Supplemental Digital Content is available in the text.
Keywords: blood-brain barrier, endothelial cells, permeability, stroke, Wnt/β-catenin
Abstract
Rationale:
The microvasculature of the central nervous system includes the blood-brain barrier (BBB), which regulates the permeability to nutrients and restricts the passage of toxic agents and inflammatory cells. Canonical Wnt/β-catenin signaling is responsible for the early phases of brain vascularization and BBB differentiation. However, this signal declines after birth, and other signaling pathways able to maintain barrier integrity at postnatal stage are still unknown.
Objective:
Sox17 (SRY [sex-determining region Y]-box 17) constitutes a major downstream target of Wnt/β-catenin in endothelial cells and regulates arterial differentiation. In the present article, we asked whether Sox17 may act downstream of Wnt/β-catenin in inducing BBB differentiation and maintenance.
Methods and Results:
Using reporter mice and nuclear staining of Sox17 and β-catenin, we report that although β-catenin signaling declines after birth, Sox17 activation increases and remains high in the adult. Endothelial-specific inactivation of Sox17 leads to increase of permeability of the brain microcirculation. The severity of this effect depends on the degree of BBB maturation: it is strong in the embryo and progressively declines after birth. In search of Sox17 mechanism of action, RNA sequencing analysis of gene expression of brain endothelial cells has identified members of the Wnt/β-catenin signaling pathway as downstream targets of Sox17. Consistently, we found that Sox17 is a positive inducer of Wnt/β-catenin signaling, and it acts in concert with this pathway to induce and maintain BBB properties. In vivo, inhibition of the β-catenin destruction complex or expression of a degradation-resistant β-catenin mutant, prevent the increase in permeability and retina vascular malformations observed in the absence of Sox17.
Conclusions:
Our data highlight a novel role for Sox17 in the induction and maintenance of the BBB, and they underline the strict reciprocal tuning of this transcription factor and Wnt/β-catenin pathway. Modulation of Sox17 activity may be relevant to control BBB permeability in pathological conditions.
Endothelial cells (ECs) are a heterogeneous cell population that has distinct structural, phenotypic, and functional properties.1 Recent studies have shown that ECs of arteries, veins, and the lymphatic system have high degree of specialization and can respond to specific hemodynamic and functional requirements.2 As compared to large vessel endothelium, the microvascular ECs can adapt and express highly specialized properties in response to the specific requirements of the different organs. Improved understanding of the molecular basis of ECs heterogeneity will help our comprehension of organ functions under physiological and pathological conditions.
Meet the First Author, see p 452
A typical example of highly specialized microvasculature is that of the brain. These vessels have the important function of regulating cerebral blood flow, oxygen delivery, and energy metabolite supply to the neural cells. Dysfunction of the brain vascular system causes major problems to brain connectivity, synaptic activity, and information processing.1 The specialized function of the brain microcirculation requires coordinated and continuous cross-talk of ECs and the other cell types that form the so-called neurovascular unit.1 In this structure, ECs are surrounded and embraced by pericytes and are in contact with the glia (ie, astrocytes, oligodendrocytes, microglia) and neurons.3 The neurovascular unit forms the blood-brain barrier (BBB), which strictly controls the entry into the central nervous system of neurotoxic plasma components, circulating inflammatory cells, and pathogens.4
While controlling general permeability, the BBB expresses multiple highly specialized transport systems that provide nutrients and other essential molecules from the blood to the neural cells.1 Thus, the integrity of the BBB is regulated by coordinated and continuous interactions of ECs with neural and vascular cells.
Wnt factors modulate cell and tissue differentiation both during development and in the adult.5 The canonical Wnt factors signal through their interactions with a complex formed by Frizzled receptors, which modulates phosphorylation of the Lrp5 (low-density lipoprotein receptor-related protein 5) and Lrp6 receptors, which, in turn, increase the stability and transcriptional activity of the downstream effector β-catenin through a complex signaling cascade.
Several groups, including ours,6–8 have reported that canonical Wnt/β-catenin signaling is responsible for brain angiogenesis and microvascular acquisition of barrier properties. However, endothelial signaling by β-catenin must be tightly regulated because long-lasting high levels of β-catenin signaling in the vasculature can cause large alterations in vascular stability and lumen malformation.9
β-catenin can also modulate the activity of other transcription factors, and in our previous studies, we showed that β-catenin can upregulate Sox17 (SRY [sex-determining region Y]-box 17), which, in turn, induces arterial differentiation in embryo10 and adult vessels. Induction of Sox17 by β-catenin was also shown for the brain microcirculation,10–12 but its role in BBB differentiation remains unknown.
In the present study, we report that endothelial-specific inactivation of Sox17 in mice (Sox17iECKO) leads to uncontrolled permeability of the brain microcirculation. The severity of this effect depends on the degree of BBB maturation: it is strong in the embryo and progressively declines after birth. RNA sequencing (RNA-Seq) analysis of gene expression of brain ECs at different stages of brain vascularization has identified candidate regulators in the control of permeability in the absence and presence of Sox17. Specifically, members of the Wnt/β-catenin pathway and a few target genes of this pathway are significantly reduced in the absence of Sox17. Consistent with this, we observed that Sox17 is a positive inducer of Wnt/β-catenin signaling and that it acts in concert with this pathway to induce and maintain the properties of the BBB. In vivo inhibition of the β-catenin destruction complex, or expression of a degradation-resistant β-catenin mutant, prevents the increase in permeability and retina vascular malformations observed in the absence of Sox17.
Overall, our data highlight a role for Sox17 in the induction and maintenance of the BBB properties, and they underline the strict reciprocal modulation of this transcription factor and the Wnt/β-catenin pathway.
Methods
The authors declare that all data and methods used in support of the findings presented in this study will be made available from the corresponding author on reasonable request.
All sequencing datasets in this article are deposited in international public repository, Gene Expression Omnibus, database under accession code GSE122564. Data can also be explored at https://edgroup.shinyapps.io/sox17_bbb/
Detailed Methods section is available in the Online Data Supplement.
Results
Endothelial Expression of Sox17 and β-Catenin Shows a Distinct Time Course
Figure 1A–1E shows the evaluation of Wnt/β-catenin signaling activity, using the Tg(BAT [β-catenin-activated transgene]-LacZ) reporter mouse (Figure 1F; anti–β-gal [β-galactosidase] immunostaining) and Sox17 nuclear localization in the brain microvasculature of embryos (E10.5, E12.5, E14, and E17), pups (P5, P9), and adults. Quantification of these results is shown in Figure 1G, and, as previously reported,7 this shows that the number of β-gal single positive nuclei (used as markers of β-catenin transcriptional activity; Figure 1G, red column) or double positive β-gal and Sox17 nuclei (Figure 1G, yellow columns) was high at E10.5, but declined markedly at later stages of embryo development and in the postnatal period. In the adult, β-gal staining of the endothelial nuclei was strongly reduced as compared to the embryo, but still detectable (positive nuclei were 5%–10% of the total counted). In contrast, the quantification of Sox17-positive nuclei (Figure 1G, yellow and green columns) had an opposite distribution, as this increased during embryo development, in the postnatal period, and in the adult, where it reaches steady state. Figure 1 also reports the actual images of β-gal and Sox17 nuclear staining of ECs of the hindbrain and cerebellum at different stages of development.
Figure 1.
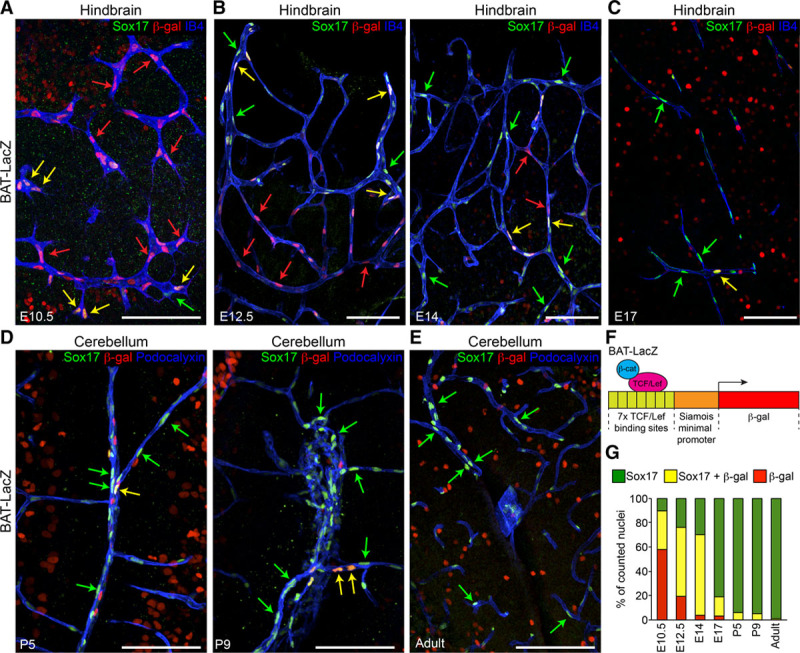
Canonical Wnt/β-cat (β-catenin) signaling and Sox17 (SRY [sex-determining region Y]-box 17) expression show distinct time courses in endothelial cells (ECs) during brain angiogenesis and vessel maturation. A–E, Confocal analysis of the brain microvasculature of BAT (β-catenin-activated transgene)-LacZ reporter embryos, pups, and adult mice. Whole-mount (A and B), vibratome sections (C), and cryosections (D and E) were stained for β-gal (β-galactosidase, red), to detect β-cat–induced expression of the Lac-Z reporter construct, Sox17 (green), and IB4 (isolectin B4, blue, A–C) or Podocalyxin (blue, D–E). Green, red, and yellow arrows indicate Sox17 staining, β-gal–positive nuclei, and double positive nuclei, respectively. Positive nuclei to β-gal outside the vascular system indicate active Wnt signaling in the brain parenchyma. Scale bar: 100 µm. F, Schematic representation of the BAT-LacZ reporter construct (see Methods). G, Quantification of nuclei positive for Sox17 only (green), β-gal only (red), or double-labeled (yellow; n=3–5 animals for each time point). These indicate different patterns of expression between Sox17 and β-cat signaling. LEF/TCF indicates lymphoid enhancer factor/T-cell factor.
