Macrophages are a heterogeneous population of cells, which show a high degree of functional plasticity, derived from their ability to integrate diverse signals from the microenvironment, and acquire distinct phenotypes. Thanks to their remarkable plasticity, macrophages participate in numerous functions, including tissue regeneration, homeostasis, and inflammatory responses.
In the last 10 years, significant advances have been made in the characterization of bone marrow (BM) macrophages and their contributions to bone and marrow homeostasis and health. Bone tissue and its adjacent marrow contain different subsets of tissue resident macrophages, which support the distinct niche environments and critically regulate erythropoiesis and hematopoietic cell stemness.1 Within the BM niche, macrophages are in direct contact with hematopoietic stem cells (HSCs) and other niche cells and exert specialized functions to maintain niche homeostasis.
The first identified BM resident macrophages were the central macrophages, which behave as nurse cells for erythroblasts in a structure called erythroblastic island (EBI).1 The interaction of central macrophages and erythroblasts within the EBI is crucial for erythroblast survival and maturation to generate functional enucleated reticulocytes. Importantly, these macrophages promote erythroid maturation by producing trophic cytokines, providing iron for hemoglobin synthesis, and removing extruded nuclei. More recently, a novel population of resident macrophages involved in the maintenance of HSCs within the HSC niche has been described.1 These cells contribute to the maintenance of HSC quiescence and the control of HSC self-renewal and proliferation. HSC-macrophage depletion leads in fact to niche alteration and HSC mobilization from BM to peripheral blood, proving the critical role of these cells in HSC retention and stemness regulation. A third subset of BM macrophages is constituted by osteal macrophages or osteomacs, which regulate bone formation and maintain the endosteal HSC niche.1,2 Osteomacs are intimately associated with bone forming osteoblasts and promote mesenchymal maturation along the osteoblast lineage and their functional differentiation.
Based on the identification of BM macrophage subsets and their critical requirement for the maintenance and function of HSC niches, the role of these cells during HSC transplantation has recently moved into focus.
HSC transplantation is the most widely used regenerative therapy for a variety of life-threatening hematologic diseases. Critical for the success of HSC transplantation is the fine adjustment of HSCs within their specialized BM niche and the interaction with supportive and nurse cells. This process favors the full restoration of hematopoiesis following transplant.
Myeloablative treatments before transplant are assumed to fully remove macrophages from BM but increasing evidence shows that recipient macrophages persist to some extent after conditioning in both humans and mice. Kaur et al took advantage of a Csf1r-eGFP mouse (MacGreen mice) model of transplantation to study the resilience of distinct BM macrophage subtypes and their contribution to the engraftment of transplanted HSCs.3 The reporter Csf1r-eGFP transgene that labels myeloid cells allowed distinction of donor from recipient macrophages in transplanted MacGreen mice. Recipient granulocytes and monocytes were ablated following conditioning therapy and fully reconstituted from donor HSCs only after transplantation. By contrast, recipient macrophages persisted and further expanded within the first weeks after transplant, in both BM and spleen. Within ablation-resistant recipient macrophages, 2 phenotypically distinct macrophage subsets were identified, EBI (defined as Ly6G+, F4/80+, VCAM1+, CD169+, ER-HR3+, Mer-TK+) and resident niche macrophages (defined as Ly6G−, F4/80+, VCAM1+, CD169+, ER-HR3+, Mer-TK+, TIM4+) (Fig. 1). The combined expansion of both recipient and donor BM macrophages led to full restoration of the BM resident macrophage population to pretransplant levels soon after transplantation. Importantly, these different macrophage subsets show a monocyte-independent self-repopulation ability, as indicated by their ability to proliferate in situ and fully reconstitute the resident macrophage pool before the appearance of donor BM monocytes. Whereas in the spleen recipient macrophages persisted temporarily during the period of extra-medullary erythropoiesis, in BM these cells self-repopulate long term after transplantation, paralleling donor macrophage expansion and HSC engraftment.
Figure 1.
