INTRODUCTION:
Whether non-alcoholic fatty liver disease (NAFLD) is associated with cardiovascular risk has still been controversial. The reasons for this disparity may be associated with subject selection, events definition, diagnostic criteria of NAFLD, or research methods. The aim of this study was to determine the relationship of NAFLD to cardiovascular disease (CVD) outcomes in patients with stable, new-onset coronary artery disease (CAD).
METHODS:
A matched case–control study based on the cohort with stable, new-onset CAD was implemented in 162 cases (patients who developed all-cause death, non-fatal myocardial infarction and stroke during an average of 11,484 patient-years of follow-up) and 162 controls without cardiovascular events matched with the same sex, the age difference ≤3 years old, and the admission date within 3 months. Abdominal ultrasonography and coronary angiography were performed at admission. COX proportional hazard models and conditional logistic regression analysis were used to assess the effect of NAFLD on CVD outcomes.
RESULTS:
NAFLD was more common in the event group than in the control group (P = 0.012). Kaplan-Meier analysis showed a significant association between NAFLD and CVD outcomes (P = 0.007). Moreover, Cox regression (hazard ratios 1.56; 95% confidence interval, 1.04–2.34, P = 0.031) and conditional logistic regression (odds ratio 2.72, 95% confidence interval, 1.16–6.39, P = 0.022) analyses further demonstrated that NAFLD was an independent risk factor for CVD outcomes.
Conclusions:
NAFLD is indeed an independent predictor of CVD outcomes in patients with stable, new-onset CAD. Further randomized controlled trials may be needed to confirm our findings.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease (CVD) are 2 diseases that are common in the general population. Additionally, NAFLD is the most common cause of chronic liver disease. It has been recognized as the hepatic manifestation of obesity and the metabolic syndrome (MS) (1) and also a marker of pathological ectopic fat accumulation combined with a low-grade chronic inflammatory state. This results in several deleterious pathophysiological processes, including fatty acid and lipoprotein metabolism, abnormal glucose, increased oxidative stress, hypercoagulability, endothelial dysfunction, and accelerated progression of atherosclerosis (2–5). Moreover, recent data suggest that NAFLD is linked to increased CVD risk, and accumulating evidence demonstrates that the clinical burden of NAFLD is not limited to liver-related morbidity and mortality, with the majority of deaths attributed to CVDs (3,6). Furthermore, it has been reported that NAFLD per se is a true risk factor for CVD, independently of classical known risk factors (3). As a result, the role of NAFLD as a potential independent CVD risk factor has gained considerable prominence and stimulated growing interest.
However, when it comes to the relationship of NAFLD to cardiovascular outcomes, the conclusions from existing studies were discordant (5,7–11). Thus, there is a hot debate on the association between NAFLD with cardiovascular outcomes nowadays. The discrepancy of previous study results may be ascribed to the different subject selection, diagnosis criteria, study strategies, and events definition. What's more, few studies were found to explore the impact of NAFLD on clinical outcomes in patients with established coronary artery disease (CAD) (5,9). In addition, to our best knowledge, there have been no studies investigating the predicting role of NAFLD for cardiovascular events in angiography-proven, stable and new-onset CAD patients or taking all-cause deaths, non-fatal myocardial infarction (MI) and stroke as the composite endpoint events in their analysis. Thus, we undertook this matched case–control study to examine the relationship of NAFLD to cardiovascular outcomes using a large Chinese cohort.
METHODS
Study design and population
From March 2011 to July 2016, 7,164 consecutive Chinese patients who received coronary angiography because of angina-like chest pain and/or positive treadmill exercise test and/or significant stenosis indicated by coronary computed tomography angiography were considered for this analysis. On admission, 43 patients declined to participate. Based on elevated myocardial enzyme levels (cardiac troponin I, creatine kinase, and creatine kinase isoenzyme), typical electrocardiogram changes, and positive findings by coronary angiography, 787 non-CAD patients and 1937 CAD patients who had acute coronary syndrome (ACS) or a history of MI, percutaneous coronary intervention or coronary artery bypass grafting were excluded. Seven hundred twenty-one patients were rejected according to the exclusion criteria as follows: patients without abdominal ultrasound examination or with positive hepatitis B surface antigen; antibody against hepatitis C virus; autoimmune hepatitis; hereditary liver disease (e.g., hereditary hemochromatosis or Wilson's disease); excessive alcohol consumption (ongoing or recent alcohol consumption >21 standard drinks on average per week in men and >14 standard drinks on average per week in women) (12); secondary causes of fatty liver (e.g., chronic use of systemic corticosteroids or methotrexate) or drug-induced liver disease. Furthermore, 24 patients were lost to follow-up during the study. Thus, the resulting population consisted of 3,623 subjects with stable, new-onset CAD. After enrollment, optimal medical treatment (aspirin, clopidogrel, statins, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, or calcium channel blockers), percutaneous coronary intervention, and coronary artery bypass grafting were provided as clinically indicated. Over an average of 11,484 patient-years of follow-up (median 3.2 years; interquartile range 2.1–4.1 years), a total of 162 patients developed hard cardiovascular events. Then, from the remaining pool of patients (n = 3,461), we randomly selected control subjects at a 1:1 ratio matched with the same sex, the age difference ≤3 years old, and the admission date within 3 months (Figure 1). Thus, there were 162 cases and 162 controls entering the final analysis.