Sox17 Is Required for Correct Brain Vascular Morphogenesis and Barrier Function
We then investigated whether endothelial Sox17 has a role in induction and maintenance of BBB function at different times during brain vascular maturation and in different brain vascular regions.
Figure 2 shows that when Sox17 was inactivated in ECs at E15.5 after tamoxifen administration (Figure 2A), the brain vasculature was severely altered (Figures 2C and 2D), as documented by magnification of the vasculature that showed extensive hemorrhage and alterations to the vascular lumen, with irregular enlargements (Figure 2D–2F). Permeability to IgG in the whole-brain was increased but failed to reach statistical significance (P=0.08; Figures 2G and 2H), probably because of the high variability of the barrier function at this developmental stage. Figure 2B and Online Figure IA show that the severity of the phenotype after Sox17 inactivation depended on the developmental stage at which tamoxifen was administered, and most importantly, on the length of time after Sox17 inactivation. As reported in Online Figure IA, the longer the time before euthanization after tamoxifen administration, the more severe the phenotype. The vascular alterations described were present in all of the different brain regions analyzed (Figure 2D), which suggests that endothelial inactivation of Sox17 during embryo development results in major defects in the entire brain vasculature as well as in BBB differentiation.
Figure 2.
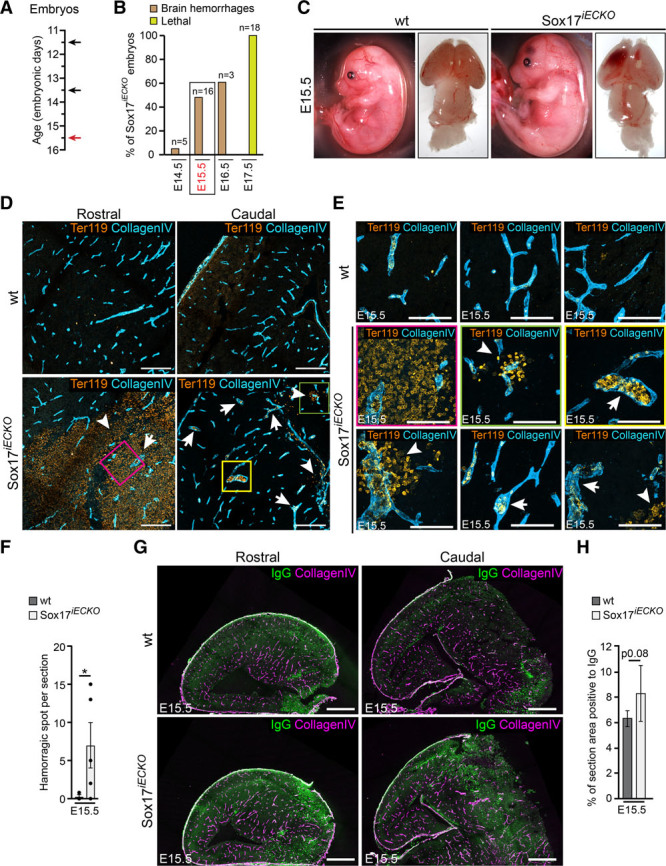
Endothelial Sox17 (SRY [sex-determining region Y]-box 17) is required for correct brain vascular morphogenesis and barrier function. A, Scheme of tamoxifen administration at E11.5, E13.5 (black arrows), and embryo analysis at E15.5 (red arrow). See Methods section for details. B, Summary assessment of Sox17iECKO embryos presenting brain hemorrhages (brown columns) after tamoxifen treatment (as indicated in A). Data are presented as percentage of total number of embryos analyzed (n). E15.5 harvesting time was selected for subsequent experiments. At E17.5 (yellow column) none of the Sox17iECKO embryos was alive. C, Sox17iECKO embryos display brain hemorrhages. D and E, Sectioning of different regions of the embryonic brain showed enlarged vessels (arrows) stained with collagen IV (light blue) and extensive hemorrhages. Ter119 (orange) indicates red blood cell (arrowheads). Scale bar: 200 μm in C, 100 μm in D. F, Average count of hemorrhagic spots per section (n=3 wild-type [wt] and 5 Sox17iECKO, mean±SEM, *P<0.05, Mann-Whitney test). G, Confocal microscopy analysis of embryonic brain cryosections. Collagen IV (magenta) and IgG (green) of wt and Sox17iECKO revealed the presence of IgG leakage in Sox17iECKO embryos. Scale bar: 500 μm. H, Quantification of IgG leakage (left; n=3 wt and 5 Sox17iECKO, mean±SD, P=0.08, 2-tailed t test).
We then investigated the BBB permeability properties in the absence of endothelial Sox17 at the postnatal stage (P9; Figure 3). The vasculature of 4 different areas of the brain and the retina were analyzed. Although the brain vascular morphology was not strongly affected at this time of development, the permeability was significantly increased. As shown in Figure 3, the permeability to IgG (Figures 3B and 3C) was increased in the cortex, striatum and in the thalamus, whereas leakage of the low molecular weight cadaverine was more marked and increased in all four of the brain regions analyzed (Figures 3D and 3E; Online Figure IIA). Consistently, PLVAP (plasmalemma vesicle–associated protein),13 a protein associated with increased endothelial permeability,12 was significantly upregulated in the brain regions considered (Figure 3F and 3G) and in the retina (Online Figure IIB).
Figure 3.
