This study aimed to assess the impact of exercise training in patients with lung cancer on several outcomes compared to a control group. Results suggest that exercise programs in patients with lung cancer are a practical and beneficial intervention for enhancing mobility and physical fitness.
Keywords: chemotherapy, exercise training, lung cancer, pulmonary function tests
Abstract
Purpose:
The aim of this study was to perform a randomized trial to assess the impact of exercise training in patients with non–small cell lung cancer during chemotherapy on several outcomes in comparison to a control group (CG).
Methods:
The exercise training group (ETG) consisted of 20 patients and the CG consisted of 10 patients. In the ETG, a 4-wk in-hospital exercise training program was performed in 2-wk cycles interspersed with consecutive rounds of chemotherapy with cytostatic drugs. The exercise training program was individualized and included warm-up, respiratory muscle exercise, training on a cycle ergometer or treadmill, and Nordic walking. CG participants were assessed before and after 6 wk of chemotherapy alone.
Results:
Comparing pre- and post-intervention values, the ETG demonstrated an increase in 6-min walk distance (486 ± 92 vs 531 ± 103 m, P = .01). In a battery of physical performance tests: Up and Go Test (6.3 ± 1.0 vs 6.0 ± 1.1 sec, P = .01); chair stand (13.3 ± 2.8 vs 14.3 ± 3.4 repetitions, P = .001); and arm curl (18.4 ± 3.1 vs 20.4 ± 3.5 repetitions, P = .001) all improved significantly. Spirometry values also improved: FEV1 % predicted (76 ± 16 vs 84 ± 15, P = .01), FVC % predicted (87 ± 14 vs 95 ± 13, P = .01), and FEV1/FVC (73 ± 13% vs 76 ± 12%, P = .04). The exercise training was well tolerated, without any adverse events due to exercise. There were no significant improvements in the CG.
Conclusions:
This study suggests that planned, individualized, and supervised exercise programs in patients with advanced lung cancer during chemotherapy are a practical and beneficial intervention for enhancing mobility and physical fitness.
Cancer is currently the second leading cause of death and disability after cardiovascular disease. Worldwide, more than 1.6 million new cases of lung cancer are diagnosed annually.1,2 Lung cancer is the most common cause of cancer-related death in men and is second in this regard in women. In Europe, the overall 5-yr survival rate among patients with lung cancer is only 10%; among those undergoing surgery, survival is 40%.3
Because of the disease process and side effects of treatment, patients with lung cancer often experience numerous symptoms, including pain, shortness of breath, weakness, weight loss, trouble sleeping, and depression.4,5 Reduction in physical activity is often observed, which contributes to a decrease in fitness and exercise capacity, a decrease in muscle mass and strength, and a decline in pulmonary function and respiratory muscle strength.6–8
It has been generally observed that patients with lung cancer can safely participate in physical activity at any stage of the disease and its treatment.9–11 Additionally, evidence has been presented that physical activity reduces the risk of several forms of cancer12 and the risk of cancer recurrence by up to 40%.13 Physical training may extend the life of patients, prevent the occurrence of other chronic diseases, and reduce the symptoms of cancer.12,14 Statements of the American Thoracic Society and the European Respiratory Society conclude that pulmonary rehabilitation (PR) reduces dyspnea and improves both exercise capacity and quality of life.15
The main objective of oncology rehabilitation is to promote functional independence and to facilitate the patient's adaptation to the changing conditions of life as a result of the disease and its treatments.16 Physical training in oncology is generally supervised; after an initial assessment, suitable exercises and tasks of movement are selected to create an individual rehabilitation program with due regard to the disease stage, the type of therapy, and the patient's motivation.17,18
The aim of this study was to assess the impact of exercise training in patients with non–small cell lung cancer during chemotherapy on several outcomes in comparison to an untrained, sedentary control group (CG).
METHODS
PARTICIPANTS
The research study took place at the Independent Public Clinical Hospital No. 3 of the Medical University of Silesia in Katowice, from October 2012 to February 2015. The study was approved by the Ethics Committee of Silesian Medical University (KNW/0022/KB1/184a/I/11/12). All patients signed a written consent to participate in the research. The research was registered in the Australian New Zealand Clinical Trials Registry (ACTRN12616001512415).
The study included 40 patients diagnosed with non–small cell lung cancer (NSCLC) at stages IIIB or IV, who were disqualified from surgery. The diagnosis was established within 6 wk prior to enrollment and was confirmed by histology. Patients were included in the study if they had the ability to perform the 6-min walk test (6MWT), were World Health Organization performance status 0-1, were able to complete questionnaires, and had willingness to participate in an exercise training program. Patients were deemed not eligible if they had uncontrolled hypertension or unstable coronary artery disease, anemia (hemoglobin <10 g/dL), severe osteoarthritis, or bone or central nervous system metastases.