Figure 1.
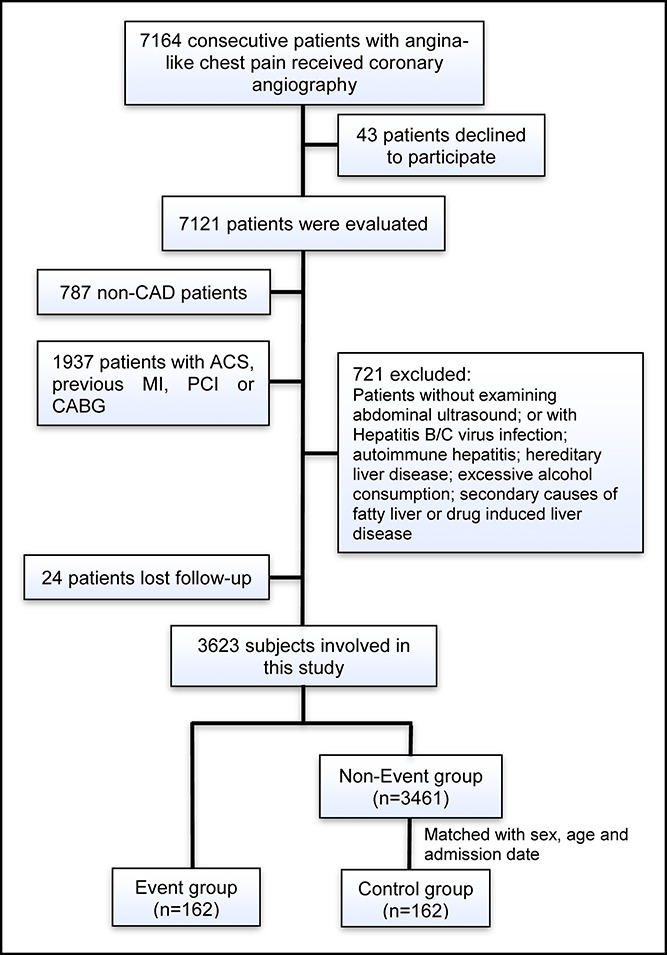
Flowchart illustrating study population. ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; PCI, percutaneous coronary intervention.
The study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (Fu Wai Hospital and National Center for Cardiovascular Diseases, Beijing, China). Each participant provided written, informed consent before enrollment.
Measurements and biochemical analysis
At baseline, during a personal interview, information on demographic factors, medical history, medication use, and personal health habits was collected from each subject. Anthropometric measurements were performed and blood pressure (BP) was measured. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Hypertension was defined as repeated BP measurements ≥140/90 mm Hg or self-reported hypertension and currently taking anti-hypertensive drugs. Diabetes mellitus (DM) was defined as a fasting plasma glucose level ≥7.0 mmol/L in multiple determinations or random plasma glucose ≥11.0 mmol/L or the 2-hour plasma glucose of the oral glucose tolerance test ≥11.0 mmol/L or using hypoglycemic medications currently. MS was diagnosed according to the Chinese Diabetes Society's advice in 2004 (13). Based on these criteria, patients were defined with MS when they had any three or all of the following items: (i) BMI ≥ 25.0 kg/m2; (ii) fasting plasma glucose level ≥6.1 mmol/L or 2-hour plasma glucose of the oral glucose tolerance test ≥7.8 mmol/L or being diagnosed with DM and under hypoglycemic medications treatment currently; (iii) BP ≥ 140/90 mm Hg or being diagnosed with hypertension and currently taking anti-hypertensive drugs; (iv) fasting plasma triglyceride ≥1.7 mmol/L or high-density lipoprotein cholesterol <0.9 mmol/L in men or <1.0 mmol/L in women. The baseline angiographic data were collected from catheter laboratory records by 3 interventional cardiologists, and the coronary severity was evaluated by calculating Gensini score (14). This score was computed by assigning a severity score to each coronary lesion according to the degree of luminal narrowing and the importance of location, and the total score equaled the sum of the severity score times the location score for all diseased segments (14).
Blood samples were collected into ethylenediaminetetraacetic acid-containing tubes from each patient after at least 12-hour fasting in the morning. Liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP) as well as lipid profiles total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, etc., were measured using an automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan) and enzymatic assay. The other related biomarkers were analyzed by standard commercial kits.
Assessment of fatty liver by ultrasonography
Abdominal ultrasonography was performed by 2 experienced sonographers who were unaware of the participants' clinical and laboratory characteristics at the time of the procedure using the Philips Ultrasound Machine (EPIQ7; Philips, Bothell, WA). The diagnosis of the fatty liver required ultrasonographic features of diffusely increased liver echogenicity greater than that of the kidney or spleen, vascular blurring, and deep attenuation of the ultrasound signal (15).
