Little data concerning childhood acute myeloid leukemia (AML) with leukemia cutis (LC) have been published. Several cases have been reported in newborns and infants suggesting that skin lesions are common in neonatal leukemia but there is no study on a larger scale in pediatric patients.1,2
We conducted a retrospective observational noninterventional study using data from the cohort of patients included in the French national clinical trial ELAM02 (NCT00149162). Inclusion criteria were: age 0 to 18 years, prior untreated AML or medullar cytology showing myelodysplasia with blasts >20% or isolated myeloid sarcoma. At diagnosis, bone marrow smears were centrally reviewed, performed according to the French-American-British (FAB) morphology classification. Cytogenetic examination and molecular investigations were also centrally reviewed. Presence of LC was verified in medical records including clinical examination and pathology reports. Treatment consisted in an induction course associating mitoxantrone and continuous intravenous standard dose aracytine followed by a first course of consolidation including high dose aracytine combined with amsacrine. Afterwards, depending on risk group of their disease and the existence of a matched related hematologic stem cell (HSC) donor, children either received 2 more consolidation courses or allogenic HSC transplant.
Among the 438 patients included in the ELAM02 study between March 2005 and December 15, 2011, 24 (5.5%) presented LC (LC+ group). Various skin lesions were observed in the LC+ group: 16 patients (67%) had nodules, 2 (8%) had papules, 2 (8%) presented both nodules and papules, and 4 (17%) had plaques. One child of LC+ group was diagnosed at birth with FAB type M2. Median age at diagnosis was 1.2 years in LC+ group and 8.7 years in LC− group. Children with LC were significantly younger than those without LC (P < 0.0001, Table 1). Infants (age under 2) represented 63% (15/24) of LC+ group. In LC+ group, FAB type M5 was significantly more frequent (P < 0.0001) than in LC− group. Three patients among the LC+ group had cutaneous relapse of AML.
Table 1.
Results of ELAM02 Study Patients Depending on Presence or Absence of LC
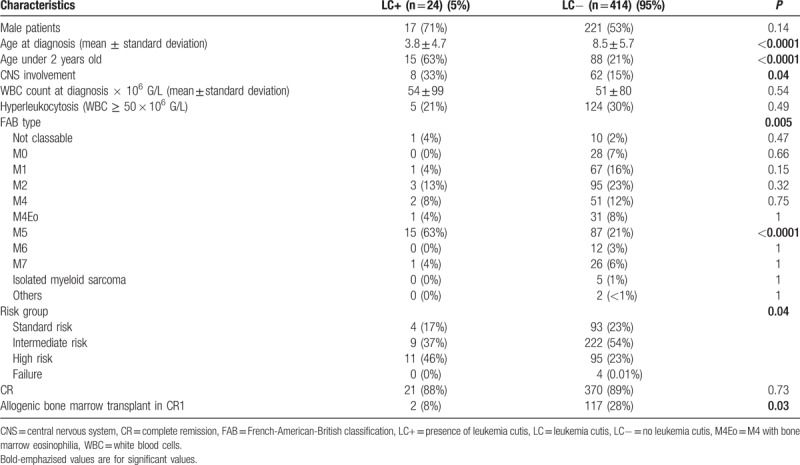
A normal karyotype was found less often in patients with LC than in those without, respectively, 8% versus 26% (P = 0.05). MLL-gene rearrangements were significantly more frequent in LC+ group than in LC− group, respectively, 46% versus 21% (P = 0.008). In infants, there was no difference of incidence of MLL-rearrangements depending on the presence or absence of LC (P = 0.27).
Molecular profiling by next-generation sequencing was performed in 19 of 24 patients with LC: the most frequently mutated genes were KRAS (16%), KIT (16%), and PTPN11 (16%). There was a nonsignificant difference of frequency of detected WT1 overexpression between patients whether they presented LC or not, respectively, 57% versus 80% (P = 0.16).
The incidence of complete remission (CR) by the end of consolidation no 1 was similar between LC+ group and LC− group, respectively, 88% versus 89% (P = 0.77). Relapse occurred more often in patients with LC (50% vs 35%) but this difference was not statistically significant (P = 0.19). Event-free survival (EFS) was not significantly different between groups but tended to be worse in patients with LC (P = 0.07; Fig. 1B). Overall survival (OS) was significantly inferior in patients with LC than in patients without LC (P = 0.006; Fig. 1A). We found no difference in risk for relapse or OS for patients with LC depending on presence or absence of MLL-rearrangement. In infants, cumulative incidence of death was more important in case of LC, 53% versus 23% (P = 0.025).
Figure 1.
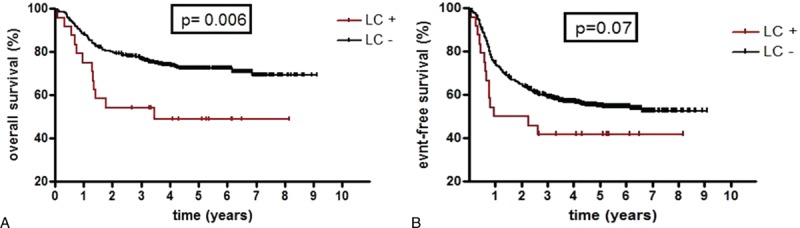
Overall (A) and event-free (B) survivals depending on the presence or absence of leukemia cutis. LC+ = presence of leukemia cutis, LC− = absence of leukemia cutis.