Participants were randomly assigned, with a 2:1 assignment, to either the exercise training group (ETG) or the CG (utilizing computer-generated random numbers): 26 patients were assigned to the ETG and 14 patients were assigned to the CG. CG participants were assessed before and after 6 wk of chemotherapy alone. In the ETG, a 4-wk in-hospital exercise training program was used on the basis of rehabilitation program in chronic obstructive pulmonary disease (COPD),19 which was performed in 2-wk cycles interspersed with consecutive rounds of chemotherapy: cisplatidiam 80 mg/m2 on the first day and vinorelbine 25 mg/m2 on the first and eighth days (patients in the CG received an identical chemotherapy regimen). This exercise program was conducted in the hospital under the supervision of a physiotherapist and contained elements of endurance training, breathing exercises, weight training, and fitness exercise.
The study was scheduled by a physiotherapist from the Institute of Physiotherapy, not related to the hospital, who generated the random allocation sequence using the hospital chart number as the identifier for each participating patient. The patients were enrolled, assigned, and supervised during the hospital stay by the doctors working in hospital departments who were not related to the study. Outcomes were obtained using chart review by a physiotherapist trained in the abstraction of the desired outcomes from the medical records who was blinded to the oncology treatment.
OUTCOME MEASURES
The study flow chart is presented in Figure 1. Potentially eligible participants were provided with a thorough review of the study by the study coordinator and willingness to participate was assessed. After obtaining consent, medical records were reviewed to determine eligibility. Eligible patients were randomized to either the ETG or CG. At baseline (Day 1) and at the end of the study (Day 42), the following factors were assessed in both groups: (1) exercise performance was assessed by the 6MWT distance, performed according to the American Thoracic Society 2002 guidelines20; (2) spirometry was performed using the Jaeger-Masterlab (Erich–Jaeger) to assess forced expiratory volume in 1 sec (FEV1), forced vital capacity (FVC), and the ratio of the 2 measures (FEV1/FVC) according to European Respiratory Society recommendations,21 and these values were compared with normal values from the European Coal and Steel Community22; (3) dyspnea was evaluated using the modified Medical Research Council (mMRC) questionnaire,23 Baseline Dyspnea Index (BDI),24 and Borg dyspnea scale25; (4) functional fitness was assessed with the Fullerton test,26 which is composed of 6 components—chair stand, arm curl, chair sit-and-reach, up and go, back scratch, and 6MWT.
Figure 1.
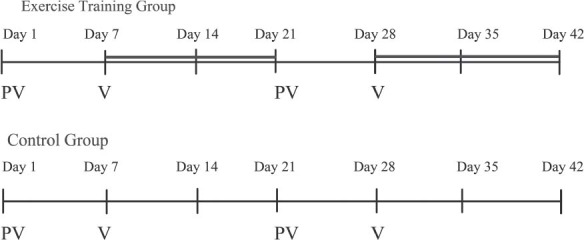
Flow of study interventions. Day 1: eligibility criteria, informed consent, baseline assessments: physical measures, lung function testing, and evaluation of dyspnea (MRC, BDI, Borg Scale), randomization, qualification for the exercise training program; afternoon: admission for chemotherapy. Day 7-20: exercise training program for the ETG (thick solid line). Day 21: admission for chemotherapy. Day 28-41: exercise training program for the ETG. Day 42, end of interventions: follow-up assessment includes physical measures, lung function testing, and evaluation of dyspnea (mMRC, BDI, Borg scale). Abbreviations: BDI, Baseline Dyspnea Index; ETG, exercise training group; mMRC, modified Medical Research Council questionnaire; PV, platidiam-vinorelbine chemotherapy treatment; V, vinorelbine chemotherapy treatment.
INTERVENTIONS
In the CG, there was a 6-wk period between the initial and final assessments; during wk 1 and wk 4, patients underwent scheduled in-hospital chemotherapy. No exercise intervention was performed in this group.
Based on the initial 6MWT and spirometry results, patients in the ETG participated in two 2-wk inpatient exercise training programs, supervised by a certified physiotherapist. In Poland, it is common practice for patients with lung cancer to be admitted to the hospital for 2 wk of in-hospital rehabilitation. Between these 2 exercise training periods of time, patients underwent scheduled in-hospital chemotherapy.
Patients enrolled in supervised exercise training were assigned different intensities based on initial 6MWT and spirometry assessments. Patients participated in exercise sessions 5 times/wk. Sessions consisted of (1) 30 min of fitness and respiratory exercises; (2) specific respiratory exercises for 30 min (relaxation exercises for breathing muscles, strengthening exercises of the diaphragm with resistance, exercises to increase costal or chest breathing, prolonged exhalation exercise, and chest percussion); (3) training on a cycle ergometer or treadmill for 20 to 30 min at an intensity of 30% to 80% of peak work rate according to individual tolerance27; (4) resistance exercise intensity 40% to 70% of the 1 repetition maximum (1RM)19; (5) Nordic walking for 45 min (depending on weather and health condition of the patient); and (6) relaxation training. Heart rate and oxygen saturation were monitored continuously throughout the sessions by pulse oximetry. Exercise blood pressure was assessed intermittently.