Follow-up
After the initial appointment, all patients were actively followed up at 6-month intervals after hospital discharge by well-trained nurses or cardiologists who were blinded to the aim of this study. Follow-up information was obtained from telephone communications and/or face-to-face interviews. The cardiovascular events were defined as all-cause death (death mainly caused by CVDs), non-fatal MI, and stroke. The follow-up time interval (months) was counted from the enrollment till the last traceable hospital inpatient or outpatient record or telephone interview before February 28, 2018. All available relevant data from any reported possible event were collected. Death of a participant was reported by the relatives, general practitioner, or specialist who treated the participant. Three experienced cardiologists who were masked to any of the study data classified the events independently.
Statistical analysis
Continuous variables are expressed as mean ± s.d. or median with interquartile range as appropriate, and differences between groups were determined using the Student's t test, analysis of variance, or nonparametric test. Categorical variables were presented as number (percentage) and analyzed by χ2 statistic test or Fisher's exact test. The cumulative event-free survival rates of patients with and without NAFLD were estimated by the Kaplan-Meier method and compared by the log-rank test. However, Kaplan-Meier curve is not a reliable test outside of randomized, evenly matched groups due to the potential influence of confounding variables and therefore cannot be reliably interpreted for survival in unevenly matched groups. Therefore, Cox proportional hazard models were further performed to calculate hazard ratios (HRs) for cardiovascular events of the presence of NAFLD and also each 10 U/L increase in liver enzymes. The analyses were initially performed adjusting for age and sex in model 1; further adjustments were subsequently made for hypertension, DM, Gensini score, left ventricular ejection fraction, creatinine, and high-sensitivity C-reactive protein in model 2, and all these risk factors plus statin use in model 3. In addition, we adjusted for age, sex, MS, Gensini score, left ventricular ejection fraction, creatinine, and high-sensitivity C-reactive protein in Cox hazard model 4. Conditional logistic regression analysis was used to further assess the impact of NAFLD and liver enzymes on 3-year cardiovascular events risk in patients who completed 3-year follow-up, adjusting for the same confounding factors with model 3 and model 4. The statistical analysis was performed with SPSS version 22.0 software (SPSS, Chicago, IL). For all analyses, 2-tailed P values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics
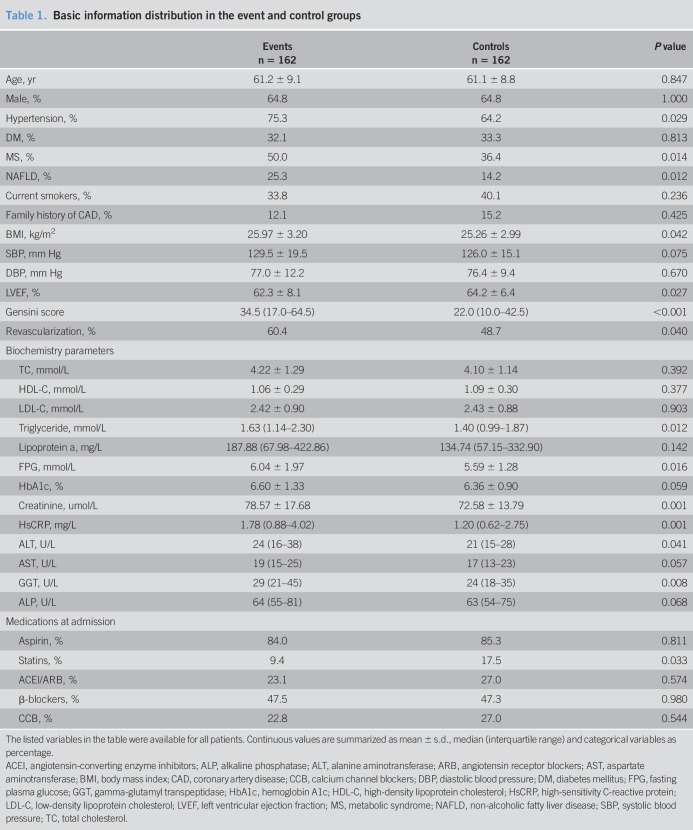
The demographics and clinical characteristics of the event and control groups are described in Table 1. The average age of the whole population was 61.2 years old and 64.8% (n = 210) of them were male. Clearly, patients in the event group had higher percentages of hypertension, MS, NAFLD, and revascularizations (all P < 0.05). Meanwhile, the event group had higher levels of BMI, Gensini score, triglyceride, fasting plasma glucose, creatinine and high-sensitivity C-reactive protein, and lower levels of left ventricular ejection fraction (P < 0.05, respectively). In addition, liver enzyme ALT (P = 0.041) and GGT (P = 0.008) levels were significantly higher in the event group compared with the control group, while the difference in AST and ALP levels between these two groups had no statistical significance (both P > 0.05). Furthermore, with respect to the prescribed medications at baseline, the event group had a much lower percentage of statin use compared with the control group (P = 0.033), while the use of the other drugs was similar between the two groups (all P > 0.05). Because of the initial diagnosis of CAD in our subjects, there was an extremely low percentage of them treated with statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
Table 1.