LC in childhood AML is a quite rare clinical feature since histological-proven LC prevalence was 3.4% in this study and global LC prevalence including clinically compatible and histological-proven LC was 4.5%. These results are similar to published data of a cohort of adult patients.3
In this study, there was no difference in gender and initial WBC count at diagnosis between patients with or without LC. A recent literature review also found no difference in gender and initial WBC count between adult patients whether they presented LC or not.4 In our cohort, patients with LC were significantly younger at diagnosis than those without LC. Even though we found only 1 patient with a congenital form of AML with LC, most of clinical case reports of childhood AML with LC concern infants and neonates suggesting LC is more frequent in very young children.2,5 Congenital forms of LC may present as blueberry muffin syndrome and can regress spontaneously before any blasts appear in blood.5
More than 60% of patients with LC had FAB type M5. Several case reports as well as previous series including both adult and pediatric patients have suggested such an association.3,5,6 Age could be a confounding factor since FAB type M5 is more common in very young children.1,7
MLL-rearrangement was the most frequent finding in patients with LC, observed in nearly half of these patients. MLL-rearrangement is a frequent cytogenetic feature in infant acute lymphoblastic leukemia and AML.8 However, we found no difference in the frequency of MLL-rearrangements in infants depending on the presence or absence of LC.
Concerning molecular work-up, our analysis is limited by many missing data since WT1 overexpression was researched in less than half of both groups. In the study published by Lapillonne et al, WT1 overexpression was observed in 78% of tested patients which was similar to our results in the LC− group but higher that the frequency found in the LC+ group.9 In that same study, WT1 values were higher in FAB type M5 as well as in AML with 11q23 rearrangements, and infants.9 In this study, patients in LC+ group presented more often with FAB type M5 and MLL-rearrangements; however, we did not find any association in this study between LC and overexpression of WT1. This could be due to insufficient data since molecular biology workup concerning WT1 overexpression was researched in less than half patients.
We found no difference concerning CR rate after induction and 1 course of consolidation whether patients presented LC or not, such as in adult patients.3 However, OS was worse in patients with LC than in patients without LC. Moreover, EFS tended to be better in patients without LC. Even though this difference was not statistically significant, the lack of significance was probably due to insufficient number of patients in both groups. A likely role of MLL-rearrangement could explain such results since MLL-rearranged AML is known to be more aggressive particularly in cases of neonatal LC with 11q23 rearrangement.1 However, we found no difference in OS for patients in LC+ group depending on presence or absence of MLL-rearrangement. Another study concluded prognosis, particularly EFS differs depending on translocation partner for MLL gene.10 A larger cohort study could be useful to study the impact of MLL-rearrangement and other molecular particularities on EFS and OS of AML associated with LC in children and infants.
In this study, we observed that children presenting LC associated with AML were more likely to be young with a median age of 1.2 years at diagnosis, had more often FAB type M5, MLL-rearrangements, and worse OS rate. To our knowledge, this is the first study showing LC could be a prognostic factor in childhood AML. Given the methodological limits of this study, a larger cohort study could be useful in order to determine the prognostic impact of LC in childhood AML and to clarify if these patients require specific treatment.
Footnotes
Citation: Gouache E, Greze V, Strullu M, Saultier P, Fenneteau O, Gandemer V, Ragu C, Auvrignon A, Boutroux H, Lapillonne H, Pasquet M, Leverger G. Leukemia Cutis in Childhood Acute Myeloid Leukemia: Epidemiological, Clinical, Biological, and Prognostic Characteristics of Patients Included in the ELAM02 Study. HemaSphere, 2018;1:1. http://dx.doi.org/10.1097/HS9.0000000000000141.
Funding/support: None.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Handler MZ, Schwartz RA. Neonatal leukaemia cutis. J Eur Acad Dermatol Venereol. 2015;29:1884–1889. [DOI] [PubMed] [Google Scholar]
- 2.Nanda A, El-Kamel MF, Al-Oneizi EM, et al. Congenital papulonodular eruption: presenting sign of congenital leukaemia cutis. Clin Exp Dermatol. 2012;37:509–511. [DOI] [PubMed] [Google Scholar]
- 3.Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002;81:90–95. [DOI] [PubMed] [Google Scholar]
- 4.Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130–142. [DOI] [PubMed] [Google Scholar]
- 5.Bacchetta J, Douvillez B, Warin L, et al. Blueberry Muffin Baby and spontaneous remission of neonatal leukaemia. Arch Pédiatr. 2008;15:1315–1319. [DOI] [PubMed] [Google Scholar]
- 6.Michel G, Boulad F, Small TN, et al. Risk of extramedullary relapse following allogeneic bone marrow transplantation for acute myelogenous leukemia with leukemia cutis. Bone Marrow Transplant. 1997;20:107–112. [DOI] [PubMed] [Google Scholar]
- 7.Webb DK, Harrison G, Stevens RF, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98:1714–1720. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury T, Brady HJM. Insights from clinical studies into the role of the MLL gene in infant and childhood leukemia. Blood Cells Mol Dis. 2008;40:192–199. [DOI] [PubMed] [Google Scholar]
- 9.Lapillonne H, Renneville A, Auvrignon A, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24:1507–1515. [DOI] [PubMed] [Google Scholar]
- 10.Balgobind BV, Raimondi SC, Harbott J, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114:2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]