Exercise training sessions were withheld in the following situations related to cancer chemotherapy: 24 hr after chemotherapy; anemia (hemoglobin <8 g/L); neutropenia (white blood cell count <0.5 × 109 cells/μL); thrombocytopenia (platelet count <50 × 109μL); participants complaining of nausea, vomiting, fatigue, disturbances of orientation, visual disturbances, weakness, and muscle or bone pain within the past 24 hr.
STATISTICAL ANALYSIS
The normality of the data was evaluated using the Shapiro-Wilk test. The Student t test was used for normally distributed data and equal variances, and the Wilcoxon signed rank test was used for non-normally distributed data with a statistically significant P value of < .05. To make intergroup comparisons of changes, the Mann-Whitney U test was adopted. The effect sizes (the Cohen d) of the differences were calculated (small ≤ 0.5, medium if ≤ 0.8, and large > 0.8). To evaluate the changes for each of the measured parameters before and after intervention in both groups, the relative (percent) change rate was used.
Prior to the start of the study, a statistical power analysis was carried out to calculate sample size. This procedure determined that 19 participants were needed to achieve a statistical power of 90% to detect a medium effect (d = 0.796) in the principal variable to be compared (Fullerton Up and Go) when assessed by a 2-tailed t test for dependent variables with a level of significance of 5%. Finally, we selected 20 participants to ensure account for dropouts. The assessment was conducted by 2-sided tests. All calculations were performed with Statistica software v.12 (Dell).
RESULTS
The characteristics of the patients in both the ETG and the CG were closely matched in terms of gender, age, weight, and height (Table 1). As indicated in the Consort diagram (Figure 2), of the 26 patients randomized to the ECG, 20 completed the 4-wk training program and underwent the final assessment. Of the 14 participants enrolled in the CG, 10 completed the study. Four in the ETG and 3 in the CG did not complete the study because of chemotherapy-related events (anemia, myalgia, asthenia, pneumonia, or renal insufficiency); 2 in the ETG did not complete the study (1 death; 1 not motivated to continue) after completing 2 wk of training. No adverse events related to exercise training were noted in the ETG group.
Table 1. Characteristics Participants Completing the Studya.
| Exercise Training Group (n = 20) | Control Group (n = 10) | |
|---|---|---|
| Age, yr | 59.1 ± 6.8 | 61.3 ± 8.8 |
| Male | 18 ± 90 | 9 ± 90 |
| BMI, kg/m2 | 25.0 ± 4.1 | 26.2 ± 2.9 |
| Comorbidities | ||
| COPD | 12 (60) | 5 (50) |
| Coronary artery disease history | 3 (15) | 1 (10) |
| Heart failure history | 0 (0) | 0 (0) |
| Diabetes mellitus | 6 (30) | 3 (30) |
| Smoking history | ||
| Current | 0 (0) | 0 (0) |
| Former | 15 (75) | 7 (70) |
| Never | 5 (25) | 3 (30) |
| WHO performance status | ||
| 0 | 3 (15) | 1 (10) |
| 1 | 17 (85) | 9 (90) |
| Diagnosis | ||
| Adenocarcinoma | 14 (70) | 7 (70) |
| Squamous cell carcinoma | 6 (30) | 3 (30) |
| TNM status | ||
| T2N2M0 | 16 (80) | 8 (80) |
| T2N2M1 | 4 (20) | 2 (20) |
| Tumor stage | ||
| IIIB | 16 (80) | 8 (80) |
| IV | 4 (20) | 2 (20) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; TNM, tumor node metastasis; WHO, World Health Organization.
aData are reported as mean ± standard deviation or number (%).
Figure 2.
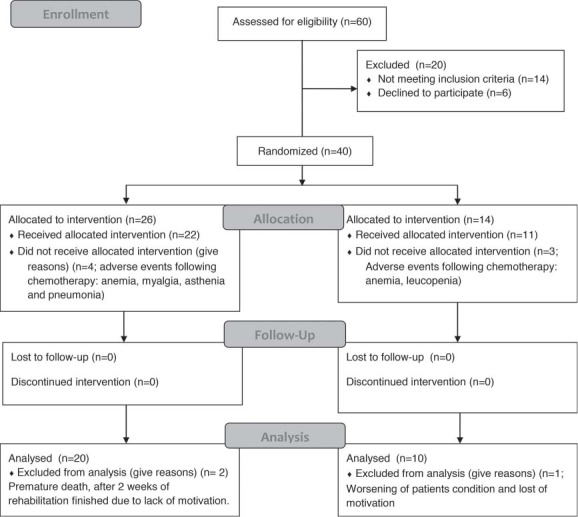
Consolidated Standards for Reporting of Trials (CONSORT) flow diagram.