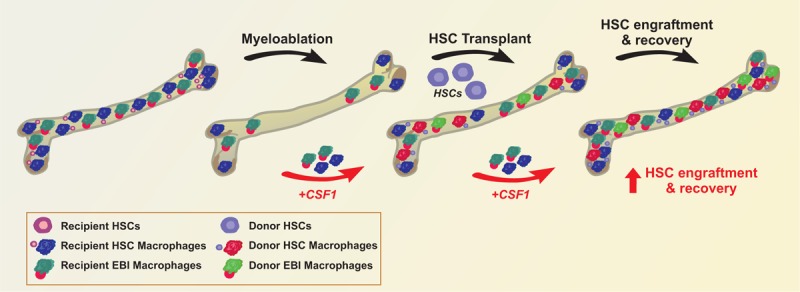
Targeting recipient BM macrophages in HSC transplantation. During myeloablative treatment, most recipient BM cells are removed. A minor proportion of recipient HSC and EBI macrophages survive, proliferate after HSC transplant, and allow proper engraftment of donor HSCs and the expansion of donor-derived macrophages. Strategies aimed at expanding the populations of recipient macrophages, such as CSF1 treatment, are expected to facilitate HSC engraftment and ameliorate the recovery of patients subjected to radiotherapy and chemotherapy as well as HSC transplantation. BM = bone marrow, EBI = erythroblastic island, HSC = hematopoietic stem cell.
Finally, to test the contribution of recipient macrophages to HSC engraftment, the authors used chimeric CD169-DTR (diphtheria toxin receptor) mice.3 Recipient BM macrophages (both EBI and resident niche macrophages) expressing CD169 were removed by diphtheria toxin treatment in CD169-DTR mice. Selective depletion of CD169+ recipient macrophages significantly impaired the engraftment of donor HSCs in the BM and translated into a reduced number of BM HSC and progenitor cell as well as total white blood cells and donor macrophages in BM and spleen.
These results highlight a critical role of recipient BM macrophages in supporting HSC engraftment and promoting hematopoietic reconstitution after transplant. The observation that recipient BM macrophages are crucial for optimal transplant outcome suggests that transplanted patients might benefit from ad hoc therapies aimed at the expansion of these cell subsets in the peritransplant period3 (Fig. 1).
The administration of macrophage-colony stimulating factor (CSF1) can be used for this purpose in the setting of BM transplantation and myelosuppressive chemotherapy4–6 (Fig. 1). In vitro CSF1 promotes the differentiation of myeloid progenitors into heterogeneous populations of monocytes, macrophages, dendritic cells, and bone-resorbing osteoclasts. The application of CSF1 as hematopoietic growth factor post-transplant or postmyeloablative treatment is currently limited due to the more common use of other myeloid growth factors, including G-CSF and GM-CSF, which successfully improve neutropenia. A few trials showed the clinical safety and benefit of CSF1 administration.4–6 Whether the effect is due to a specific action of CSF1 on BM macrophages after transplant and myeloablation is unknown and requires further investigation.
These findings unravel a novel relevant mechanism with the exciting potential to improve the outcome of HSC engraftment in transplanted patients, as well as to ameliorate the function of the BM microenvironment in patients subjected to radiotherapy and chemotherapy. Preclinical and clinical studies focused on testing therapeutic strategies targeting recipient macrophages and their expansion will eventually open the way to improved approaches for BM transplant.
Footnotes
Citation: Vinchi F. Targeting Bone Marrow Niche Macrophages: The Novel Frontier in Bone Marrow Transplant. HemaSphere, 2018;1:1. http://dx.doi.org/10.1097/HS9.0000000000000148
Funding/support: None.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kaur S, Raggatt LJ, Batoon L, et al. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin Cell Dev Biol. 2017;61:12–21. [DOI] [PubMed] [Google Scholar]
- 2.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. [DOI] [PubMed] [Google Scholar]
- 3.Kaur S, Raggatt LJ, Millard SM, et al. Self-repopulating recipient bone marrow resident macrophages 1 promote long-term hematopoietic stem cell engraftment. Blood. 2018;132:735–749. [DOI] [PubMed] [Google Scholar]
- 4.Kandalla PK, Sarrazin S, Molawi K, et al. M-CSF improves protection against bacterial and fungal infections after hematopoietic stem/progenitor cell transplantation. J Exp Med. 2016;213:2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masaoka T, Shibata H, Ohno R, et al. Double-blind test of human urinary macrophage colony stimulating factor for allogeneic and syngeneic bone marrow transplantation: effectiveness of treatment and 2-year follow-up for relapse of leukaemia. Br J Haematol. 1990;76:501–505. [DOI] [PubMed] [Google Scholar]
- 6.Hume DA, MacDonald KP. Therapeutic applications of 531 macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. [DOI] [PubMed] [Google Scholar]