Basic information distribution in the event and control groups
NAFLD, liver enzymes, and cardiovascular outcomes
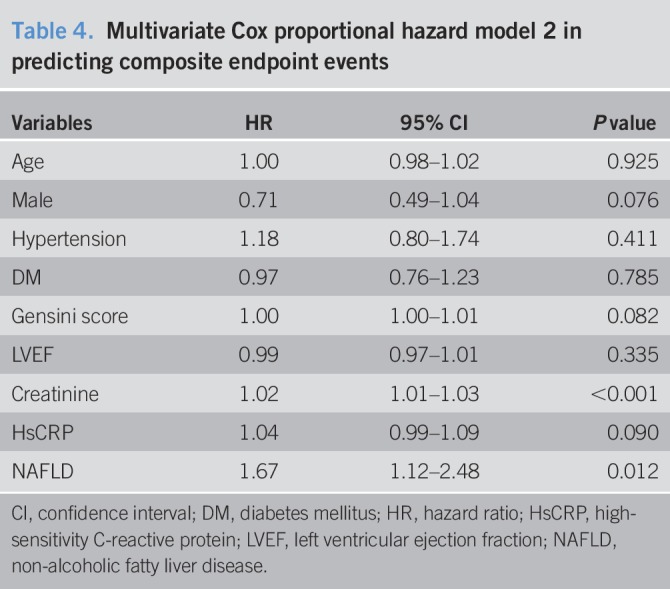
Over an average of 11,484 patient-years of follow-up, 162 hard endpoint events were recorded, including 44 (27.16%) deaths, 39 (24.07%) non-fatal MIs, and 79 (48.77%) strokes. Among all mortality events, 30 (68.18%) patients died of CVD, 11 (25.00%) patients died of cancer, and 3 (6.82%) patients died of accident. Meanwhile, there were 10 deaths, 11 non-fatal MIs, and 20 strokes among patients with NAFLD, while 34 deaths, 28 non-fatal MIs, and 59 strokes among patients without NAFLD. The Kaplan-Meier analysis demonstrated that there was a significant difference in the event-free survival rates between patients with and without NAFLD (P = 0.007, Figure 2.). As shown in Table 2, a baseline NAFLD had a 1.66 × higher risk of the occurrence of cardiovascular events compared with the non-NAFLD group in the univariate Cox proportional hazards regression analysis. Additional adjustments for multiple variables in 3 models did not change this association (all P < 0.05; Tables 3–5), and NAFLD was still significantly and independently associated with the risk of cardiovascular events (HRs, 1.56; 95% confidence interval (CI), 1.04–2.34); Table 5). Moreover, after adjusting for MS and other potential confounding factors, the predicting role of NAFLD in cardiovascular outcomes remained unchanged (HR, 1.62; 95% CI, 1.09–2.39; Table 6). Among liver enzymes ALT, AST, GGT, and ALP, only ALT had a significant and independent association with cardiovascular outcomes, with an adjusted HR of 1.09 (95% CI, 1.02–1.17) for each 10 U/L increase in it. The following conditional logistic regression analysis further demonstrated the association of NAFLD and each 10 U/L increase in ALT with 3-year risk of cardiovascular events (137 events and 137 controls) after adjusting for the same confounding factors with model 3 (NAFLD: odds ratio (OR), 2.72; 95% CI, 1.16–6.39; ALT: OR, 1.19; 95% CI, 1.01–1.40) and model 4 (NAFLD: OR, 2.23; 95% CI, 1.15–4.34; ALT: OR, 1.13; 95% CI, 1.00–1.27). Moreover, a subanalysis exploring the association of NAFLD (HR, 1.56; 95% CI, 1.02–2.38) and ALT (HR, 1.08; 95% CI, 1.01–1.15; for each 10 U/L increase of ALT) with composite endpoint events inclusive of specific cardiovascular death, non-fatal MI, and stroke showed similar results with the above analysis of primary endpoint events after adjusting for the confounding factors.
Figure 2.
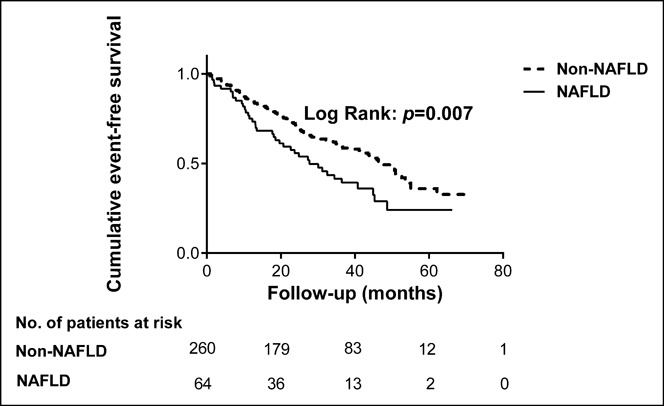
The cumulative event-free survival analysis according to the presence or absence of NAFLD. NAFLD, non-alcoholic fatty liver disease.
