Abstract
Supplemental Digital Content is available in the text
Hereditary xerocytosis (HX) is an autosomal dominant hemolytic anemia characterized by red blood cells (RBC) with elevated mean corpuscular hemoglobin concentration (MCHC), osmotic resistance and occasional stomatocytes, and variably associated with systemic iron overload, pseudohyperkalemia, and transient neonatal edema.1 HX is caused by missense mutations in mechanosensitive cation channel gene PIEZO1 or Ca2+-gated K+ channel (Gardos channel) gene KCNN4,2 increasing red cell rigidity and susceptibility to hemolysis.1–3
We present here the molecular basis for sporadic HX in a Brazilian female with hemolytic anemia (Proband II:1 in pedigree of Fig. S1, Supplemental Digital Content) characterized by macrocytic stomatocytosis (Fig. S2, Supplemental Digital Content), resistance to osmotic lysis, and hyperferritinemia in the absence of HFE mutations.4 Following neonatal ascites and transfusion-dependence since childhood, the proband experienced jaundice and underwent splenectomy and cholecystectomy without known subsequent thrombotic events. Proband's liver biopsy indicated hepatosiderosis, and she later presented with cardiac failure secondary to parenchymal iron deposition responsive to chelation therapy.4
We have found Proband II.1 to be heterozygous for the (then novel) PIEZO1 exon 14 mutation c.1792G>A encoding the amino acid substitution V598M. The mutation affects an evolutionarily conserved residue (Fig. S2, Supplemental Digital Content), and is predicted to be damaging (PolyPhen-2 score 1.000). None of the proband's unaffected parents and siblings shared the V598M mutation (Figs. S1–S3, Supplemental Digital Content). The proband had no missense variants in cDNA sequences of the only other known HX gene, KCNN4, or in the familial pseudohyperkalemia gene, ABCB6.
The V598M mutation in PIEZO1 was also unusual insofar as previously reported HX-associated PIEZO1 mutations resided in the C-terminal third of the PIEZO1 polypeptide. Here, we extend the hematological and functional phenotypes of the PIEZO1 V598M mutant polypeptide. While this work was being completed, Rapetti-Mauss et al5 reported an unrelated French HX patient of similar hematological phenotype with the same PIEZO1 mutation.
At age 35, proband's hemoglobin was 9.9 g/dL, hematocrit 32.8%, and reticulocytes 14.8% with MCV 115 fL and RDW 18.1% (values more abnormal than observed in many HX patients with PIEZO1 mutations), but MCHC and CHCM were normal, and dense cells (>41 g/dL) were rare (Table S1, Supplemental Digital Content), despite moderate stomatocytosis (Fig. S3, Supplemental Digital Content). Ektacytometry in this splenectomized patient revealed reduced elongation index upon increasing shear stress (Fig. S4A, Supplemental Digital Content) and reduced isotonic and hypertonic deformability (Fig. S4B, Supplemental Digital Content).
Proband II:1 RBC exhibited higher total unstimulated K+ influx (Fig. 1A, P < 0.05) and senicapoc-sensitive unstimulated K+ influx (Fig. 1B, P < 0.01) than observed in RBC from the proband's unaffected (wild-type [WT]) mother (I:1). These results were consistent with the <2-fold-increased higher senicapoc-sensitive whole cell current from RBC of the French HX patient with the same mutation,5 as well as with properties of other HX-associated mutations in the C-terminal third of the PIEZO1 polypeptide.1 Treatment of PIEZO1 V598 RBC with the PIEZO1 agonist YODA-16 produced larger acute decreases in proband MCV within 2 minutes after exposure (Fig. 1E; 10 fL) than in unaffected maternal MCV (Fig. 1C; 4 fL). The same YODA-1 exposure increased CHCM in proband RBC by ∼4 g/dL within 2 minutes (Fig. 1F), but only ∼2.5 g/dL in unaffected maternal RBC (Fig. 1D).
Figure 1.
