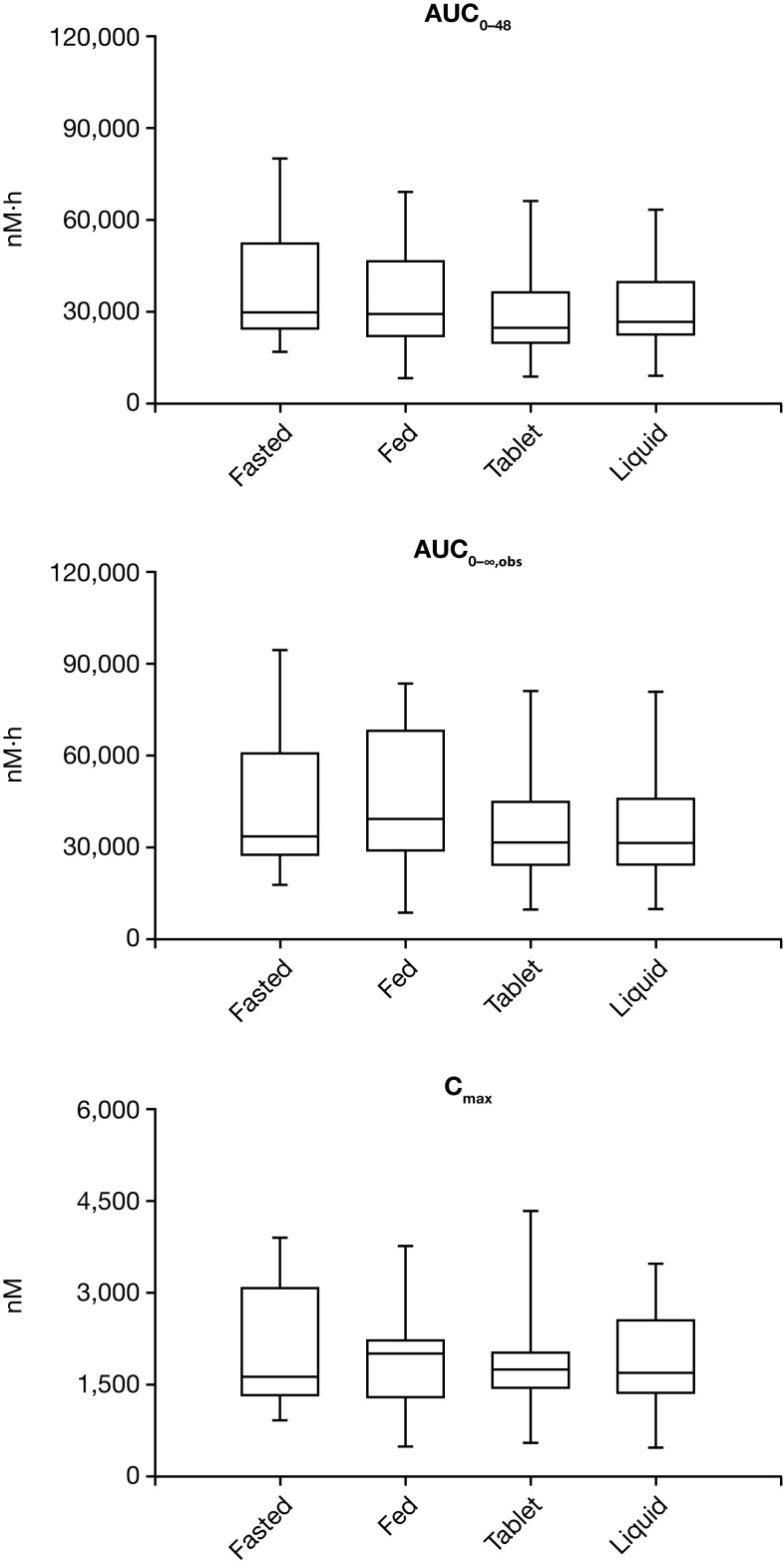
The plasma profile, tmax and t½ of BI 853520, when taken after a high-calorie meal, were not markedly different from those in fasted patients. The 90% CI for the adjusted GMRs for , AUC0–∞,obs, and Cmax all crossed the lower of the 80–125% boundaries. However, we do not consider the reductions to result in clinically meaningful differences in exposure. Our data, therefore, seem to support the view that BI 853520 may be administered orally without the need for stringent conditions regarding food intake. Interestingly, the geometric mean value for Cmax decreased slightly when BI 853520 was administered with food (Table 2), while the median value increased (Fig. 3).
Fig. 3.