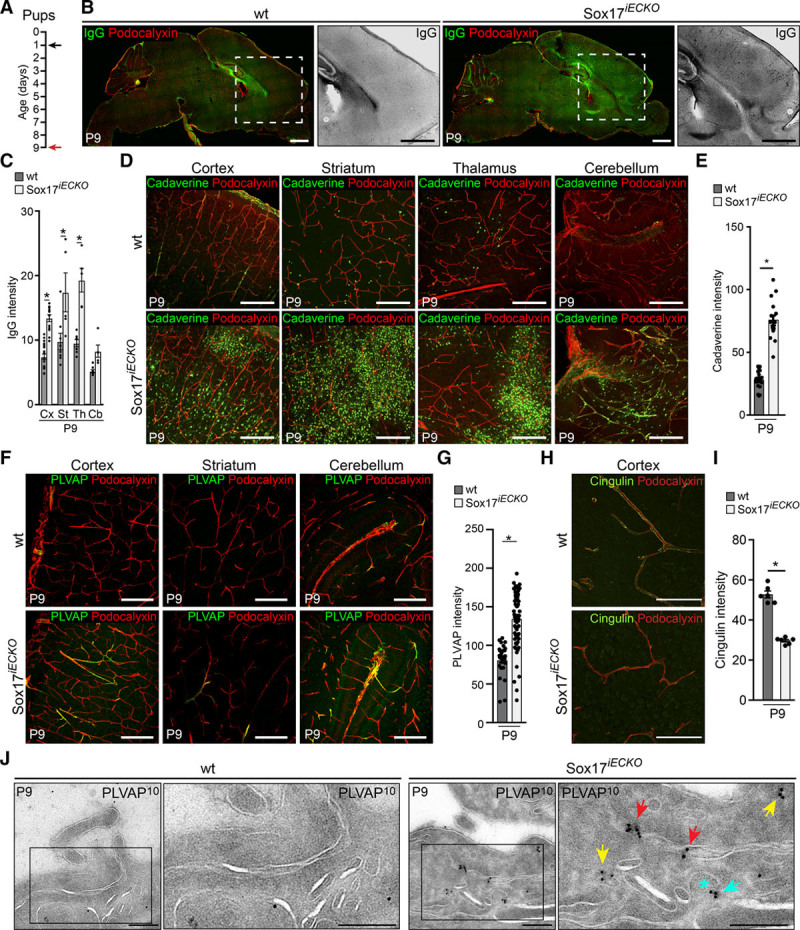
Endothelial Sox17 (SRY [sex-determining region Y]-box 17) deficiency affects blood-brain barrier integrity. A, Scheme for tamoxifen injections in the pups at P1 (black arrow), with analysis at P9 (red arrow). B, Confocal images of vibratome sections of wild-type (wt) and Sox17iECKO brains stained with Podocalyxin (red) and IgG (green). The presence of IgG leakage (black [magnified]) reveals high permeability in Sox17iECKO brains. Scale bar: 1 mm. C, Quantification of signal intensities for IgG extravasation indicated more prominent leakage in the cortex (Cx), striatum (St) and thalamus (Th) than in the cerebellum (Cb; n=5 wt and 8 Sox17iECKO, mean±SEM, *P<0.01, 1-way ANOVA and Bonferroni post hoc test). D, Confocal analysis of Alexa555-conjugated cadaverine leakage (green) in brain sections stained with Podocalyxin (red). Sox17iECKO present cadaverine accumulation in the brain parenchyma in all of the regions analyzed (lower). Scale bar: 200 μm. E, Quantification of cadaverine leakage (n=4 wt and 4 Sox17iECKO, mean±SEM, *P<0.01, Mann-Whitney test). F–G, Confocal analysis of sections and quantification of PLVAP (plasmalemma vesicle–associated protein) expression (green) in the brain microvasculature (Podocalyxin in red) in wt and Sox17iECKO mouse. PLVAP expression is increased in Sox17iECKO mice. Scale bar: 200 μm. (n=6 wt and 6 Sox17iECKO, mean±SEM, *P<0.01, Mann-Whitney test). H–I, Confocal images and quantification of Cingulin (green) in the microvasculature (Podocalyxin in red) of cortex in wt and Sox17iECKOmouse. Scale bar: 100 μm. (n=3 wt and 3 Sox17iECKO, mean±SEM, *P<0.01, Mann-Whitney test). J, Electron microscopy of PLVAP immunogold-labeled (10 nm gold, PLVAP10) ultrathin sections of brain capillaries from wt and Sox17iECKO mice (P9). PLVAP is detectable only in the ECs of Sox17iECKO and localizes in the overlapping interendothelial contacts (red arrows) in the endosomal structures (yellow arrows) and in the neck of clathrin-coated buds (light blue arrow and asterisk).
The efficiency of Sox17 recombination in the different brain regions studied is reported in Online Figure IIC and IID. Sox17 is essentially absent in the 4 regions of the brain. Sox17 downregulation in the brain microvasculature has been further analyzed by real-time quantitative polymerase chain reaction, and data are presented in Online Figure VIIIB.
By electron microscopy, we did not find fenestrae in the brain microcirculation of controls or Sox17iECKO mice. However, the analysis of immunolabeled cryosections showed that PLVAP localized in the regions of endothelial junctions (Figure 3J), in endosomal structures and at the neck of clathrin-coated buds. As expected, PLVAP immunolabeling was absent in wild-type ECs.
Confirming the data obtained with other techniques, by electron microscopy, we observed edema around the Sox17iECKO vessels (Online Figure VA). In addition, we found that the junctions of ECs in Sox17iECKO brains were abnormal. The typical junction morphology of control ECs shows that contacting cells extend intraluminal microvilli in the vascular lumen (Online Figure VB).14,15 In contrast, the contact points between Sox17iECKO ECs presented a different morphology with large overlapping regions, with either any or mono-cell intraluminal microvilli (Online Figure VB). We also found that, compared with wild-type, ECs of Sox17iECKO mice were devoid of caveolae but presented higher number of clathrin-coated buds and abundant endosomal structures which can be identified (by electron microscopy appearance) as late endosomes (Online Figure VC). The absence of caveolae parallels the downregulation of caveolin-1 observed by RNA-Seq (Figure 6E).
Figure 6.
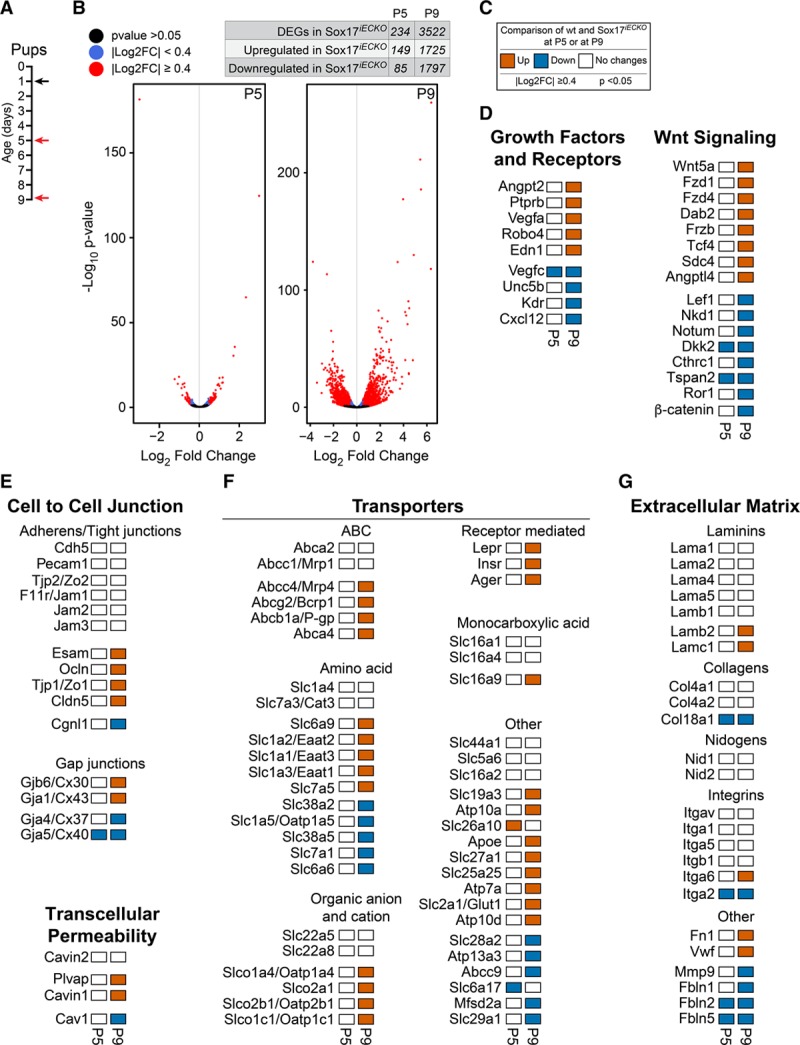
Role of Sox17 (SRY [sex-determining region Y]-box 17) expression on blood-brain barrier (BBB)-related genes at P5 and P9. A, Scheme of tamoxifen injection in the pups at P1 (black arrow) and analysis of endothelial cells isolated from brains at P5 and P9 (red arrows). B, Volcano plots are used to display the magnitude of the differential expression between wild-type (wt) and Sox17iECKO either at P5 (left) or at P9 (right; n=3 wt and 3 Sox17iECKO for each time point). Each dot represents 1 gene that has detectable expression both in wt and Sox17iECKO cells. Black dots represent genes that are not significantly different (adjusted P>0.05). Significant differentially expressed genes (DEGs; adjusted P≤0.05) are colored either in red (Log2FC≥0.4) or in blue (Log2FC<0.4). The number of DEGs in Sox17iECKO are reported in the table on top of the Volcano plots. C, Description of thresholds and colors used in D–G. D, Selected angiogenesis and Wnt/β-cat (β-catenin) related genes, differentially expressed, either up or down, in Sox17iECKO. E–G, Regulation of the expression of gene relevant for key BBB features (cell to cell junction [E], transcellular permeability [E], transporters [F], and extracellular matrix [G]), as in Figure 5, modulated by the inactivation of Sox17 expression. The genes that do not have any expression changes are displayed by white box. FC indicates fold change.