Table 2 summarizes the Fullerton test results. In the ETG, values for the chair sit and reach (P = .45, effect size 0.04) and back scratch (P = .39, effect size 0.04) tended to improve, but these changes did not reach statistical significance. The 6MWT distance improved significantly in the ETG (P = .01, effect size 0.47). In the Up and Go Test, there was a statistically significant reduction in the time needed to complete the task (P = .01, effect size 0.23). In the chair stand (P < .001, effect size 0.33) and arm curl (P = .001, effect size 0.61), there was a statistically significant improvement in repetitions.
Table 2. Baseline and 6-wk Fullerton Test Results for Both Groupsa.
| Exercise Training Group | Control Group | Between-Group Comparison, % Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before PR | After PR | P Value | Cohen's d | Baseline | 6-wk Follow-up | P Value | Cohen's d | ETG CG | P Valueb | |
| Arm curl, repetitions | 18.4 ± 3.1 | 20.4 ± 3.5 | .001 | 0.61 | 15.2 ± 3.0 | 16.2 ± 3.3 | .06 | 0.33 | 11.8 ± 13.4 6.9 ± 9.7 |
.36 |
| Chair stand, repetitions | 13.3 ± 2.8 | 14.3 ± 3.4 | .01 | 0.33 | 11 ± 1.8 | 11.2 ± 1.5 | .34 | 0.12 | 7.2 ± 9.2 2.4 ± 6.0 |
.17 |
| Chair sit and reach, cm | 1.9 ± 6.6 | 2.2 ± 6.2 | .45 | 0.04 | −3.3 ± 3.6 | −4.4 ± 3.4 | .02 | 0.33 | −3.3 ± 15.1 −14.7 ± 105.1 |
.20 |
| Up and go, sec | 6.3 ± 1.0 | 6.0 ± 1.1 | .01 | 0.23 | 6.0 ± 0.4 | 6.3 ± 0.8 | .046 | 0.54 | 3.8 ± 6.4 −5.3 ± 7.3 |
.01 |
| Back scratch, cm | − 1.8 ± 7.1 | −1.5 ± 7.2 | .39 | 0.04 | −9.1 ± 6.1 | −8.1 ± 4.6 | .36 | 0.2 | −5.0 ± 45.9 3.8 ± 20.6 |
.52 |
| 6MWT, m | 486 ± 92 | 531 ± 103 | .01 | 0.47 | 487 ± 100 | 490 ± 124 | .92 | 0.02 | 10.4 ± 17.4 0.3 ± 15.4 |
.09 |
Abbreviations: CG, control group; ETG, exercise training group; PR, rehabilitation program; 6MWT, 6-min walk test.
aData reported as mean ± standard deviation.
bMann-Whitney U test was used the calculate P values.
In the CG, none of the changes in the component tests showed a statistically significant improvement. In the chair sit and reach (P = .02, effect size 0.33) and up and go (P = .046, effect size 0.54), there was a statistically significant decrease in performance. In between-group comparisons, the Up and Go Test differences achieved statistical significance; the 6MWT distance differences approached significance (Table 2).
In the ETG, a statistically significant improvement in FEV1 % pred. (P = .01, effect size 0.56), FVC % pred. (P = .01, effect size 0.64), and FEV1/FVC (P = .04, effect size 0.29) were observed (Table 3). Significant changes were seen in the CG only in the FEV1/FVC (P = .03, effect size 0.79) (Table 3).
Table 3. Baseline and 6-wk Spirometry Test Results for Both Groupsa.
| Exercise Training Group | Control Group | Between-Group Comparison, % Change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before PR | After PR | P Value | Cohen's d | Baseline | 6-wk Follow-up | P Value | Cohen's d | ETG | CG | P Valueb | |
| FEV1, % pred | 76 ± 16 | 84 ± 15 | .01 | 0.56 | 70 ± 23 | 68 ± 24 | .68 | 0.11 | 14 ± 21 | −0.9 ± 15.1 | .08 |
| FVC, % pred | 87 ± 14 | 95 ± 13 | .01 | 0.64 | 80 ± 21 | 80 ± 22 | .83 | 0.04 | 9 ± 13.9 | 0.4 ± 10.1 | .06 |
| FEV1/FVC, % | 70 ± 13 | 76 ± 12 | .04 | 0.29 | 82 ± 17 | 71 ± 12 | .03 | 0.79 | 9,3 ± 15.8 | −9,5 ± 11.7 | .01 |
Abbreviations: FEV1, forced expiratory volume in 1 sec; FEV1/FVC, forced expiratory volume in 1 sec as a percent of vital capacity; FVC, forced vital capacity; PR, pulmonary rehabilitation.
aData are reported as mean ± standard deviation.
bMann-Whitney U test was used to calculate P values.