AUC0–48, AUC0–∞,obs, and Cmax of BI 853520 (200 mg) in the food-effect and liquid formulation studies. Boxplots of AUC0–48, AUC0–∞,obs, and Cmax of BI 853520 following a single 200 mg dose administered as a liquid or tablet in the liquid formulation study, and under fed or fasted conditions (both as a tablet) in the food effect study. AUC0–∞,obs observed area under the plasma concentration–time curve extrapolated from time zero to infinity, AUC0–48 area under the plasma concentration–time curve from time zero to 48 h, area under the plasma concentration–time curve from time zero to the last quantifiable concentration at tz, Cmax maximum plasma concentration
Administration of BI 853520 after dispersion of the tablet in a reconstitution solvent did not appear to have any clinically important impact on the measured pharmacokinetic parameters. None of the 90% CIs of the calculated pharmacokinetic parameters crossed the predefined 80–125% limits. This suggests that the bioavailability of BI 853520 is not strongly affected by liquid dispersion and supports the use of the reconstitution liquid to facilitate drug administration in patients who have problems swallowing.
Overall, the results of our study suggest that the pharmacokinetic profile of BI 853520 is unlikely to be greatly influenced by concomitant administration with food or the type of formulation. These properties should allow for a patient-friendly posology, without strict requirements for administration under fasted conditions. In addition, administration of BI 853520 as a liquid dispersion may be particularly convenient for patients who experience problems swallowing.
As is apparent from Fig. 3, we observed large inter-individual pharmacokinetic variability in our study; this was also observed in the dose-finding study (see de Jonge et al. [21]). This variability cannot yet be explained; however, it may relate to differing plasma levels of α-1-acid glycoprotein (AGP), the major binding partner of BI 853520 in human plasma.
Conclusion
These randomized, open-label, crossover studies suggest that a high-calorie meal did not appear to have a large impact on BI 853520 pharmacokinetics. Further, our data suggest that the formulation of BI 853520 as a liquid dispersion has no important effects on the pharmacokinetics of BI 853520 relative to the tablet formulation. These pharmacokinetic properties may help make BI 853520 dosing more convenient and flexible, improving treatment compliance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing and editing assistance, supported financially by Boehringer Ingelheim, was provided by Lynn Pritchard of GeoMed, an Ashfield Company, part of UDG Healthcare plc, during the preparation of this article.
Funding
This work was supported by Boehringer Ingelheim, Ingelheim am Rhein, Germany. BI 853520 is an asset of Boehringer Ingelheim. This article was published open access under a Springer Compact agreement with Dutch universities and Academy institutes.
Conflict of interest
Remy B. Verheijen is an employee of AstraZeneca. Lillian L. Siu reports clinical trial funding (for her institution) for this study provided by from Boehringer Ingelheim. Linda C. Pronk and David Schnell are employees of Boehringer Ingelheim. Filip Y. F. L. De Vos has been paid for expert testimonial by Bristol-Myers Squibb, and received grants from Novartis. Hal W. Hirte has received honoraria from AstraZeneca, Roche, and Merck. Diane A. J. van der Biessen, Sebastien J. Hotte, Anna Spreafico, Maja J. A. de Jonge, Neeltje Steeghs, and Martijn P. Lolkema declare no conflicts of interest.
Contributor Information
Remy B. Verheijen, Email: r.verheijen@nki.nl
Diane A. J. van der Biessen, Email: a.vanderbiessen@erasmusmc.nl
Sebastien J. Hotte, Email: Hotte@HHSC.CA
Lillian L. Siu, Email: lillian.siu@uhn.ca
Anna Spreafico, Email: anna.spreafico@uhn.ca.
Maja J. A. de Jonge, Email: m.dejonge@erasmusmc.nl
Linda C. Pronk, Email: linda-christina.pronk@boehringer-ingelheim.com
Filip Y. F. L. De Vos, Email: f.devos@umcutrecht.nl
David Schnell, Email: david.schnell@boehringer-ingelheim.com.
Hal W. Hirte, Email: hirteh@HHSC.CA
Neeltje Steeghs, Email: n.steeghs@nki.nl.