These data reflect alterations in cell contact morphology and intracellular protein trafficking in Sox17. We then examined the distribution of highly expressed tight junctions (TJ) associated proteins (ZO-1 [zonula occludens-1], Claudin-5, and Cingulin) in the brain vasculature of wild-type and Sox17iECKO. Despite the upregulation of ZO-1 and Claudin-5 revealed by RNA-Seq (Figure 6E), the protein levels of these proteins, measured by immunofluorescence of junctional localization, were unchanged in the Sox17iECKO ECs (Online Figure IIIB–IIIE). However, we observed a strong and significant downregulation of Cingulin (Figure 3H and 3I and Online Figure IIIA) consistent with the RNA-Seq result (Figure 6E). Cingulin is of particular interest because it can bind to and bundle actin filaments and interact with myosin II and several TJ proteins, including ZO-1 and JAM (junction adhesion molecule). Overall, these morphological data suggest a defective association of TJ with the cytoskeleton that may alter the capacity of these structures to control permeability.16
In the Online Figure IVA, we also measured the pericyte coverage of the microvessels, and we found that the coverage is reduced in the absence of Sox17iECKO. The loss of surrounding pericytes may contribute to the reduced barrier function of the vessels.3
In contrast, the deletion of Sox17 does not affect the endothelial survival. As reported in Online Figure IVB, we found no differences between wild-type and Sox17iECKO cleaved caspase 3 staining.
Sox17 Protects the Adult Vasculature From Ischemic Injury in an Experimental Model of Stroke
As reported in Online Figure VIA–VIE, when Sox17 was inactivated in the adult, we did not see major alterations in the organization of the microvasculature or increase in permeability in the 4 brain regions. However, when we induced a focal cerebral ischemia (Figure 4), by insertion of a silicon-coated nylon filament, of the middle cerebral artery occlusion, Sox17 expression was increased in the ipsilesional area of the brain (Figure 4A), but not in the contralesional area.
Figure 4.
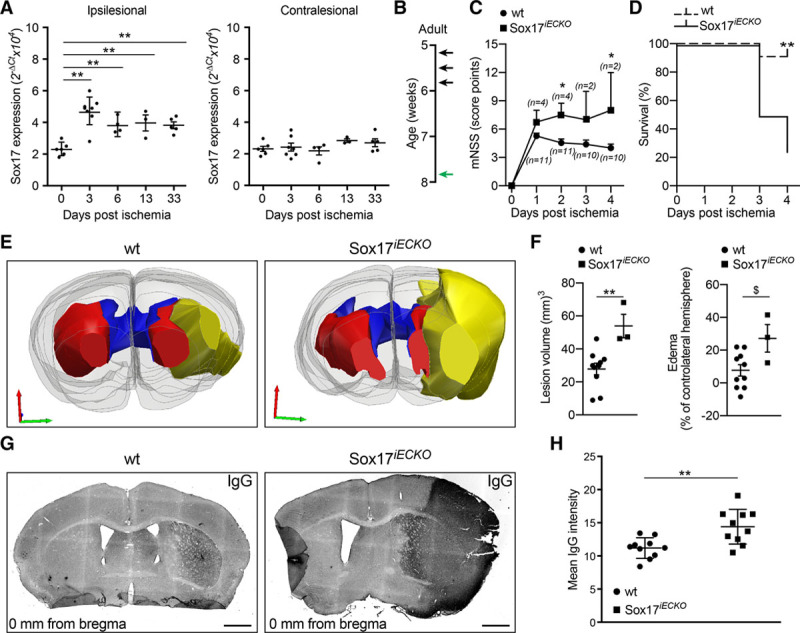
Endothelial Sox17 (SRY [sex-determining region Y]-box 17) deficiency exacerbates stroke outcome. A, Real-time quantitative polymerase chain reaction analysis of Sox17 expression in the ischemic forebrain of wild-type (wt) animals. Expression was determined in the ischemic brain hemisphere (ipsilesional) and in the nonischemic controlateral hemisphere (contralesional) at different days postischemia (dpi; n=3–8 mice per time point, mean±CI95%, **P≤0.01, compared with 0 dpi, 1-way ANOVA, and Bonferroni post hoc test). B, Tamoxifen was injected in 5-wk-old mice (3 injections every other day; black arrows). Two weeks later, mice underwent left middle cerebral artery occlusion (MCAO; green arrow) and analysed at the time points indicated in the specific panels. C, Modified Neurological Severity Score in wt and Sox17iECKO mice (mean±SEM, *P≤0.05, 2-way ANOVA followed by Bonferroni post hoc test). D, Kaplan-Meier survival curve recorded in wt (n=11) and Sox17iECKO (n=4) mice subjected to MCAO up to 4 dpi (n=11 wt and 4 Sox17iECKO, **P≤0.01, Log-rank (Mantel-Cox) Test). E, Representative serial 3D reconstructions of the forebrains of a wt and a Sox17iECKO mouse at 4 dpi. Yellow represents the ischemic lesion tissue. Red represents the healthy striatum. Blue represents the ventricles. Gray represents the contours of the hemispheres. F, Graphs representing the lesion volume (left) and the edema of the ipsilesional hemisphere (right; n=10 wt and 3 Sox17iECKO, mean±SEM), (for lesion volume **P≤0.01, Mann-Whitney test), (for edema $P<0.05, 2-tailed t test and P=0.08, Mann-Whitney test). G, Confocal images of 1 representative cryosection from wt and Sox17iECKO at 4 dpi stained for IgG (black). The presence of IgG leakage reveals loss of BBB integrity in Sox17iECKO brains. Sections correspond to the bregma (0 mm). Scale bar: 1 mm. H, Quantification of IgG leakage from cryosections (n=5 wt and 3 Sox17iECKO, mean±SEM, **P<0.01, Mann-Whitney).
When this model was applied to adult Sox17iECKO mice, the lesions were significantly larger compared with control animals (Figure 4E–4G) and many typical parameters of ischemic stroke were strongly increased by the lack of Sox17, including modified Neurological Severity Score, survival (Figure 4C and D), edema, and IgG permeability (Figure 4F–4H). These data indicate that although in a resting state the vessels appear normal, in the absence of Sox17, they are more vulnerable to pathological conditions.
To further investigate this aspect, we asked whether Sox17iECKO mice could be more sensitive to ischemia-induced VEGF (vascular endothelial growth factor). We used adeno-associated-virus to obtain low but constant release of VEGF in a specific region of the brain mimicking, at least in part, the condition of stroke-induced ischemia (Online Figure VIIA–VIID). We found that, in response to these low levels of VEGF, control mice did not show any significant increases in permeability to IgG or local hemorrhage. In contrast, Sox17iECKO mice reacted dramatically to VEGF showing a strong increase in permeability and diffuse hemorrhage (Online Figure VIIA–VIID). Overall, these data suggest that Sox17 expression counteracts the vascular response to VEGF, thus, maintaining vascular stability and control of permeability in ischemic conditions.
Analysis of Gene Expression During BBB Maturation
To study the signaling pathways involved in BBB differentiation and maintenance and the role of Sox17 in these processes, we performed genome-wide analysis of the brain endothelial transcriptome in the embryo, at different postnatal stages, and in adult mice. The different times selected corresponded to the different stages of BBB maturation, which include the onset of angiogenesis (E15.5), the early times of BBB maturation (P5 and P9), and the adult brain vasculature. As reported in Online Figure VIIIA, the ECs preparation shows strong enrichment of ECs-specific markers, such as Cdh5, Cldn5, and Pecam1/CD31, with barely detectable amounts of pericytes and astrocytes or epithelial cell markers, such as Acta2, Pdgfrb, Gfap, and Cdh1. Cre-induced downregulation of Sox17 was monitored using real-time quantitative polymerase chain reaction (Online Figures VIIIB and VIG).
From the RNA-Seq analysis, the genes that are significantly regulated over time were clustered into 6 groups (Figure 5A) on the basis of their expression profiles. Genes that encode proteins related to angiogenesis, such as growth factors, vascular remodeling, matrix adhesion, cell migration, and others, were highly enriched in clusters 5 and 6 (Figure 5B; Online Table I). Genes more strictly related to BBB permeability properties, such as transmembrane transporters, cell to cell junctions, and matrix proteins, were better represented in clusters 1, 5, and 6 (Figure 5B; Online Table I), with sustained expression in the adult.
Figure 5.

Key signaling pathways governing the maturation of a functional blood-brain barrier (BBB). A, Normalized expression of 10 783 differentially expressed genes (DEGs, obtained by Next-maSigPro analysis: P<0.05 and R2>0.6) during BBB development (E15.5), maturation (P5 and P9), and maintenance (adult; n=3 for each time point). Heat map shows gene expression levels (transcript per million [TPM]) in clusters (color-coded as indicated). Trajectory plots show the gene expression pattern of each cluster by displaying the average gene expression level at each time point in that cluster. B, Enriched gene ontology (GO) terms related to BBB development in clusters. Bar plot shows the number of genes that belong to enriched GO terms in each cluster. C, The top 5 most enriched GO terms in cluster 6. D, Heatmap shows expression levels over time of key signaling pathway endothelial cell (EC) genes during BBB development. E–G, Heat maps are used to display the expression levels of genes relevant for key features of BBB formation and maturation (cell to cell junction [E], transcellular permeability [E], transporters [F] and extracellular matrix [G]) at different developmental stages. In D–G, the genes are categorized based on time course analysis: DEGs (P<0.05 and R2>0.6) are grouped by the clusters that they belong to, and nondifferentially expressed genes are grouped into not significant (NS) category (R2<0.6 or P<0.05). VEGF indicates vascular endothelial growth factor.