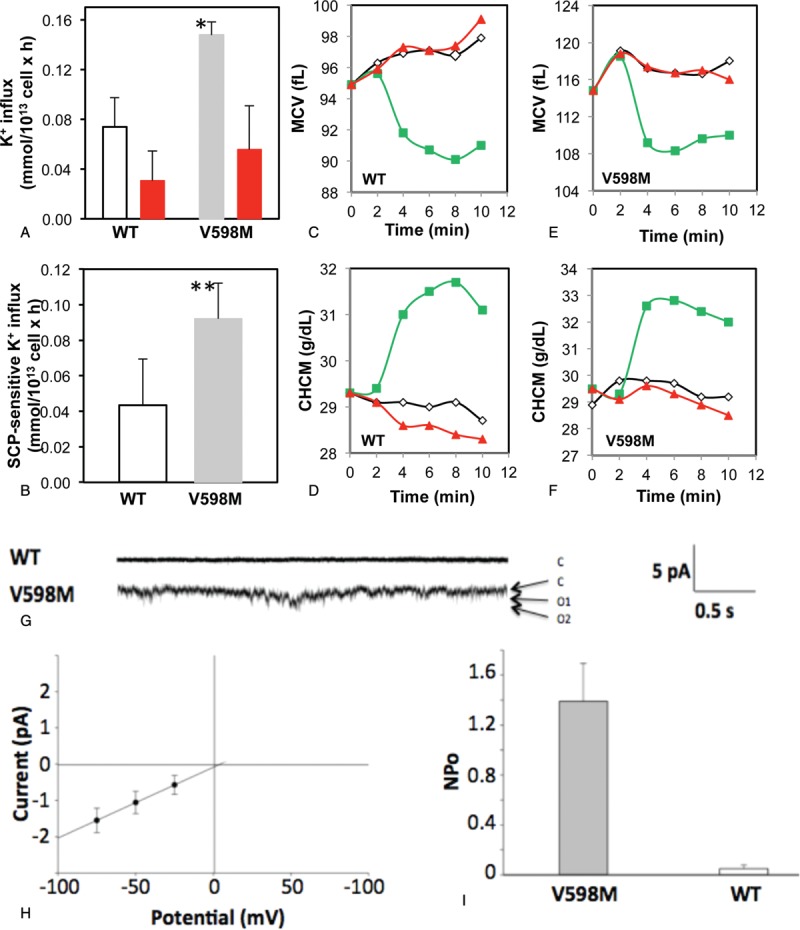
Functional properties of PIEZO1 V598M erythrocytes. (A–B) K+ influx into wild-type (WT) and heterozygous mutant PIEZO1 V598M RBC was measured as baseline unidirectional 86Rb+ influx at 37°C in the presence of ouabain (0.1 mM) and bumetanide (1 mM), and in the absence (white bars, WT; gray bars, V598M) or presence (red bars) of 200 nM senicapoc (SCP). Rates were calculated from 86Rb+ uptakes measured at 2 and 10 minutes after initiation of influx. The senicapoc-sensitive fraction of K+ influx (B) represents KCNN4 activity, and was elevated in V598M RBC. Values in panels A and B are means from 2 triplicate experiments (∗P < 0.05; ∗∗P < 0.01 comparing V598M vs WT). (C–F) ADVIA120 hemoanalyzer profiles showing YODA-1 effects on RBC mean cell volume (MCV; C, E) and mean corpuscular hemoglobin concentration (MCHC; D, F). Freshly isolated and washed RBC (WT [C, D] or heterozygous PIEZO1 V598M [E, F]) were resuspended at 10% cytocrit in normal saline containing 1.5 mM CaCl2, 10 mM glucose, 0.1 mM ouabain, and 1 mM bumetanide and incubated at room temperature in the presence of 15 μM YODA-1 in the absence (green squares) or presence of 200 nM senicapoc (red triangles). Control red cells of both genotypes were exposed to neither YODA-1 nor senicapoc (black diamonds). Results in panels C–F are single experiments, each representative of 2 with similar results. (G-I) Single channel characteristics of cell-attached patches on RBC of the affected proband II:1 (V598M) and of an unaffected family member (WT). Symmetric bath and pipette solutions included (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 mM Na HEPES, pH 7.4.1 (G) Representative cell-attached RBC patch traces (2.5 seconds) in unstimulated conditions show low-channel activity in a patch from an unaffected family member (WT, upper trace), in contrast to the high spontaneous channel activity in a patch from the HX proband II:1 heterozygous for PIEZO1 V598M (lower trace). −Vp = −50 mV. (H) Single channel current-voltage relation of a representative cell-attached patch on an HX proband II:1 red cell heterozygous for PIEZO1 V598M. Single channel slope conductance was 19.6 pS with extrapolated reversal potential at +1 mV, r2 = 0.99. (I) NPo (measured from 10 seconds sweeps) was 1.39 ± 0.30 in cell-attached patches on 3 RBC from HX proband II:1, in contrast to an NPo of 0.05 ± 0.03 in patches on RBC from an unaffected family member (P = 0.02). Single channel recordings from RBC were performed as previously1 with seal resistances of >5 Gigohms. Blood samples used in these studies were shipped on coldpack from Sao Paulo and arrived in Boston on the 2nd day post-venisection. Cells were immediately washed and used for experiments. Washed cells were also stored in choline storage solution until additional experimental use on the 3rd and 4th day post-venisection. RBC = red blood cells.