Martijn P. Lolkema, Phone: +31 10 704 19 06, Email: m.lolkema@erasmusmc.nl
References
- 1.Lim ST, Mikolon D, Stupack DG, Schlaepfer DD. FERM control of FAK function: implications for cancer therapy. Cell Cycle. 2008;7(15):2306–2314. doi: 10.4161/cc.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Hochwald SN. The role of FAK in tumor metabolism and therapy. Pharmacol Ther. 2014;142(2):154–163. doi: 10.1016/j.pharmthera.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14(9):598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34(3):240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- 5.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55(13):2752–2755. [PubMed] [Google Scholar]
- 6.Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, et al. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9(1):215–222. [PubMed] [Google Scholar]
- 7.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86(8):1551–1556. doi: 10.1002/(SICI)1097-0142(19991015)86:6<1551::AID-CNCR23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68(2):164–171. doi: 10.1002/(SICI)1097-0215(19961009)68:2<169::AID-IJC4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342(8878):1024–1025. doi: 10.1016/0140-6736(93)92881-S. [DOI] [PubMed] [Google Scholar]
- 10.Ilic D, Kovacic B, McDonagh S, Jin F, Baumbusch C, Gardner DG, et al. Focal adhesion kinase is required for blood vessel morphogenesis. Circ Res. 2003;92(3):300–307. doi: 10.1161/01.RES.0000055016.36679.23. [DOI] [PubMed] [Google Scholar]
- 11.Golubovskaya VM, Figel S, Ho BT, Johnson CP, Yemma M, Huang G, et al. A small molecule focal adhesion kinase (FAK) inhibitor, targeting Y397 site: 1-(2-hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo [3.3.1.1(3,7)]decane; bromide effectively inhibits FAK autophosphorylation activity and decreases cancer cell viability, clonogenicity and tumor growth in vivo. Carcinogenesis. 2012;33(5):1004–1013. doi: 10.1093/carcin/bgs120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golubovskaya VM, Ho B, Zheng M, Magis A, Ostrov D, Morrison C, et al. Disruption of focal adhesion kinase and p53 interaction with small molecule compound R2 reactivated p53 and blocked tumor growth. BMC Cancer. 2013;13:342. doi: 10.1186/1471-2407-13-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. 2010;9(10):778–790. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68(6):1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 15.Tavora B, Reynolds LE, Batista S, Demircioglu F, Fernandez I, Lechertier T, et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature. 2014;514(7520):112–116. doi: 10.1038/nature13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SF, Siu LL, Bendell JC, Cleary JM, Razak AR, Infante JR, et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Investig New Drugs. 2015;33(5):1100–1107. doi: 10.1007/s10637-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 18.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol. 2012;30(13):1527–1533. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 19.Soria JC, Gan HK, Blagden SP, Plummer R, Arkenau HT, Ranson M, et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann Oncol. 2016;27(12):2268–2274. doi: 10.1093/annonc/mdw427. [DOI] [PubMed] [Google Scholar]
- 20.Roy-Luzarraga M, Hodivala-Dilke K. Molecular pathways: endothelial cell FAK-A target for cancer treatment. Clin Cancer Res. 2016;22(15):3718–3724. doi: 10.1158/1078-0432.CCR-14-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jonge MJA, Steeghs N, Lolkema MP, Hotte SJ, Hirte HW, van der Biessen Diane AJ, et al. Phase I study of BI 853520, an inhibitor of focal adhesion kinase, in patients with advanced or metastatic nonhematologic malignancies. Target Oncol. 2019 doi: 10.1007/s11523-018-00617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi T, Yang JC-H, Shitara K, Naito Y, Cheng A-L, Sarashina A, et al. Phase I study of the focal adhesion kinase inhibitor BI 853520 in Japanese and Taiwanese patients with advanced or metastatic solid tumors. Target Oncol. 2019 doi: 10.1007/s11523-019-00620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–1156. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Parsad S, Ratain MJ. Food effect studies for oncology drug products. Clin Pharmacol Ther. 2017;101(5):606–612. doi: 10.1002/cpt.610. [DOI] [PubMed] [Google Scholar]
- 25.Szmulewitz RZ, Ratain MJ. Playing Russian roulette with tyrosine kinase inhibitors. Clin Pharmacol Ther. 2013;93(3):242–244. doi: 10.1038/clpt.2012.245. [DOI] [PubMed] [Google Scholar]
- 26.Heath EI, Chiorean EG, Sweeney CJ, Hodge JP, Lager JJ, Forman K, et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88(6):818–823. doi: 10.1038/clpt.2010.199. [DOI] [PubMed] [Google Scholar]
- 27.Chi KN, Spratlin J, Kollmannsberger C, North S, Pankras C, Gonzalez M, et al. Food effects on abiraterone pharmacokinetics in healthy subjects and patients with metastatic castration-resistant prostate cancer. J Clin Pharmacol. 2015;55(12):1406–1414. doi: 10.1002/jcph.564. [DOI] [PubMed] [Google Scholar]
- 28.Kovarik JM, Hartmann S, Figueiredo J, Rordorf C, Golor G, Lison A, et al. Effect of food on everolimus absorption: quantification in healthy subjects and a confirmatory screening in patients with renal transplants. Pharmacotherapy. 2002;22(2):154–159. doi: 10.1592/phco.22.3.154.33542. [DOI] [PubMed] [Google Scholar]
- 29.Koch KM, Reddy NJ, Cohen RB, Lewis NL, Whitehead B, Mackay K, et al. Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol. 2009;27(8):1191–1196. doi: 10.1200/JCO.2008.18.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Food and Drug Administration. Guidance for Industry. Food-effect bioavailability and fed bioequivalance studies. 2002. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070241.pdf. Accessed 21 Dec 2018.
- 31.Food and Drug Administration. Bioavailability and bioequivalence studies submitted in NDAs or INDs - general considerations (draft). 2014. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm389370.pdf. Accessed 21 Dec 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.