The mean values of the mMRC questionnaire (P = .18, effect size 0) and BDI scale in the ETG were not significantly improved (P = .83, effect size 0). The Borg Dyspnea Scale (P = .04, effect size 0.12) showed a significant improvement in the perception of dyspnea in the ETG. In the CG, the mMRC (P = 1, effect size 0.38), BDI (P = .72, effect size 0.04), and the Borg dyspnea scale (P = .42, effect size 0.83) showed nonsignificant decreases (Table 4).
Table 4. Evaluation of Dyspneaa.
| Exercise Training Group | Control Group | Between-Group Comparison, % Change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before PR | After PR | P Value | Cohen's d | Baseline | 6-wk Follow-up | P Value | Cohen's d | ETG | CG | P Valueb | |
| mMRC | 0.7 ± 0.9 | 0.7 ± 1.0 | .18 | 0 | 0.6 ± 1.0 | 0.3 ± 0.7 | 1 | 0.38 | 0.7 ± 0.9 | 0.7 ± 1.0 | .18 |
| FI | 3.2 ± 0.8 | 3.1 ± 0.9 | 1 | 0.06 | 3.3 ± 0.9 | 3.3 ± 0.8 | .72 | 0 | 3.2 ± 0.8 | 3.1 ± 0.9 | 1 |
| MT | 3.3 ± 0.7 | 3.3 ± 0.9 | 1 | 0 | 3.3 ± 0.9 | 3.3 ± 0.8 | 1 | 0 | 3.3 ± 0.7 | 3.3 ± 0.9 | 1 |
| ME | 3.1 ± 0.8 | 3.1 ± 0.9 | .80 | 0 | 3.3 ± 0.8 | 3.2 ± 0.9 | .96 | 0.12 | 3.1 ± 0.8 | 3.1 ± 0.9 | .80 |
| BDI | 9.5 ± 2.1 | 9.5 ± 2.4 | .83 | 0 | 9.9 ± 2.6 | 9.8 ± 2.4 | .72 | 0.04 | 9.5 ± 2.1 | 9.5 ± 2.4 | .83 |
| Borg | 1.7 ± 2.2 | 1.5 ± 2.1 | .04 | 0.12 | 1.1 ± 1.0 | 2.6 ± 2.5 | .42 | 0.83 | 1.7 ± 2.2 | 1.5 ± 2.1 | .04 |
Abbreviations: BDI, Baseline Dyspnea Index (BDI=FI+MT+ME); Borg, Borg Dyspnea Scale; FI, functional impairment; ME, magnitude of effort; mMRC, modified Medical Research Council Questionnaire; MT, magnitude of task; PR, pulmonary rehabilitation.
aData are reported as mean ± standard deviation.
bMann-Whitney U test.
DISCUSSION
This study is among the few studies evaluating the effects of an exercise training program in patients with advanced NSCLC during chemotherapy.28 In the last decade, growing interest has been noted in research on different forms of PR, including physiotherapy, in patients with lung cancer. Most of these studies have focused on perioperative physiotherapy, including the Cochrane database review.29–32
Chronic obstructive pulmonary disease often coexists with lung cancer.33 A PR program designed for patients with COPD19 was adopted in this study. Research conducted over many years in the field of physiotherapy in COPD clearly shows its positive impact.12,19,34–38
Several systematic reviews conclude that exercise training is safe in lung cancer and even increases functional capacity,28,39–41 but only a few studies in patients with newly diagnosed advanced lung cancer during chemotherapy were evaluated.17,42–47 From 2004 to 2007, Temel et al42 enrolled 25 patients with NSCLC, of whom only 11 (44%) completed the entire rehabilitation program. The authors used aerobic training and weight training for 90 to 120 min twice weekly for 8 wk. The authors demonstrated nonsignificant increases in the average 6MWT distance and muscle strength.42 A similar study was performed by a Polish group of investigators assessing the utility of a PR program on mobility, dyspnea, and lung function in patients with advanced lung cancer during chemotherapy. The study group consisted of 20 patients (a study group of 12, a CG of 8). Only 12 underwent the final assessment (60%). The 8-wk inpatient rehabilitation program was based on Nordic walking exercise training and respiratory muscle training 5 times/wk. The authors observed an increase in the average distance in the 6MWT, FEV1, and perception of dyspnea with the MRC questionnaire.43 Hwang et al47 enrolled 24 patients with NSCLC (stages IIIa-IV), who were randomly assigned to either the CG or the exercise group. The exercise training consisted of treadmill or cycling ergometer performed 3 times/wk for 24 sessions; each exercise session was 30 to 40 min in length. The authors demonstrated a significant increase in the peak oxygen uptake and % predicted peak oxygen uptake and decreased dyspnea.47
Tarumi et al48 investigated the changes in respiratory function because of a perioperative intensive PR program in patients with NSCLC who underwent induction chemoradiotherapy. A group of 82 patients with NSCLC IIB-IV stage underwent a PR program for an average of 10 wk. Significant increases were observed in FEV1 and FVC.48