Table 2.
Univariate Cox proportional hazards regression analysis of the composite endpoint events
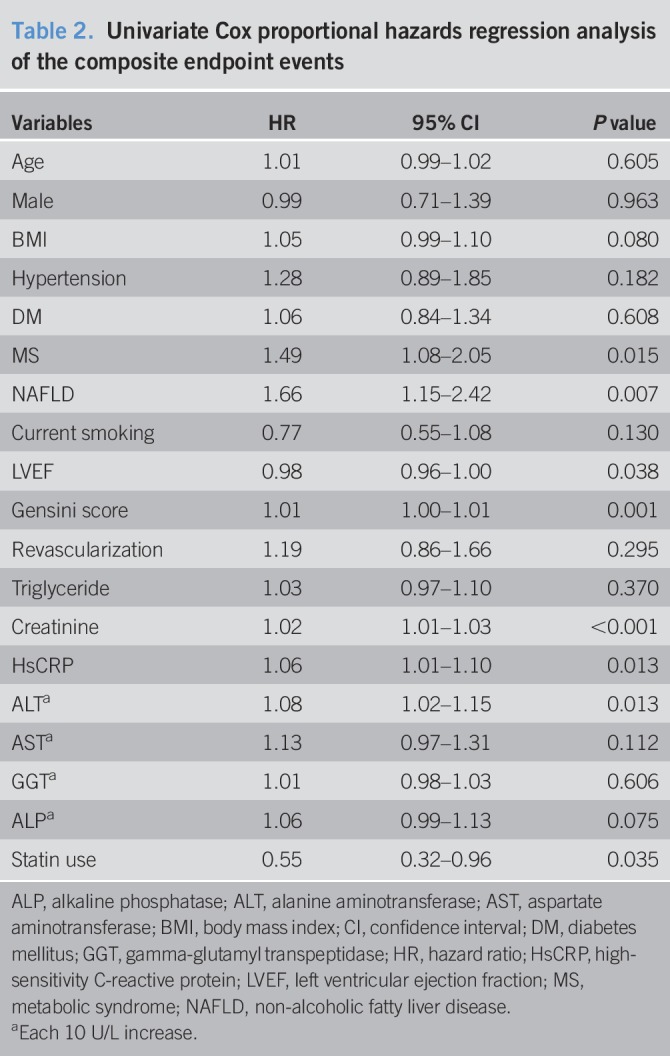
Table 3.
Multivariate Cox proportional hazard model 1 in predicting composite endpoint events
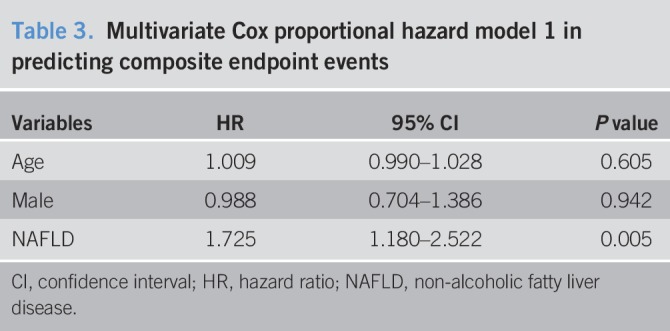
Table 5.
Multivariate Cox proportional hazard model 3 in predicting composite endpoint events
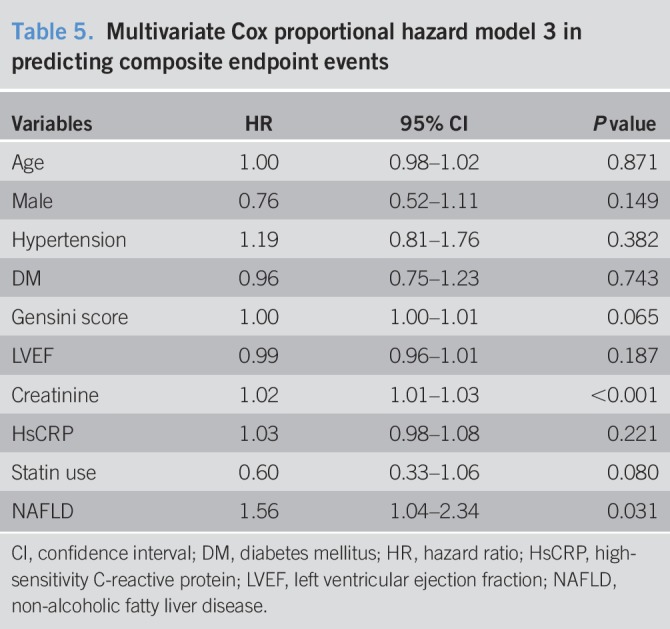
Table 6.