Overrepresentation analysis of gene ontology showed that the most enriched ontology for cluster 6 was angiogenesis (Figure 5C). Sox17 expression follows the kinetics of this cluster, as do those of several other ECs signaling pathways, such as Dll4, Angpt2, receptors Tie-1 and Tek, Vegfc, and Flt-1 with increasing levels of expression after birth (Figure 5D). In many cases, different members of the same signaling pathway might present different kinetics during BBB development (Figure 5D). For instance, whereas Notch1 and Jag1 follow the time course typical of cluster 5, Notch4 and Dll4 belong to cluster 6. Similarly, whereas canonical Wnt signaling declines at later times, some members of the noncanonical pathways (such as Wnt5a) increase (Figure 5D). It is of interest that Sox17 belongs to same cluster of several other transcription factors which have been described as essential regulators of ECs specification as, for instance, Ets1, Erg, Klf4, and Edn1 (Figure 5D).
These first observations emphasize the complexity of the signaling network that modulates brain vascularization, and they suggest that fine coordination of the different pathways involved is required for correct maturation and maintenance of the BBB.
We also observed that several genes essential for the key features of the BBB, such as cell to cell junctions and transporters, mainly belongs to clusters 1 and 6. These molecules present expression levels that increase over BBB development and maturation (E15.5, P5, and P9) and either stabilize (cluster 6) or further increase after P9 (cluster 1; Figure 5E and 5F), indicating a continuous maturation of the barrier transcriptome. Genes involved in extracellular matrix deposition and stabilization present an expression profile characterized by a transient increase in the postnatal stage (P5, P9) followed by lower expression levels in the adult (cluster 5; Figure 5G). In cluster 3 and 5, we also found a small number of transporters that are already expressed in the embryo (E15.5) and in the pups (P5, P9), while declining in the mature barrier of the adult brain (Figure 5G).
The expression profiles of these genes, which are fundamental for the proper barrier functions of brain ECs, reflect the complex dynamics of the developing barrier.
Role of Sox17 on Expression of BBB Related Genes
To define the Sox17 mechanism of action in the maintenance of BBB permeability, we next compared gene expression profiles in the absence and presence of Sox17 at E15.5 (Online Figure IB), P5 and P9 (Figures 6A–6G), and in the adult (Online Figure VIH).
Several BBB transporters were up or downregulated by inactivation of Sox17 expression (Figure 6F). It is conceivable that, during brain vascularization, ECs have different needs and, as a consequence, express specific transporters for their growth and maturation. Once the barrier is stabilized, these transporters are downregulated and reach a steady state. In contrast, genes related to increased permeability, such as PLVAP and Angpt2, were upregulated in the absence of Sox17 (Figure 6D and Online Figure VIIIB). Many genes involved in angiogenesis were downregulated by Sox17, which suggested vascular stabilization. Importantly, the expression of genes implicated in canonical and noncanonical Wnt signaling pathways was modulated by Sox17. Wnt/β-catenin target genes, such as Nkd1 and Lef1, and also β-catenin itself, were significantly reduced by the absence of Sox17 (Figure 6D and Online Figure VIIIB), which suggested a positive interaction of this transcription factor with the canonical Wnt/β-catenin pathway. The members of the SoxF subgroup of transcription factors, and in particular Sox17, might act as positive or negative modulators of Wnt/β-catenin signaling.17,18 In our gene expression analysis, we detected a reduction in a few Wnt/β-catenin targets in the absence of Sox17. We, therefore, tested whether in this system Sox17 has a positive role in canonical Wnt/β-catenin signaling. Figure 7A–7D shows that both in the embryo and at the postnatal stage (P9), the number of β-gal–positive nuclei was significantly reduced in Sox17iECKO embryos and pups, in comparison to control mice. In the adult (Figure 7D and Online Figure VIF), we could not detect a significant difference in β-gal–positive endothelial nuclei in the presence or absence of Sox17, possibly because the number of β-gal–positive nuclei was relatively low already in the control mice (about 10% of the total ECs nuclei).
Figure 7.
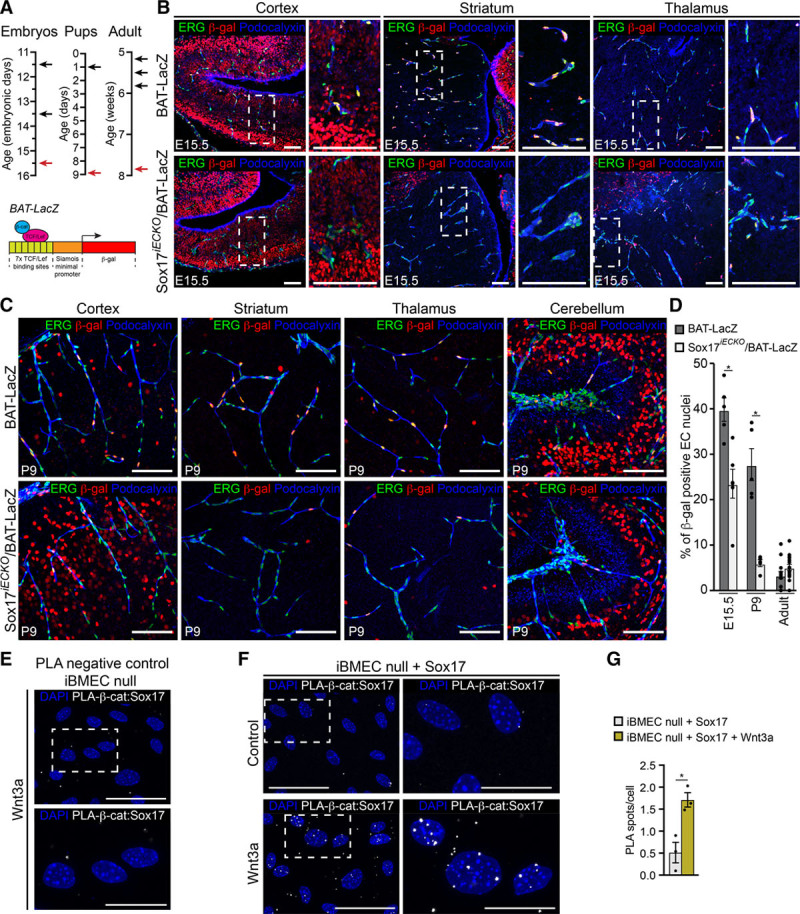
Endothelial Sox17 (SRY [sex-determining region Y]-box 17) is required for Wnt/β-catenin signaling in the brain. A, Scheme of tamoxifen administration and analysis. Pregnant females were injected (E11.5 and E13.5; black arrows) and the embryos dissected and analyzed at E15.5 (red arrow); pups were injected at P1 (black arrow), and analyzed at P9 (red arrow); 5-wk-old mice received 3 injections every other day (black arrows) and were analyzed at 8-wk old (red arrow). See Methods for details. Schematic representation of the BAT (β-catenin-activated transgene)-LacZ reporter construct as in Figure 1F. B and C, Confocal analysis of wild-type and Sox17iECKO/BAT-LacZ mice (see Methods for details) at the indicated ages. Brains sections were stained for ERG (ETS transcription factor; green), β-gal (β-galactosidase; red) and Podocalyxin (blue). In the mutant embryos and pups (lower B and C, respectively), the number of β-gal–positive endothelial nuclei (ERG-positive) are significantly reduced in all of the brain regions. For the embryonic brain, higher magnification of the insets is also shown. Scale bar: 100 μm. D, Quantification of β-gal–positive nuclei with respect to total ERG-positive endothelial cells (ECs; percentages) as in B (embryos), C (pups), and Online Figure VIF (adult). β-gal–positive nuclei outside the vascular system indicate active Wnt signaling in the brain parenchyma (n=3–4 animals for each experimental condition, mean±SEM, *P<0.01, Mann-Whitney test). E–F, In situ proximity ligation assay (PLA). iBMEC (immortalized brain microvascular endothelial cells) null for Sox17 was used as negative control (E). In iBMEC null transduced with Sox17, Sox17/β-cat (β-catenin) interaction (white) was observed. Nuclei were counterstained with DAPI (4’,6-diamidino-2-Phenylindole; blue). Magnification of boxed areas are shown. Note the increase in nuclear spot, induced by Wnt3a activation. G, Quantification of PLA nuclear signal expressed as the average number of PLA spots per cell; n=3 for each condition, mean±SEM, *P<0.01, 2-tailed t test. LEF/TCF indicates lymphoid enhancer factor/T-cell factor.