Compared to previous studies, 26 patients were randomized to the intervention group, and 20 underwent the final assessment (77%) in our study. Our results suggest good tolerance of exercise training by patients with lung cancer. The favorable outcomes indicated by our results could be because of the exercise training program at the study hospital, which consisted of high-intensity exercise (activities that occurred 5 times/wk; daily patient participation ranged from 120 to 170 min, depending on the model of rehabilitation used) over a shorter duration (the exercise training program lasted 4 wk). In our study, the statistically significant improvement in the 6MWT distance is the result of the rehabilitation adhering to the recommendations of the American College of Sport Medicine,19 which posits that aerobic training should be carried out 3 to 5 times/wk to have a positive impact on improving physical capacity. The results of our study correspond to the results of Licker et al,49 who investigated the effects of high-intensity interval training in patients awaiting lung cancer surgery. Those results showed a significant improvement in aerobic performance expressed as 6MWT distance.49 Appropriate qualifications for rehabilitation, including a functional assessment of the patient, contributed to the proper selection of load and intensity of training, and thereby to the choice of the appropriate model of rehabilitation according to the individual abilities of the patient, which seems to be lacking in previous studies.
In the assessment of the results of exercise training in patients with lung cancer, the Fullerton test was used because of the advanced age of the patients. A statistically significant improvement was observed in the Up and Go Test, which is also commonly used for patients with COPD to assess dynamic balance and coordination, directly affecting the risk of falls.50–52 This is important in patients with lung cancer treated with chemotherapy, in whom balance problems are often observed as a side effect of chemotherapy. A statistically significant improvement was also observed in the arm curl and chair stand tests. This is consistent with the study by Quist et al,44 which also reported a significant improvement in muscle strength in a nonrandomized study with 71 patients with advanced-stage lung cancer (NSCLC, stage IIIb-IV). These findings also correspond with the results described by Adamsen et al,53 Salhi et al,54 and Kuehr et al.55 These results are in contrast to those of previous studies reporting no change in muscle strength in patients with NSCLC. In a study by Temel et al,42 which included 25 patients with advanced NSCLC who underwent a structured exercise program, the authors found no deterioration in their 6MWT or muscle strength. Arbane et al30 proposed a 12-wk rehabilitation program for patients undergoing lung resection for NSCLC. Post-operative data showed a change in quadriceps strength from baseline to 12 wk, but this was not statistically significant.30
We observed a deterioration in functional fitness within the CG. A significant decrease in the Up and Go Test and the Chair Sit and Reach Test compared with baseline was observed.
This study has revealed that exercise training is beneficial for lung capacity. The patients in the exercise group displayed significant improvements in FEV1, FVC, and FEV1/FVC. In contrast to our data, Andersen et al46 reported no change in FEV1 and FEV1 % predicted after 7 wk of twice weekly training in 21 patients with lung cancer.
Decreased dyspnea was also noted in the ETG; however, a significant improvement in dyspnea was observed only in the Borg scale in contrast to the CG, who showed a nonsignificant increase of dyspnea.
The strength of our study was the inclusion of a CG. A limitation of this study was the relatively small number of people enrolled and the lack of biochemical marker measurements in the ETG compared with the CG. Indeed, we cannot exclude the effect of exercise training on reducing fatigue symptoms and increasing exercise tolerance. Our results require confirmation by a larger multicenter study. The individual exercise training program could be introduced in the first phase of treatment in patients with lung cancer. The results of this study on the role of exercise training in reducing fatigue symptoms show that this method could be an important part of the rehabilitation program in patients with advanced lung cancer during chemotherapy.
CONCLUSION
This study suggests that planned, individualized, and supervised exercise programs in patients with advanced lung cancer during chemotherapy are a practical and beneficial intervention for enhancing mobility and physical fitness.
Footnotes
ORCID ID numbers: Dr Rutkowska (0000-0002-2456-8499); Dr Jastrzebski (0000-0002-8598-1930); Dr Rutkowski (0000-0002-8504-0129); and Dr Zebrowska (0000-0001-7446-528X).
All authors declare no conflicts of interest.
REFERENCES
- 1.Cancer Research UK. Lung cancer—UK incidence statistics. January 2012. http://www.cancerresearchuk.org/. Accessed September 1, 2016.