Multivariate Cox proportional hazard model 4 in predicting composite endpoint events
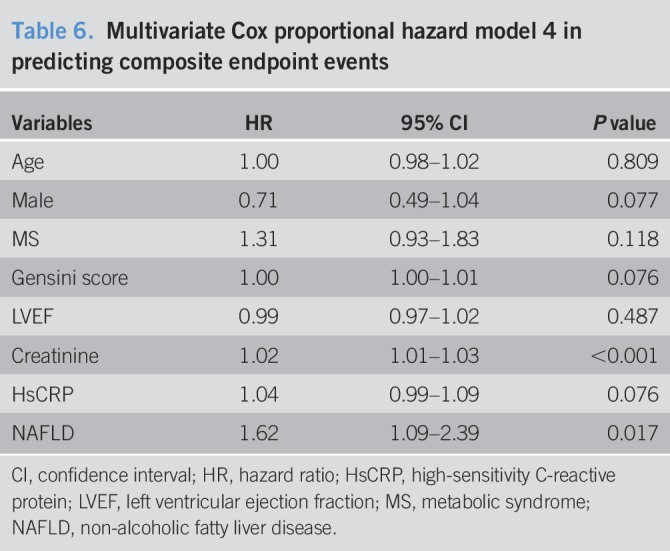
Table 4.
Multivariate Cox proportional hazard model 2 in predicting composite endpoint events
DISCUSSION
In this matched case–control study on new-onset CAD patients undergoing coronary angiography, we found that NAFLD was associated with cardiovascular outcomes independently of other demographic and metabolic factors and that NAFLD-induced increase in ALT also had a significant association with cardiovascular prognosis. These results may provide additional and novel information for the association of NAFLD with clinical prognosis in patients with stable, new-onset CAD.
NAFLD is a common liver disease, affecting as high as a third of the population worldwide, and may confer increased cardiometabolic risk with consequent adverse cardiovascular outcomes independent of traditional CVD risk factors and the MS. It is usually characterized by insulin resistance and is strongly associated with obesity and type 2 DM (3) and has been regarded as the liver manifestation of MS, a highly atherogenic condition (1). Since NAFLD and MS are closely related, previous studies have suggested that patients with NAFLD have an increased CVD risk. There is a growing body of epidemiological and experimental evidence suggesting that NAFLD predisposes to atherogenic dyslipidemia, deteriorates hepatic or peripheral insulin resistance, and releases a variety of proinflammatory, procoagulant, thrombogenic factors that may promote the development of CVD, type 2 DM, and so on (11). In fact, Choi et al. (16) indicated that NAFLD is associated with an elevated 10-year risk of developing CAD as estimated using Framingham risk score and independently related to the risk of developing CAD, regardless of classical risk factors and other components of MS. Moreover, NAFLD is also demonstrated to be associated with coronary artery calcification, endothelial dysfunction, and coronary stenosis (2,9,17,18).
However, studies on the association between NAFLD and cardiovascular outcomes have not reached a consensus conclusion yet. The discordance of these study results may be explained by the differences in their subject selection, events definition, diagnostic criteria of NAFLD, or research methods. In the general population, Fracanzani et al. (4) indicated that NAFLD was independently related to the cardiovascular events including ACS, revascularization, and stroke. Hamaguchi et al. (19) reported that NAFLD was a strong predictor of CVD outcomes and might play a central role in the cardiovascular risk of MS. Treeprasertsuk et al. (20) demonstrated that NAFLD was associated with a 10-year CVD risk defined by congestive heart failure, ACS, a flow-limiting stenosis from angiography, or angina requiring revascularization. Meanwhile, in a multiethnic study, researchers found that NAFLD could predict all-cause mortality and incident cardiovascular events (MI, resuscitated cardiac arrest, angina, or coronary revascularizations) (21). Nonetheless, there was only a trend towards association between hepatic steatosis and cardiovascular prognosis in Mellinger et al.'s study (10) with the events defined by non-fatal MI, stroke, transient ischemic attack, heart failure, or peripheral arterial disease and no significant relationship between them in Pickhardt et al.'s study (22) with the events diagnosed as MI, cerebrovascular accident, transient ischemic attacks, and coronary bypass grafting or stenting. In type 1 diabetic patients, Mantovani et al.'s (23) study showed that NAFLD is associated with an increased risk of incident cardiovascular events (nonfatal ischemic heart disease, nonfatal ischemic stroke, or coronary or peripheral artery revascularizations). NAFLD can also predict the risk of cardiovascular events including nonfatal MI, revascularizations, ischemic stroke, and cardiovascular deaths in type 2 diabetic adults (24). At the same time, Perera et al. (25) suggested that patients with NAFLD had a higher predicted mortality from acute MI (AMI), and Keskin et al. (26) demonstrated that NAFLD revealed a higher incidence of major adverse cardiac events consisting of all-cause death, non-fatal AMI, and/or target lesion revascularization in patients with ST-segment elevation MI. Moreover, in patients with chronic heart failure, NAFLD fibrosis score was independently associated with cardiovascular events including cardiovascular deaths, MI, stroke, sudden cardiac death, and rehospitalization due to worsening heart failure (27). However, Karajamaki et al. (28) found that NAFLD with MS implied a considerable risk of cardiovascular events (coronary artery revascularizations, cardiovascular death, and stroke), whereas NAFLD without MS did not. Moreover, in patients with angiography-proven CAD, Wong et al. (5,9) reported that NAFLD could not predict cardiovascular mortality, ACS/non-fatal MI, secondary coronary interventions, and hospitalization for congestive failure. Excepting a handful of researches diagnosing NAFLD with histology, most of the previous studies defined NAFLD through abdominal ultrasound. Meanwhile, there were also some studies using abdominal computed