The increased PIEZO1 activity evidenced in measurements of isotopic influx and hematological indices was reflected in experiments with cell-attached patch recording of ion channel activity. Whereas maternal WT red cells revealed minimal basal activity in cell-attached patches, proband II:1 red cells (V598M) exhibited increased ion channel activity (Fig. 1G) with single channel conductance of 19.6 pS (Fig. 1H), and patch NPo of 1.39 ± 0.30 compared with NPo of 0.05 ± 0.03 in unaffected (WT) maternal RBC (Fig. 1I; n = 3, P = 0.02), similar to HX RBC from patients with C-terminal PIEZO1 mutations.
Cell-attached patch recordings from HEK-293 cells previously transfected with PIEZO1 V598M cDNA (Fig. 2) revealed single channel openings in response to brief negative pressure pulses of 20 mm Hg (Fig. 2A). However, short-lived channel openings were also evident between pressure pulses during periods when the patch was experiencing only intrinsic stress (gray-boxed portions of Fig. 2A trace). The amplitude histogram revealed a predominant unitary (single channel) current of 2.5 pA (Fig. 2C, left panel). Figure 2B presents the variable responses of a single, long-lived, cell-attached patch to 3 consecutive negative pressure pulses of 40 mm Hg, each of 50 seconds duration. The traces show multiple channel openings and quiescent periods of varying duration. The amplitude histogram for this patch (Fig. 2C, right panel) shows a predominant current amplitude of 3.06 pA, with an additional, less frequently populated subconductance state of 1 to 1.5 pA.
Figure 2.
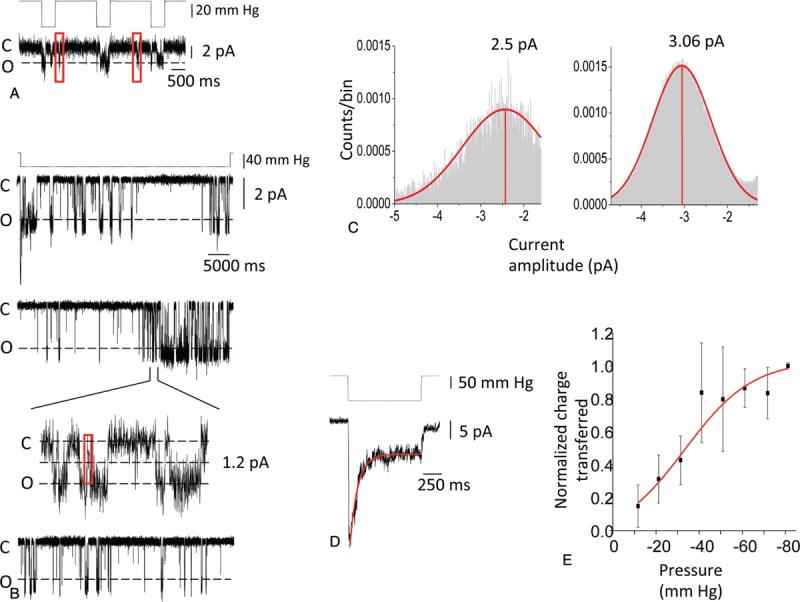
Single channel properties of PIEZO1 V598M transiently expressed in HEK-293 cells. (A) Representative cell-attached patch from a cell transiently transfected 24 hours previously with cDNA encoding human PIEZO1 V598M. Channels opened predominantly during the pressure pulse and occasionally during the interpulse intervals (red boxes) with only residual intrinsic stress remaining in the patch. (B) Responses of the same cell-attached patch to 3 subsequent pulses of prolonged stimulation (50 seconds). The time-resolved inset for pulse 2 shows a subconductance level of 1 to 1.5 pA (red box). Holding potential in A and B was −60 mV. (C) Left, amplitude histogram with predominant amplitude at 2.5 pA, corresponding to trace of panel A; right, amplitude histogram with predominant amplitude of 3.06 pA, corresponding to traces of panel B. (D) Representative cell-attached patch at holding potential of −60 mV subjected to −50 mm Hg pressure step of 1 second duration, showing rapid activation of multiple channels with slow, partial inactivation to a lower steady-state activity level. Panels A–D are each representative of similar recordings from 3 cells. (E) Relationship between applied negative pressure (conditions as in panel D) and normalized magnitude of charge transferred during the entire pressure pulse, as fit with the Boltzmann equation for 3 cells. For all panels, bath (in mM) was 150 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4; pipette solution (in mM) was 150 KCl, 10 HEPES, pH 7.4. Additional single channel recording conditions were as described.10.