By proximity ligation assay experiment, we found that in brain ECs, Sox17 can associate to β-catenin, and this interaction is increased by Wnt stimulation (Figure 7E–7G).
Restoration of β-Catenin Signaling Rescues Permeability and Morphological Alterations Induced by Sox17 Inactivation
We then asked whether the effect of Sox17 in the maintenance of BBB permeability properties was indirectly mediated by upregulation of Wnt/β-catenin signaling. As gene expression analysis showed that β-catenin was reduced in the absence of Sox17 (Figure 6D, Online Figure VIIIB), we tested whether restoring the level and activity of this transcriptional regulator would rescue the Sox17iECKO phenotype. To this end, we treated pups with (2′Z,3′E)-6-bromoindirubin-3′-acetoxime (6-BIO), a known inhibitor of GSK-3β (glycogen synthase kinase-3β) and β-catenin destruction complex.19 As we reported in our previous study,10 in the absence of Sox17, the vasculature of the retina presents a strongly altered horizontal growth and increased PLVAP, which is used here as a marker of permeability (Online Figure XI). The treatment of pups with 6-BIO (Figures 8A) almost completely reverted the retina vascular phenotype, restoring the correct vascular morphogenesis and significantly reducing PLVAP (Online Figure XIA and XIB).
Figure 8.
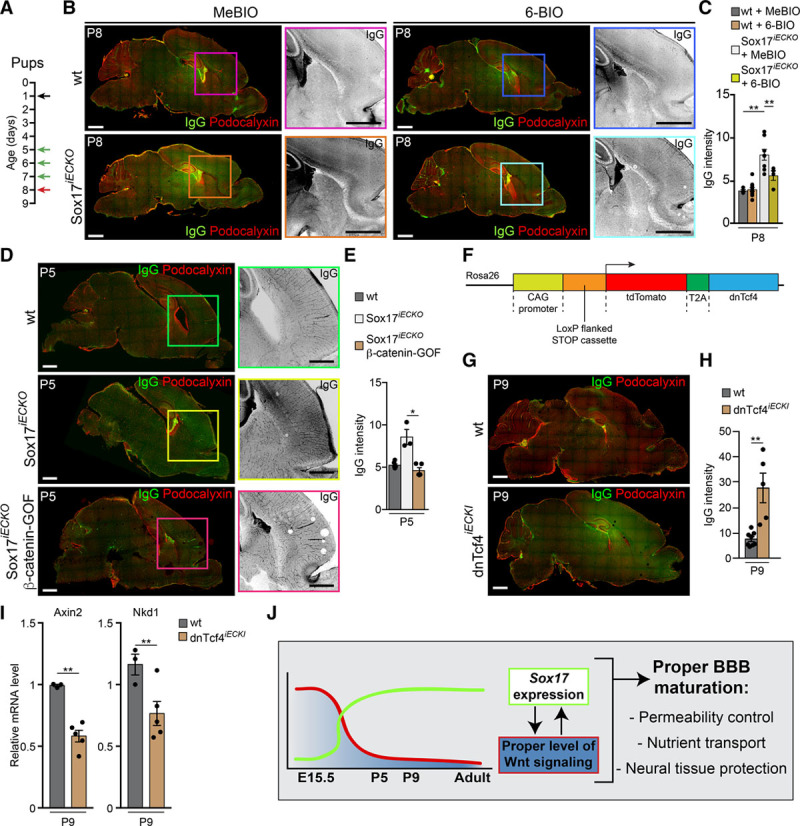
Stabilization of β-cat (β-catenin) signaling restores vascular defects induced by Sox17 (SRY [sex-determining region Y]-box 17) inactivation. A, Scheme of tamoxifen injections in the pups at P1 (black arrow), (2′Z,3′E)-6-bromoindirubin-3′-acetoxime (6-BIO; or 1-methyl- bromoindirubin [MeBIO]) injections at P5-7 (green arrows), and analysis of brain P8 (red arrow). B, Confocal images of sections stained with Podocalyxin (red) and IgG (green or black in magnification) of wild-type (wt) and Sox17iECKO pups revealed a complete rescue of IgG leakage in mutant brains treated with 6-BIO. Scale bar: 1 mm. C, Quantification of IgG leakage in brain as in B (n=3 for each experimental condition, mean±SEM, **P<0.01 ANOVA and Tukey post hoc analysis). D, β-cat stabilization rescue Sox17iECKO blood-brain barrier (BBB) defect. Confocal images of section stained with Podocalyxin (red) and IgG (green) from wt, Sox17iECKO, and Sox17iECKO/β-cat–gain of function (GOF) brains from P5 pups. The presence of IgG leakage (black [magnified]) reveals high permeability in Sox17iECKO brains. In the double mutant Sox17iECKO/β-cat GOF pups, IgG leakage is reduced to control levels. Scale bar 1 mm. E, Quantification of IgG extravasation indicates more prominent leakage in Sox17iECKO brains (n=6 wt and 3 Sox17iECKO and 5 Sox17iECKO/β-cat-GOF, mean±SEM, *P<0.05, Mann-Whitney test). F, Schematic representation of the td-Tomato-T2A–dominant-negative Tcf4 (transcription factor 7 like 2, T cell-specific, HMG box; dnTcf4) construct. See Methods for details (CAG, CMV immediate enhancer/β-actin). G, Confocal images of brain microvasculature. IgG (green) and Podocalyxin (red) staining of sections of wt and inducible endothelial-specific dominant-negative Tcf4 (dnTcf4iECKI) P9 pups. The presence of IgG reveals higher permeability in dnTcf4iECKI. Scale bar 1 mm. H, Quantification of IgG leakage indicates more prominent leakage in dnTcf4iECKI brains (n=8 wt and 5 dnTcf4iECKI, mean±SEM, **P<0.01, Mann-Whitney test). I, Real-time quantitative polymerase chain reaction analysis of 2 Wnt/β-cat target genes (Axin2 and Nkd1) expressed in brain endothelial cells isolated from dnTcf4iECKI P9 pups (n=3 wt and 4 dnTcf4iECKI, mean±SD **P<0.01, 2-tailed t test). J, Schematic model of the cross-talk between Wnt/β-cat and Sox17 signaling. Wnt signaling declines after birth and triggers activation of Sox17 that in turn control brain vascular permeability. A calibrated cross-talk of these 2 transcription pathways is necessary to prevent uncontrolled effect and pathogenic conditions.
Consistent with this, the increase in permeability of the Sox17iECKO brain vasculature to endogenous IgG (Figures 8B and 8C) was also significantly reduced by treatment of the mice with 6-BIO. As GSK3-β takes part in other signaling pathways, we also tested Sox17iECKO mice intercrossed with mice expressing an inducible endothelial-specific constitutively active β-catenin (ie, gain of function [GOF]).9,10 The recombined β-catenin mutant lacks the exon 3 domain and cannot be phosphorylated and subsequently ubiquitinated and degraded in proteasomes. Therefore, it is free to translocate to the nucleus and activate cell transcription, functioning as a GOF mutant. Using these double mutants, Sox17iECKO/β-catenin GOF, we saw a decrease in IgG permeability of the brain microvasculature (Figure 8D and 8E).
Finally, we analyzed the brain vasculature of pups (P9) expressing an inducible endothelial-specific dominant-negative Tcf4 (transcription factor 7 like 2, T cell-specific, HMG box) that inhibits β-catenin transcriptional signaling (Figure 8F). Endothelial-specific activation of inducible endothelial-specific dominant-negative Tcf4 reduced the expression of β-catenin target genes as Axin2 and Nkd1 (Figure 8I). Under this condition, the permeability control of the BBB was significantly reduced (Figure 8G and 8H). Thus, inhibition of β-catenin transcriptional signaling after birth disrupts the BBB function in a way comparable to inactivation of Sox17. Overall, these data support the concept that Sox17 maintains the correct BBB function by sustaining the activity of Wnt/β-catenin signaling.
Notch and Sox17 Signaling
As reported in Figure 5D, we first observed that multiple members of the Notch signaling pathway are upregulated with a time course that paralleled the differentiation of the brain microvasculature.
We then tested whether Sox17 and Notch could cross-talk during brain vascularization.
We analyzed 2 genetically modified mouse strains with endothelial-specific loss of function mutations of Notch signaling: RbpjiECKO (recombination signal binding protein for immunoglobulin kappa J region), Dll4iECKO (delta-like canonical Notch ligand 4), and mice treated with DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) to inhibit Notch signaling.9,10 In addition, we studied a mouse strain expressing active Notch1 intracellular domain in ECs, thus acting as GOF mutation of Notch pathway (NotchICiECKI).