- 2.Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 3.Shokouh Z, Maryammosadate M. Survival of patients with lung cancer. Asian Pac J Cancer Prev. 2012;13:4387–4391. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Crowley JJ, IASLC International Staging Project. The International Association for the Study of Lung Cancer International Staging Project on Lung Cancer. J Thorac Oncol. 2006;1:281–286. [Google Scholar]
- 5.Davis KM, Kelly SP, Luta G, Tomko C, Miller AB, Taylor KL. The association of long-term treatment-related side effects with cancer-specific and general quality of life among prostate cancer survivors. Urology. 2014;84(2):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracos V, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;4:1133–1137. [DOI] [PubMed] [Google Scholar]
- 7.Vainshelboim B, Fox BD, Saute M, et al. Limitations in exercise and functional capacity in long-term postpneumonectomy patients. J Cardiopulm Rehabil Prev. 2015;35(1):56–64. [DOI] [PubMed] [Google Scholar]
- 8.Shannon VR. Role of pulmonary rehabilitation in the management of patients with lung cancer. Curr Opin Pulm Med. 2010;16(4):334–339. [DOI] [PubMed] [Google Scholar]
- 9.Janssen SM, Abbink JJ, Lindeboom R, Vliet Vlieland TP. Outcomes of pulmonary rehabilitation after treatment for non-small cell lung cancer stages I to IIIa: an observational study. J Cardiopulm Rehabil Prev. 2017;37(1):65–71. [DOI] [PubMed] [Google Scholar]
- 10.Spruit MA, Janssen PP, Willemsen SC, et al. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. 2006;52:257–260. [DOI] [PubMed] [Google Scholar]
- 11.Jastrzebski D, Zebrowska A, Rutkowski S, et al. Pulmonary rehabilitation with a stabilometric platform after thoracic surgery: A preliminary report. J Hum Kinet. 2018;65:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajarajeswaran P, Vishmpriya R. Exercise in cancer. Indian J Med Pediatr Oncol. 2009;30:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florian J, Rubin A, Mattiello R, Fontoura FF, Camargo Jde J, Teixeira PJ. Impact of pulmonary rehabilitation on quality of life and functional capacity in patients on waiting lists for lung transplantation. J Bras Pneumol. 2013;39(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spruit M, Singh S, Garvey C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;8:1011–1027. [DOI] [PubMed] [Google Scholar]
- 16.Nwosu A, Bayly J, Gaunt K, Mayland C. Lung cancer and rehabilitation—what are the barriers? Results of a questionnaire survey and the development of regional lung cancer rehabilitation standards and guidelines. Support Care Cancer. 2012;20:3247–3254. [DOI] [PubMed] [Google Scholar]
- 17.Büntzel J, Kusterer I, Rudolph Y, Kubin T, Micke O, Hübner J. Cancer patients' knowledge and acceptance of physical activities for rehabilitation. In Vivo. 2017;31(6):1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolan LB, Barry D, Petrella T, et al. The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. J Cardiopulm Rehabil Prev. 2017;38(4):246–252. [DOI] [PubMed] [Google Scholar]
- 19.Garvey C, Bayles MP, Hamm LF, et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: an Official Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(2):75–83. [DOI] [PubMed] [Google Scholar]
- 20.ATS statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2016;193(10):1185. [DOI] [PubMed] [Google Scholar]
- 21.Miller M, Hankinson J, Brusasco V, et al. ATS/ERS task force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 22.Quanjer P, Tammeling G, Cotes J, Pedersen O, Peslin R, Yernault J. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 23.Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahler D, Weinberg D, Wells C, Feinstein A. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. [DOI] [PubMed] [Google Scholar]
- 25.Kendrick K, Baxi S, Smith R. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26(3):216–222. [DOI] [PubMed] [Google Scholar]
- 26.Hesseberg K, Bentzen H, Bergland A. Reliability of the senior fitness test in community-dwelling older people with cognitive impairment. Physiother Res Int. 2015;20(1):37–44. [DOI] [PubMed] [Google Scholar]
- 27.Hill K, Jenkins SC, Cecins N, Philippe D, Hillman D, Eastwood P. Estimating maximum work rate during incremental cycle ergometry testing from six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2008;89:1782–1787. [DOI] [PubMed] [Google Scholar]
- 28.Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2011;72:139–153. [DOI] [PubMed] [Google Scholar]
- 29.Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non-small cell lung cancer—a Cochrane systematic review. Cancer Treat Rev. 2014;40(4):585–594. [DOI] [PubMed] [Google Scholar]
- 30.Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer. 2011;71(2):229–234. [DOI] [PubMed] [Google Scholar]
- 31.Crandall K, Maguire R, Campbell A, Kearney N. Exercise intervention for patients surgically treated for non-small cell lung cancer (NSCLC): a systematic review. Surg Oncol. 2014;23(1):17–30. [DOI] [PubMed] [Google Scholar]
