Abstract
Purpose
To report the technical aspects, systemic, and ocular safety of a novel, low-cost, wide-field, infant retinal camera for use on premature infants.
Methods
The device, the “3nethra Neo” (Neo) is a 120° portable, contact, wide-field, unibody camera, with a CMOS sensor (2040 × 2040 resolution) and a warm light-emitting diode (LED) illumination source. The Neo was used to image 140 awake, preterm infants between postmenstrual age (PMA) of 28 to 37 weeks, undergoing retinopathy of prematurity (ROP) screening. Baseline, ‘during procedure', at 5 minutes, and for 60 minutes postprocedure, readings of oxygen saturation and heart rate were recorded. The device design, optics, illumination, and software specifications were compared with the RetCam 3.
Results
Study defined bradycardia (9 infants, 6.4%), tachycardia (3 infants, 2.1%), and hypoxia (2 infants, 1.4%) were observed but there were no clinically significant systemic changes that required intervention during or following any of the study time intervals. There was a transient increase in heart rate by 9.68 (7.53–11.83; P < 0.0001) and marginal decrease in oxygen saturation (−1.94 [−1.60 to −2.28], P < 0.0001), which started to return to baseline 5 minutes after the procedure. Transient redness was seen in two eyes (0.7%) of two infants. No other ocular adverse effects were observed.
Conclusions
The Neo is easy to use in preterm infants and being compact was readily portable. There were no significant ocular or systemic adverse effects, potentially allowing it to be a viable low-cost device for ROP screening in low resource settings.
Translational Relevance
The camera provides a safe and affordable alternative to image the retina of infants by using novel illumination and lens mechanics and has the potential of worldwide acceptance.
Keywords: retinopathy of prematurity, RetCam, Neo, wide field imaging, low cost
Introduction
Every year, 15 million babies are born premature globally, with India and China collectively contributing to a third of that number.1 With improving neonatal care, survival of these infants is increasing, and with it, the burden of retinopathy of prematurity (ROP) management. In India, with a greater proportion of these premature deliveries occurring in the remote and rural areas, the unmet challenge of unscreened ROP has added to the burden of preventable childhood blindness.2 The situation is similar in many of the middle-income countries in Asia, Eastern Europe, and Latin America, leading to the global ‘third epidemic' of this disease.3,4
In India, the awareness about ROP has recently increased. A recent judgment by the Supreme Court of India against a government managed, tertiary-care hospital for failing to provide a female child timely ROP screening awarded the family a compensation of USD 300,000.5 This landmark judgment ruled that ROP screening is ‘standard of care' and part of the ‘essential services' to be provided to ‘at risk' premature infants. Unfortunately, currently, neither the private nor the public health care delivery systems are capable of handling the burden of screening.6,7 With over 3.5 million infants born premature annually,1 and less than 100 ROP specialists across the nation, screening for ROP is an important unmet public health challenge.8
The traditional gold standard of ROP screening using the binocular indirect ophthalmoscope has been challenged even in the West.9 In countries like India, the lack ROP specialists makes this even more unfeasible. Since 2008, wide-field imaging by trained and accredited nonphysicians who use a telemedicine platform has been developed, validated, and reported from India.8,10–12 An Australian report evaluating the Indian tele-ROP model based on the Centers for Disease Control (CDC) guidelines, observed that any ‘deviation from the wide-field imaging and photodocumentation model could be fraught with the danger of suboptimal care'.13 This has resulted in the Government of India accepting wide-field imaging as a viable and practical alternative method for screening for ROP in the rural and district special neonatal care units and has invited innovations of cost-effective devices and solutions to fill this gap. Despite the successful deployment of the RetCam Shuttle (Clarity MSI, Pleasanton, CA, USA) in the rural program of Karnataka Internet Assisted Diagnosis of Retinopathy of Prematurity (KIDROP) and some other states, the cost of the device and the technology has prevented the scaling up of the National ROP Program, which is imperative in the current scenario.
This study reports a new, wide-field infant retinal camera primarily developed for ROP screening. This device, the “3nethra Neo” (Neo), invented by Forus Health, Bangalore, India was evaluated for its systemic and ocular safety for the use on premature infants. The device has also been compared with the RetCam 3 in a multicenter prospective study, which will be reported subsequently.14
Methods
This is a clinical, observational, hospital-based study describing the safety of a new retinal imaging device while using it on infants undergoing ROP screening.