As reported in Online Figure IX, with the exception of Dll4iECKO, all the Notch loss of function or GOF murine strains presented areas of hemorrhages in the brain microvessels that were particularly large in the GOF NotchICiECKI mutant mice.
Sox17 signaling did not change in the brain microcirculation of all the strains of Notch mutant mice (Online Figure XA–XD). The β-catenin signaling (Online Figure XE and XF) also remained unaffected by inhibiting Notch signaling by DAPT. Thus, Notch signaling does not seem to be upstream of Sox17 in this system.
Furthermore, we analyzed the expression of members of the Notch pathway (Dll4, Notch4, Notch1, Hey1, and Hey2[hairy/enhancer-of-split related with YRPW motif 1 and 2]) known to be downstream of Sox1710 in Sox17iECKO mice. By real-time quantitative polymerase chain reaction, we could not detect significant modifications in the expression levels of these Notch signaling molecules, suggesting that Notch signaling is not downstream of Sox17 in the brain microcirculation (Online Figure VIIIC). Indeed, the brain vasculature phenotypes of Notch gain and loss of function look different from those induced by the loss of function of Sox17, showing strong enlargement of the vascular lumen and hemorrhages that are not present on Sox17 deficient embryos or pups.
Overall, these data strongly suggest that although Notch may be important in the development and differentiation of the brain microcirculation, the effects of Sox17 are independent from the activation of this signaling system.
Discussion
The brain microvasculature forms the BBB that strictly controls permeability to circulating toxic agents and immune/inflammatory cells to prevent any damage to the delicate neural microenvironment.
The development of the BBB is a complex, highly coordinated, multistep process that is modulated by different intracellular and extracellular signaling pathways.4
A few groups, including us,6–8,12 showed that Wnt/β-catenin signaling has an important role in the induction of brain angiogenesis and BBB differentiation. Activation of Wnt/β-catenin seems to be an early phenomenon in the development of the BBB, with strong activation during embryo development that declines quite rapidly after birth.
The Wnt/β-catenin signaling pathway is active in many tissues in which the Sox proteins are expressed.18,20 In our previous studies,10 we showed that Sox17 is one of the major transcriptional targets of Wnt/β-catenin during vascular development in the retina and of the brain microvasculature. Sox17, in turn, might modulate Wnt/β-catenin transcriptional activity in different ways and through different types of interactions.21,22
In the present study, we show that β-catenin and Sox17 have partially overlapping time courses. Sox17 expression increases during development and after birth, whereas Wnt/β-catenin signaling declines after birth. However, a significant percentage of ECs showed double transcriptional activation of both Sox17 and β-catenin, which supports the concept of cross-talk between these 2 signaling pathways. This was further supported by the observation that β-catenin signaling was significantly reduced in the absence of endothelial Sox17 and that vascular malformations and permeability of the retina and brain vasculature were rescued by upregulation of β-catenin signaling.10
Sox17 exerts a positive role on β-catenin signaling during brain vascularization to maintain the correct vascular morphology and control of permeability. As reported in previous articles (see for review Kormish et al20), Sox transcription factors may act both as activators and inhibitors of canonical Wnt signaling depending on the cell context. In general, Sox genes have Wnt independent roles, but there are several examples where they may be involved in the same biological process such as shown here for BBB differentiation.
As reported in other systems,20 active Wnt signaling may promote the expression of Sox transcription factors, and Sox may cooperatively stimulate β-catenin activity in an autoregulatory amplification. Considering that Sox and Tcf are structurally related and they can bind β-catenin, it is perhaps not surprising that they may, in concert, regulate Wnt signaling.
Taken together, the results presented here support the idea that Sox17 and β-catenin transcription factors act in synergy to maintain the integrity of the brain microcirculation. β-catenin signaling increases Sox17 that, in turn, increases and maintain the correct β-catenin signal. In this particular system the lack of expression of Sox17, which causes BBB disruption, can be compensated by increasing β-catenin signaling.
Other studies have shown that although low, β-catenin signaling is necessary to maintain the integrity of the adult BBB and to prevent pathogenic conditions that increase the risk of hemorrhagic stroke or brain inflammatory reactions.23 It is tempting to speculate that although indispensable for the first steps of brain vascularization and stability, high activation of the canonical Wnt system must be then reduced and maintained at the minimal active levels to prevent undesirable and uncontrolled effects. Consistent with this concept, previous studies have shown that long-lasting high levels of β-catenin signaling in the vasculature causes strong alterations in vascular stability and lumen malformations.9,12
We still do not know the factors that induce the drop in Wnt/β-catenin signaling in the BBB at late embryo development and postnatally. Few inhibitors of this signaling pathway have been identified previously, although among these, Apcdd1 is of particular interest in this context. Apcdd1 is expressed during angiogenesis and barrier organization in the retina vasculature.24,25 In the present study, the RNA-Seq analysis indicated that Apcdd1 is upregulated with a time course compatible with the decline of Wnt/β-catenin signaling. We also observed upregulation of Angptl4, a Wnt signaling antagonist that acts by promoting the internalization of the Wnt coreceptor Lrp6.26 Finally, other potential Wnt/β-catenin inhibitors, that are triggered during BBB development in the absence of Sox17, are members of the so-called noncanonical Wnt signaling pathway. In this group, Wnt5a might act as an inhibitor of canonical Wnt signaling, depending on the cellular context.27,28
We have previously reported10 that Sox17 is required for the arterial differentiation of the vasculature. This effect was mediated by the transcriptional upregulation of Dll4 and Notch4 and the after activation of Notch signaling.
Our previous data were performed mostly on large vessels of mice at different ages and in different organs, including aorta, intestine, bladder, diaphragm, and the retina.
In contrast to large vessels, brain microcirculation presents organ-specific characteristics largely different from the microcirculation of other organs.12,29 In this vascular system, arteries and veins are not clearly detectable. As shown here at different times of BBB development, multiple members of the Notch signaling pathway are upregulated with a time course that paralleled the differentiation of the brain microvasculature.
To test whether Notch could modulate Sox17 activity and vascular permeability in the brain, we analyzed genetically modified mouse strains expressing loss of function or GOF mutations of members of the Notch pathway. Although these mutants presented alterations in the brain vascular development and hemorrhages, Sox17 signaling did not change in the brain microcirculation of all the strains of Notch mutant mice. The β-catenin signaling also remained unaffected by inhibiting Notch signaling. These data strongly suggest that Notch signaling is not upstream of Sox17 in this system.
Furthermore, we analyzed the expression of members of the Notch pathway known to be downstream of Sox17 (Dll4, Notch 1, Notch 4, Hey1, Hey2).10 We could not detect significant modifications in Sox17 deficient mice suggesting that Notch signaling is not downstream of Sox17 in the brain microcirculation. Finally, the brain vasculature phenotypes of Notch GOF and loss of function look largely different from the one induced by the loss of function of Sox17. Overall, these data strongly suggest that in the brain microcirculation the effects of Sox17 are not mediated by Notch activation.
An important observation from the study presented here is that although the brain vessels of adult Sox17iECKO mice do not show increased permeability under resting conditions, they react strongly when an experimental ischemic stroke is applied. This leads to much larger brain lesions, strong increase in modified Neurological Severity Score, edema, and mortality. Therefore, these data underline that Sox17 is not required to maintain the integrity of the brain vasculature in resting conditions, but it is necessary to protect the vessels from pathological ischemic conditions. The analysis of junction morphology of tight and adherens junction components or growth factor production did not show major changes comparing Sox17 deficient and control mice (data not shown). Future and more extensive studies are needed to identify the specific causes of the strong response of Sox17 null adult mice in ischemic conditions.
Two recent studies also reported on the dynamic gene expression changes during vascular development of the retina30 and of the embryo brain vasculature.31
In the first article, the authors identified the critical growth factors responsible for angiogenesis of the retina vasculature, whereas the second study describes the embryonic development of the brain vascular system identifying endothelial-specific signaling pathways implicated in BBB maturation. The work reported here is complementary to these 2 studies and adds other elements to our understanding of the pathways regulating BBB differentiation.
More and more, it seems that the maturation of the BBB is a multidimensional process acting through several mediators that work in well-orchestrated ways. Taken together, the data presented also underline the concept that the development of the BBB occurs as a continuum as shown schematically in Figure 8J and varies with time. The cross-talk between Wnt/β-catenin and Sox17 signaling indicates, for the first time, the fine-tuning between these 2 pathways in BBB differentiation and maintenance.