tomography (10,20–22) or liver enzymes (29–31) to diagnose NAFLD. Different diagnostic methods of NAFLD may also influence the study results. Moreover, throughout the studies on the association between NAFLD and cardiovascular outcomes, most of them were prospective, but there were still some retrospective ones (7,23), especially the studies conducted 10 years ago. The difference in research methods may contribute to the disparity of existing studies as well. Based on these situations and similar to most studies, we observed a significant association between NAFLD and cardiovascular events defined by all-cause death/cardiovascular death, non-fatal MI, and stroke independently of other metabolic factors including MS. It is noteworthy that differing from Karajamaki et al. ' study (28), we did not find that MS could interfere with the association between NAFLD and cardiovascular outcomes. Nevertheless, MS was defined using the criteria of Chinese Medical Association (13) but not revised National Cholesterol Education Program Adult Treatment panel III (NCEP-ATP III) (32) or International Diabetes Federation (33) in our study, since we did not have data on waist circumference, which may be a minor flaw. But above all, our study population was a cohort of patients with stable, new-onset CAD, which has not been explored before. Thus, the findings of the present study may provide more evidence for the conception that NAFLD is an independent risk factor for cardiovascular outcomes.
In addition, aminotransferase, especially ALT, has been regarded as a representative marker of NAFLD after the exclusion of other liver diseases (34–36). Martin-Rodriguez et al. (35) indicated that serum ALT seems to be a pretty good biomarker of liver fat accumulation and is positively correlated with liver triglyceride quantification. However, many other studies suggested that ALT levels are relatively insensitive markers of NAFLD (11) and that it cannot be used to predict the severity of NAFLD (37, 38). Furthermore, when it comes to the association between ALT and cardiovascular outcomes, the conclusions of related studies have also been discordant (6,31,36,39,40). In the present study, we found that patients with cardiovascular events had higher baseline ALT levels and that ALT was significantly associated with cardiovascular outcomes.
Our study is limited by several facts. First, as inherent to the nature of any prospective and observational study, our findings are subject to confounding factors, and also the level of risk factors at the baseline examination might change during the follow-up. Second, the sample size and follow-up time of this study were relatively small and short. However, a case–control study just does not require that large sample size. Moreover, our study is still continuing in order to better examine the prognostic value of NAFLD in the long-term cardiovascular outcomes in the future. Third, given the restrictions of the cardiovascular specialist hospital, we could not perform liver biopsy, which is considered as the gold standard to diagnose NAFLD. However, we minimized bias by restricting the examination to 2 experienced operators. In addition, we could also evaluate the cardiovascular outcomes professionally and reliably as cardiologists.
In this matched case–control study with a Chinese cohort of stable, new-onset CAD, data suggested that NAFLD was an independent predicator of cardiovascular outcomes and that NAFLD-induced elevation of ALT was also positively associated with cardiovascular prognosis. This result is undoubtedly significant as it provides important clinical implications for screening and surveillance strategies of NAFLD in patients with stable CAD. Thus, for CAD patients with NAFLD, medical management and moderate lifestyle modification with regard to NAFLD may be appropriate and should be recommended to improve long-term clinical prognosis.
CONFLICTS OF INTEREST
Guarantor of the article: All authors have had access to the data and have control of the decision to publish.
Specific author contributions: H.-H.L. designed the study, analyzed the data, and prepared the original draft. Y.-X.C., D.S., and J.-L.J. conducted the study and edited the manuscript. Y.-L.G., N.-Q.W., and C.-G.Z. monitored the study, analyzed the data, and reviewed the manuscript. Y.G. and Q.-T.D. contributed to the data interpretation and discussion of the manuscript. X.Z., S.L., Y.Z., and G.L. collected the data and conducted statistical analysis. J.-J.L. designed and monitored the study and made critical revisions of the manuscript. All authors have approved the final draft submitted.
Financial support: This work was partially supported by the Capital Health Development Fund (grant number 201614035) and CAMS Major Collaborative Innovation Project (grant number 2016-I2M-1-011) awarded to J.-J.L. The funding organizations did not participate in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Potential competing interests: None.
Study Highlights
WHAT IS KNOWN
✓ NAFLD and CVD are common in the general population.
✓ Whether NAFLD is associated with cardiovascular risk has still been controversial.
WHAT IS NEW HERE
✓ This is a strictly matched case–control study.