‘Neo' Specifications
The Neo was developed in India by Forus Health, a technology-led company focused on ophthalmic care. The research and development for the device was initiated in 2012 and evolved through four prototypes with inputs from ROP specialists. Significant enhancements through the prototypes included the replacement of the illumination source from an optical fiber to a light-guided light-emitting diode (LED) illumination system and from a motorized focusing component to a liquid-lens focusing system in the current study version. The weight of the hand piece was reduced during each version and currently weighs 310 grams. The device, cables, foot switch, and accessories are placed in a single portable suitcase (Fig. 1). The design, optics, illumination, and software specifications are summarized in Table 1 and are compared with the RetCam 3 (Clarity MSI). Data about the Neo was provided by the device manufacturer.
Figure 1.
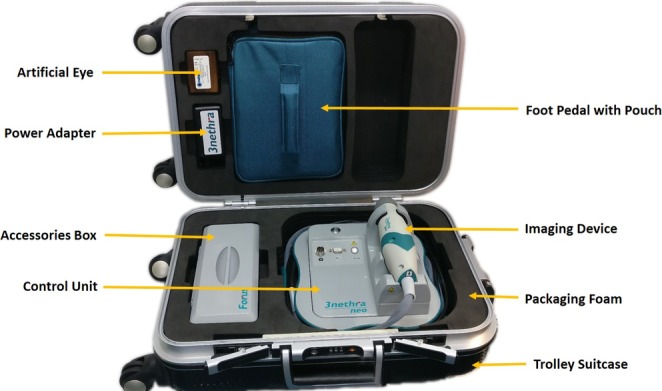
The Neo device with the cables, foot switch, and accessories placed in a single portable suitcase.
Table 1.
Technical Comparisons Between the Novel Infant Retinal Camera ‘Neo' and the Most Commonly Used Camera (Gold Standard) “RetCam”
| Neo |
RetCam |
|
| Design | ||
| Probe design | Single, monolithic hand-held probe | Detachable front optical hand-held probe |
| Weight (with lens and wire) | 740 g | 800 g (measured manually) |
| Image capture | Video and still | Video and still |
| Image size | 300 KB–9 MB | 150 KB–1.6 MB |
| Image resolution | 2040 × 2040 | 1600 × 1800 |
| Image shape | Square, complete circular image | Rectangle, cropped image |
| Optics | ||
| Field of view | Maximum 120° field of view | Maximum 130° field of view with additional attachment lens |
| Focus | Noiseless, motionless focus mechanism using liquid lens | Motorized focus |
| Image capture | Foot pedal and on screen | Foot pedal and on keyboard |
| Illumination | ||
| Light source | LEDs with waveguide optics | Halogen with optical fibers |
| Position of light source | Internal | External |
| Intensity of light source | 100–6000 lux | 100–6000 lux |
| Wavelength | Warm LED | Halogen light |
| Software and service | ||
| Live zoom | Available | Not available |
| Telemedicine integration | Neocare | RetCam Review Software |
| Cost | USD 25,000 (India) | USD 125,000 (Retcam Shuttle) (in India) |
The device was used for a safety assessment study at our institute. This study fulfilled the requirements of the hospital safety committee and the clinical research department and adhered to the tenets of the Declaration of Helsinki and was accepted by the Institute Research Board and the Institute Ethics Committee after the manufacturer provided a device safety compliance report for electrical and light safety. The device was tested initially on healthy, adult volunteers before being used in this study on infants.