Acknowledgments
We thank Christopher P. Berrie for editorial assistance; Prof Kari Alitalo, University of Helsinki for his contribution to scientific discussion and for sharing adeno-associated-viral-vectors; Dr Gaetano Finocchiaro and Dr Serena Pellegatta, Fondazione Istituto di Ricerca e Cura a Carattere Scientifico Istituto Neurologico C. Besta (Milan, Italy) for technical advice and for sharing of equipment. We acknowledge the technical help of Andrea Bergamaschi, San Raffaele Scientific Institute, and Vita Salute San Raffaele University (Milan, Italy). M. Corada, F. Orsenigo, and G.P. Bhat conceived the project, design, performed the experiments, and analysed the results; L.L. Conze performed the bioinformatic analyses; F. Breviario performed real-time quantitative polymerase chain reaction and immunohistochemistry analyses; G.V. Beznoussenko and A.A. Mironov performed the electron microscopy experiments; M. Bacigaluppi and G. Martino conducted the stroke experiments; M.E. Pitulescu and R.H. Adams provided Notch mutant mice and contributed to scientific discussion; L. Claesson-Welsh, P. Magnusson, and S.I. Cunha contributed to the scientific design and provided critical discussion and comments to the article; and E. Dejana designed the research and wrote the article.
Sources of Funding
This study was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC; investigator grant [IG] 16683), by AIRC IG2016 18683 and by the Special Programme in Molecular Clinical Oncology 5x1000 to Myeloproliferative Neoplasm Research Venture AIRC (21267), by the European Research Council (project EC-ERC-VEPC (Inherited Disfunctions of Brain Microcirculation, contract 742922), by Initial Training Networks BtRAIN (Brain Barriers Training) grant 675619, by the CARIPLO (Cassa di Risparmio delle Provincie Lombarde) Foundation (2008.2463), by the Special Programme in Molecular Clinical Oncology 5x1000 to AIRC Gruppo Italiano Malattie Mieloproliferative (10005), and by the Swedish Science Council and the Knut and Alice Wallenberg Foundation. M.E. Pitulescu and R.H. Adams were supported by Deutsche Forschungsgemeinschaft grant FOR2325. The sequencing was performed with the SNP&SEQ Technology platform in Uppsala. This Facility is part of the National Genomics Infrastructure Sweden and Science for Life Laboratory. The SNP&SEQ Platform is also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. M. Bacigaluppi was supported by the Ministero della Salute Italiana (Progetto Giovani Ricercatori 58/ GR-2011-02348160).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- β-gal
- β-galactosidase
- BBB
- blood-brain barrier
- ECs
- Endothelial cells
- GOF
- Gain of function
- iECKO
- inducible endothelial cells knock-out
- IgG
- immunoglobulin G
- MCAO
- middle cerebral artery occlusion
- RNA-Seq
- RNA sequencing
- VEGF
- Vascular endothelial growth factor
In November 2018, the average time from submission to first decision for all original research papers submitted to Circulation Research was 12.76 days.
These authors contributed equally to this article.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.118.313316.
Novelty and Significance
What Is Known?
The brain microvasculature (blood-brain barrier, BBB) is a highly specialized area of the vascular tree characterized by a strict control of permeability that protects the central nervous system from toxic agents and inflammatory cells.
Dysfunction of the brain microcirculation leads to pathological conditions, such as hemorrhagic or ischemic stroke.
Wnt/β-catenin transcriptional signaling orchestrates brain vascularization and BBB differentiation.
What New Information Does This Article Contribute?
Wnt/β-catenin signaling is increased during development of the BBB but declines after birth.
While declining, Wnt/β-catenin signaling triggers the activation of another transcription factor called Sox17 (SRY [sex-determining region Y]-box 17) that, in turn, controls BBB permeability.
In the absence of Sox17, BBB permeability is increased but can be restored by increasing Wnt/β-catenin signaling.
The BBB maintains a strict control of permeability limiting the passage of toxic agents and inflammatory cells and regulating the entrance of nutrients to the brain. Pathological modifications of the BBB may cause important neurological disorders.
It has been previously reported that Wnt/β-catenin signaling orchestrates brain vascularization and BBB differentiation in the embryo and newborns. After this period, Wnt signaling declines and other signaling pathways are needed to maintain BBB specialized properties. Here, we show that Wnt activates another transcriptional factor called Sox17. Endothelial-specific loss of Sox17 increases brain vascular permeability. The severity of this effect depends on the degree of BBB maturation with profound effects seen in the embryo but declining in newborns and being undetectable in the adult. Sox17 deficiency exacerbated injury because of ischemic stroke in mice. The lesions were significantly larger compared with control animals, and many typical parameters of ischemic stroke were strongly increased by the lack of Sox17. Although in a resting state the vessels appear normal, in the absence of Sox17, they are more vulnerable to a pathological insult. Our results open new therapeutic opportunities by identifying pharmacological targets downstream of Wnt or Sox17 signaling pathways.
References
- 1.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357:eaal2379. doi: 10.1126/science.aal2379. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 9.Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–949. doi: 10.1016/j.devcel.2010.05.006. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corada M, Orsenigo F, Morini MF, Pitulescu ME, Bhat G, Nyqvist D, Breviario F, Conti V, Briot A, Iruela-Arispe ML, Adams RH, Dejana E. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun. 2013;4:2609. doi: 10.1038/ncomms3609. doi: 10.1038/ncomms3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stan RV, Tse D, Deharvengt SJ, et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203–1218. doi: 10.1016/j.devcel.2012.11.003. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makarov V, Zueva L, Sanabria P, Wessinger WD, Golubeva T, Khmelinskii I, Inyushin M. On the role of the blood vessel endothelial microvilli in the blood flow in small capillaries. J Biophys. 2015;2015:529746. doi: 10.1155/2015/529746. doi: 10.1155/2015/529746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citi S, Pulimeno P, Paschoud S. Cingulin, paracingulin, and PLEKHA7: signaling and cytoskeletal adaptors at the apical junctional complex. Ann N Y Acad Sci. 2012;1257:125–132. doi: 10.1111/j.1749-6632.2012.06506.x. doi: 10.1111/j.1749-6632.2012.06506.x. [DOI] [PubMed] [Google Scholar]
- 17.Chew LJ, Gallo V. The Yin and Yang of Sox proteins: activation and repression in development and disease. J Neurosci Res. 2009;87:3277–3287. doi: 10.1002/jnr.22128. doi: 10.1002/jnr.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engert S, Burtscher I, Liao WP, Dulev S, Schotta G, Lickert H. Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development. 2013;140:3128–3138. doi: 10.1242/dev.088765. doi: 10.1242/dev.088765. [DOI] [PubMed] [Google Scholar]
- 19.Meijer L, Skaltsounis AL, Magiatis P, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Luo M, Xie W, Wells JM, Goodheart MJ, Engelhardt JF. Sox17 modulates Wnt3A/beta-catenin-mediated transcriptional activation of the Lef-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2010;299:L694–L710. doi: 10.1152/ajplung.00140.2010. doi: 10.1152/ajplung.00140.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Göthert JR, Malik AB, Valyi-Nagy T, Zhao YY. Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation. 2016;133:177–186. doi: 10.1161/CIRCULATIONAHA.115.015982. doi: 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzoni J, Smith JR, Shahriar S, Cutforth T, Ceja B, Agalliu D. The Wnt inhibitor Apcdd1 coordinates vascular remodeling and barrier maturation of retinal blood vessels. Neuron. 2017;96:1055.e6–1069.e6. doi: 10.1016/j.neuron.2017.10.025. doi: 10.1016/j.neuron.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimomura Y, Agalliu D, Vonica A, Luria V, Wajid M, Baumer A, Belli S, Petukhova L, Schinzel A, Brivanlou AH, Barres BA, Christiano AM. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2010;464:1043–1047. doi: 10.1038/nature08875. doi: 10.1038/nature08875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirsch N, Chang LS, Koch S, Glinka A, Dolde C, Colozza G, Benitez MDJ, De Robertis EM, Niehrs C. Angiopoietin-like 4 is a Wnt signaling antagonist that promotes LRP6 turnover. Dev Cell. 2017;43:71.e6–82.e6. doi: 10.1016/j.devcel.2017.09.011. doi: 10.1016/j.devcel.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanlandewijck M, He L, Mäe MA, et al. Author correction: a molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;560:E3. doi: 10.1038/s41586-018-0232-x. doi: 10.1038/s41586-018-0232-x. [DOI] [PubMed] [Google Scholar]
- 30.Jeong HW, Hernández-Rodríguez B, Kim J, Kim KP, Enriquez-Gasca R, Yoon J, Adams S, Schöler HR, Vaquerizas JM, Adams RH. Transcriptional regulation of endothelial cell behavior during sprouting angiogenesis. Nat Commun. 2017;8:726. doi: 10.1038/s41467-017-00738-7. doi: 10.1038/s41467-017-00738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hupe M, Li MX, Kneitz S, Davydova D, Yokota C, Kele-Olovsson J, Hot B, Stenman JM, Gessler M. Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci Signal. 2017;10:eaag2476. doi: 10.1126/scisignal.aag2476. doi: 10.1126/scisignal.aag2476. [DOI] [PubMed] [Google Scholar]