- 32.Tokunaga Y, Tarumi S, Yokomise H. Can pulmonary rehabilitation during preoperative chemoradiotherapy for non-small cell lung cancer improve the respiratory function? Kyobu Geka. 2016;69(1):47–52. [PubMed] [Google Scholar]
- 33.Logathan R, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in woman compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129:1305–1312. [DOI] [PubMed] [Google Scholar]
- 34.Yan J, Guo Y, Yao H, Wang Z, Hu J, Yan J. Effects of Tai Chi in patients with chronic obstructive pulmonary disease: preliminary evidence. PLoS One. 2013;8(4):e61806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Gestel A, Clarenbach C, Stöwhas A, et al. Predicting daily physical activity in patients with chronic obstructive pulmonary disease. PLoS One. 2012;7(11):e48081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro A, Porto E, Iamonti V, de Souza G, Nascimento O, Jardim J. Oxygen and ventilatory output during several activities of daily living performed by COPD patients stratified according to disease severity. PLoS One. 2013;8(11):e79727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko FW, Dai DL, Ngai J, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology. 2011;16(4):617–624. [DOI] [PubMed] [Google Scholar]
- 38.Revitt O, Sewell L, Morgan MD, Steiner M, Singh S. Short outpatient pulmonary rehabilitation programme reduces readmission following a hospitalization for an exacerbation of chronic obstructive pulmonary disease. Respirology. 2013;18(7):1063–1068. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Larrad A, Lascurain-Aguirrebena I, Abecia-Inchaurregui LC, Seco J. Perioperative physiotherapy in patients undergoing lung cancer resection. Interact CardioVasc Thorac Surg. 2014;19:269–281. [DOI] [PubMed] [Google Scholar]
- 40.Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact CardioVasc Thorac Surg. 2011;13:300–302. [DOI] [PubMed] [Google Scholar]
- 41.Pouwels S, Fiddelaers J, Teijink JA, Woorst JF, Siebenga J, Smeenk FW. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med. 2015:109(12):1495–1504. [DOI] [PubMed] [Google Scholar]
- 42.Temel TS, Greer JA, Goldberg S, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(5):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jastrzębski D, Maksymiak M, Kostorz S, et al. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. Adv Exp Med Biol. 2015;861:57–64. [DOI] [PubMed] [Google Scholar]
- 44.Quist M, Adamsen L, Rørth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015;14(4):341–349. [DOI] [PubMed] [Google Scholar]
- 45.Riesenberg H, Lubbe AS. In-patient rehabilitation of lung cancer patients—a prospective study. Support Care Cancer. 2010;18(7):877–882. [DOI] [PubMed] [Google Scholar]
- 46.Andersen AH, Vinther A, Poulsen LL, Mellemgaard A. Do patients with lung cancer benefit from physical exercise? Acta Oncol. 2011;50(2):307–313. [DOI] [PubMed] [Google Scholar]
- 47.Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169–3177. [DOI] [PubMed] [Google Scholar]
- 48.Tarumi S, Yokomise H, Gotoh M, et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg. 2015;149(2):569–573. [DOI] [PubMed] [Google Scholar]
- 49.Licker M, Karenovics W, Diaper J, et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12(2):323–333. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira C, Lee A, Grange C, Miller K, Irving L, Denehy L. Postural control and fear of falling assessment in people with chronic obstructive pulmonary disease: a systematic review of instruments, international classification of functioning, disability and health linkage and measurement properties. Arch Phys Med Rehabil. 2013;94:1784–1799. [DOI] [PubMed] [Google Scholar]
- 51.Tudorache E, Oancea C, Avram C, Fira-Mladinescu O, Petrescu L, Timar B. Balance impairment and systemic inflammation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1847–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bisca G, Morita A, Hernandes N, Probst V, Pitta F. Simple lower limb functional tests in patient with chronic obstructive pulmonary disease: a systematic review. Arch Phys Med Rehabil. 2015;96(12):221–230. [DOI] [PubMed] [Google Scholar]
- 53.Adamsen L, Stage M, Laursen J, Rorth M, Quist M. Exercise and relaxation intervention for patients with advanced lung cancer: a qualitative feasibility study. Scand J Med Sci Sports. 2012;22:804–815. [DOI] [PubMed] [Google Scholar]
- 54.Salhi B, Huysse W, Van Maele G, Surmont VF, Derom E, van Meerbeeck JP. The effect of radical treatment and rehabilitation on muscle mass and strength: a randomized trial in stages I-III lung cancer patients. Lung Cancer. 2014;84(1):56–61. [DOI] [PubMed] [Google Scholar]
- 55.Kuehr L, Wiskemann J, Abel U, Ulrich CM, Hummler S, Thomas M. Exercise in patients with non-small cell lung cancer. Med Sci Sports Exerc. 2014;46(4):656–663. [DOI] [PubMed] [Google Scholar]