The cell-attached patch of Figure 2D shows application of a 1 second duration negative pressure pulse of 50 mm Hg leading to rapid activation of multiple channels, followed by rapid mono-exponential decay to a sustained plateau current of ∼25% of peak current value. Cessation of the pressure pulse triggers further rapid inactivation to a nonzero baseline current. In Figure 2E, recombinant PIEZO1 V598M polypeptide expressed in HEK-293 cells was subjected in cell-attached patches to repeated negative pressure pulses of increasing magnitude, each of 1 second duration. PIEZO1 V598M exhibited a P50 of ∼32 mm Hg for pressure-activation of transmembrane charge transfer. This value is left-shifted compared to reported P50 values of WT PIEZO1 ranging between 38 and 45 mm Hg,7,8 but comparable to the left-shifted P50 values of WT PIEZO1 in cell-attached patches from cytoskeleton-depleted plasmalemmal blebs and from plasma membrane of cells treated with the nonmuscle myosin inhibitor, blebbistatin.9 This increased sensitivity to the incremental stretch imposed by hydrostatic pressure on the prestressed membrane patch is consistent with increased PIEZO1 activity associated with the HX mutant V598M.
Thus, we have demonstrated in HX RBC from a patient heterozygous for PIEZO1 mutant V598M increased senicapoc-sensitive 86Rb+ fluxes and increased currents recorded in cell-attached patch mode. These data extend a previous demonstration of increased whole cell K+ currents in RBC from a patient heterozygous for the same mutation.5 Our report further suggests (Fig. 1C-F) enhanced YODA-1 responsiveness of PIEZO1 V598M RBC versus control RBC. We have also documented in PIEZO1 V598M the first evidence for spontaneous single channel openings without imposed pressure, for the existence of a substate conductance, and for enhanced responsiveness to negative hydrostatic pressure within the patch membrane (membrane stretch), comparable to that of WT PIEZO1 recorded in osmotically generated plasmalemmal blebs or in patches pharmacologically depleted of cytoskeleton.9 These properties are consistent with the postulated location of the V598M missense substitution within the “blade” of the trimeric PIEZO1, believed to contribute to the channel's membrane tension sensor function.
Our report confirms that mutations throughout the PIEZO1 polypeptide can cause HX, and suggests that the HX mutation V598M may lower the channel activation threshold to the mechanical perturbation of increased hydrostatic pressure applied to patch membranes in cell-attached mode. This increased sensitivity of mechanically stimulated PIEZO1 in RBC is accompanied by increased downstream activity of the volume-regulatory K+ channel, KCNN4.
Supplementary Material
Footnotes
Citation: Gnanasambandam R, Rivera A, Vandorpe DH, Shmukler BE, Brugnara C, Snyder LM, Andolfo I, Iolascon A, Silveira PA, Hamerschlak N, Gottlieb P, Alper SL. Increased Red Cell KCNN4 Activity in Sporadic Hereditary Xerocytosis Associated With Enhanced Single Channel Pressure Sensitivity of PIEZO1 Mutant V598M. HemaSphere, 2018;00:00 http://dx.doi.org/10.1097/HS9.0000000000000055.
Funding/support: SLA, PG, and NH designed the study. NH contributed essential patient-derived samples. RG, AR, DHV, PAS and BES performed the research and analyzed the data. SLA, RG, AR, DHV, and PG wrote the paper. All coauthors edited the paper. SLA was supported by research funds from the Doris Duke Charitable Foundation and QUEST Diagnostics. LMS was an employee of QUEST Diagnostics. Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article. PG was supported by NIH R01HL054887 to Dr Frederick Sacks.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
RG and AR have equally contributed as first authors.
PG and SLA have equally contributed as senior authors.
Supplemental Digital Content is available for this article.
References
- 1.Andolfo I, Alper SL, De Franceschi L, et al. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 2013; 121:3925–3935. [DOI] [PubMed] [Google Scholar]
- 2.Andolfo I, Russo R, Manna F, et al. Novel Gardos channel mutations linked to dehydrated hereditary stomatocytosis (xerocytosis). Am J Hematol 2015; 90:921–926. [DOI] [PubMed] [Google Scholar]
- 3.Cahalan SM, Lukacs V, Ranade SS, et al. Piezo1 links mechanical forces to red blood cell volume. eLife 2015; 4:e07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assis RA, Kassab C, Seguro FS, et al. Iron overload in a teenager with xerocytosis: the importance of nuclear magnetic resonance imaging. Einstein 2013; 11:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapetti-Mauss R, Picard V, Guitton C, et al. Red blood cell Gardos channel (KCNN4): the essential determinant of erythrocyte dehydration in Hereditary Xerocytosis. Haematologica 2017; 102:e415–e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syeda R, Xu J, Dubin AE, et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015; 4:e07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae C, Gnanasambandam R, Nicolai C, et al. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci USA 2013; 110:E1162–E1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae C, Gottlieb PA, Sachs F. Human PIEZO1: removing inactivation. Biophys J 2013; 105:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox CD, Bae C, Ziegler L, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 2016; 7:10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnanasambandam R, Bae C, Gottlieb PA, et al. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS ONE 2015; 10:e0125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