✓ NAFLD was common in patients with cardiovascular events.
✓ NAFLD could predict clinical prognosis in patients with stable CAD.
TRANSLATIONAL IMPACT
✓ NAFLD may be used for risk stratification and a novel treatment target in CAD patients.
ACKNOWLEDGMENTS
The authors thank the doctors, nurses, and laboratory technicians of the Division of Dyslipidemia for their important contributions to the study and the enrolled patients for their agreement to participant in the studies.
REFERENCES
- 1.Goessling W, Massaro JM, Vasan RS, et al. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008;135:1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42:473–80. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia LS, Curzen NP, Calder PC, et al. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor? Eur Heart J 2012;33:1190–200. [DOI] [PubMed] [Google Scholar]
- 4.Fracanzani AL, Tiraboschi S, Pisano G, et al. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis 2016;246:208–13. [DOI] [PubMed] [Google Scholar]
- 5.Wong VW, Wong GL, Yeung JC, et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology 2016;63:754–63. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Byrne CD. Circulating markers of liver function and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 2015;35:2290–6. [DOI] [PubMed] [Google Scholar]
- 7.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–73. [DOI] [PubMed] [Google Scholar]
- 8.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009;7:234–8. [DOI] [PubMed] [Google Scholar]
- 9.Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 2011;60:1721–7. [DOI] [PubMed] [Google Scholar]
- 10.Mellinger JL, Pencina KM, Massaro JM, et al. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol 2015;63:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 13.Chinese Diabetes Society Metabolic Syndrome Research Group. Recommendations of the Chinese Diabetes Society on metabolic syndrome. Chin J Diabetes 2004;12:156–61. [Google Scholar]
- 14.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [DOI] [PubMed] [Google Scholar]
- 15.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011;54:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SY, Kim D, Kim HJ, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol 2009;104:1953–60. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012;56:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assy N, Djibre A, Farah R, et al. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology 2010;254:393–400. [DOI] [PubMed] [Google Scholar]
- 19.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007;13:1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treeprasertsuk S, Leverage S, Adams LA, et al. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int 2012;32:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeb I, Li D, Budoff MJ, et al. Nonalcoholic fatty liver disease and incident cardiac events: The multi-ethnic study of atherosclerosis. J Am Coll Cardiol 2016;67:1965–6. [DOI] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, Hahn L, Munoz del Rio A, et al. Natural history of hepatic steatosis: Observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol 2014;202:752–8. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Mingolla L, Rigolon R, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol 2016;225:387–91. [DOI] [PubMed] [Google Scholar]
- 24.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007;30:2119–21. [DOI] [PubMed] [Google Scholar]
- 25.Perera N, Indrakumar J, Abeysinghe WV, et al. Nonalcoholic fatty liver disease increases the mortality from acute coronary syndrome: An observational study from Sri Lanka. BMC Cardiovasc Disord 2016;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keskin M, Hayiroglu MI, Uzun AO, et al. Effect of nonalcoholic fatty liver disease on in-hospital and long-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2017;120:1720–6. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Watanabe T, Shishido T, et al. The impact of non-alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels 2017;33(7):733–9. [DOI] [PubMed] [Google Scholar]
- 28.Karajamaki AJ, Bloigu R, Kauma H, et al. Non-alcoholic fatty liver disease with and without metabolic syndrome: Different long-term outcomes. Metabolism 2017;66:55–63. [DOI] [PubMed] [Google Scholar]
- 29.Ravichandran L, Goodman SG, Yan AT, et al. Non-alcoholic fatty liver disease and outcomes in persons with acute coronary syndromes: Insights from the GRACE-ALT analysis. Heart Asia 2012;4:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608–12. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 33.Alberti KG, Zimmet P, Shaw J, et al. The metabolic syndrome—A new worldwide definition. Lancet 2005;366:1059–62. [DOI] [PubMed] [Google Scholar]
- 34.Miyake T, Kumagi T, Hirooka M, et al. Metabolic markers and ALT cutoff level for diagnosing nonalcoholic fatty liver disease: A community-based cross-sectional study. J Gastroenterol 2012;47:696–703. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Rodriguez JL, Gonzalez-Cantero J, Gonzalez-Cantero A, et al. Diagnostic accuracy of serum alanine aminotransferase as biomarker for nonalcoholic fatty liver disease and insulin resistance in healthy subjects, using 3T MR spectroscopy. Medicine (Baltimore) 2017;96:e6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: A 10-year follow-up of the Hoorn study. Atherosclerosis 2007;191:391–6. [DOI] [PubMed] [Google Scholar]
- 37.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 38.Maximos M, Bril F, Portillo Sanchez P, et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology 2015;61:153–60. [DOI] [PubMed] [Google Scholar]
- 39.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009;136:477–85 e11. [DOI] [PubMed] [Google Scholar]
- 40.Ford I, Mooijaart SP, Lloyd S, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. Int J Epidemiol 2011;40:1530–8. [DOI] [PubMed] [Google Scholar]