Study Cohort
The study cohort comprised of consecutive Asian Indian premature infants enrolled for ROP screening under the KIDROP program. All study infants were less than 2000 g and/or 34-weeks gestational age or less and were less than 30 days of life when first screened, as per the national guidelines.15 The program screens in 104 neonatal intensive care units, but for the purpose of this study, only infants screened at Bangalore were enrolled to allow better monitoring of the systemic and ocular parameters. Parents or legal guardians accompanying the infant were counseled and an informed consent was obtained from all cases. Wide-field imaging performed by a trained and certified imager is the screening method of choice in the KIDROP program. This differs from indirect ophthalmoscopy performed by an ophthalmologist in that imaging is performed by a contact camera, which is placed over the cornea of the infant's eye using a coupling agent (Lubic Gel; Neon Pharma, Mumbai, India. Additional material on the safety and experience of this coupling agent can be found in Supplementary File S1). The eye is pried open using an infant wire speculum. Eyes are imaged one at a time. With indirect ophthalmoscopy, the ophthalmologist also uses a speculum to pry open one eye at a time. In addition, a Flynn infant speculum is used to visualize each quadrant using a 20-diopter (D) double aspheric lens. The method is subjective and cannot be recorded as images. The process of wide-field imaging has been reported in several reports of our group. Over 100,000 sessions have been completed in the past decade.6–8,10–13
Only the first ROP screening session was included in this analysis. Infants were monitored by an anesthetist and a pediatric nurse throughout the study session. Pupillary dilatation was started 1 hour prior to the imaging session and was achieved with a commercially available combination of phenylephrine 2.5% and cyclopentolate 0.5% (Auropent Plus; AuroLab, Tamil Nadu, India) one drop in each eye, repeated two to three times, 10 minutes apart. Babies were not fed an hour before and until an hour after the imaging session.
Ocular adverse outcomes that were looked for included conjunctival hemorrhage, laceration, corneal abrasion, ocular infection, hyphema, and retinal or vitreous hemorrhages. Systemic adverse effects that were looked for included bradycardia, tachycardia, hypoxia, apnea, cyanosis, seizure, and vomiting. Any other abnormal behavior was also documented, if present. The study definitions for the systemic adverse events were as per previously published criteria and are summarized in Table 2.
Table 2.
Study Definitions of Systemic Factors
| Bradycardia | Heart rate <80 bpm sustained for >30 sec16 | |
| Tachycardia | Heart rate >200 bpm sustained for >30 sec16 | |
| Hypoxia | Oxygen saturation <80% sustained for at least 30 sec16 | Drop in oxygen saturation ≥20% of baseline17,18 |
| Apnea of prematurity | Breathing pause lasting for 10 sec16 | |
| Others | Cyanosis, vomiting, seizure activity | |
Readings of oxygen saturation and heart rate were recorded using a portable multiparameter infant monitor (Mindray, VS-800 NIBP; Absolute Medical Services Inc, Stony Point, NY) for 60 minutes before the start of procedure with the last reading considered as baseline, ‘during the procedure', 5 minutes after, and for 60 minutes after procedure, with the last reading recorded as the reading at 60 minutes postprocedure. Heart rate was monitored continuously though the period and any episode above or below cut off was monitored to see if it lasted for greater than 30 seconds to record it as an ‘episode'. Similarly, oxygen was monitored to see if it fell below 80% saturation and if it lasted for 30 seconds or more was labelled an ‘episode' of hypoxia. As per the other study definition of hypoxia, any reading 20% or less of baseline was counted as an ‘event'. Because it was difficult to monitor oxygen saturation during the procedure, we had readings in less than 50% of babies and hence not included for analysis. For analysis therefore, recordings 1 hour before the procedure, immediately after the procedure, and an hour after the procedure were used for outcome analysis.
Ocular adverse effects were noted during, immediately after the imaging and at the end of 1 hour by the ROP specialist. The anterior segment was examined with the magnification of the 20-D lens and the retina using indirect ophthalmoscopy. The baby was monitored by the parents and a nursing staff prior to discharge. The caregivers were asked to report any adverse effects even if they did not attribute it to the eye imaging, and included eye discharge, redness, watering, or stickiness. Information of systemic and ocular adverse effects were collected doing a chart review for admitted cases and phone-in for discharged babies after 24 hours.
Imaging With the Neo
Imaging with the Neo was performed after pupillary dilatation was confirmed by the ROP specialist. Topical anesthesia was achieved by proparacaine hydrochloride 0.5% (Paracain, Sunways, India). An infant wire speculum was used on one eye at a time. Imaging was performed by one of two senior ROP imaging specialists (level 3 technicians).10 Scleral depressor was not used during the imaging in this study.
Sample Size and Statistical Analysis
Descriptive analysis was performed for means of continuous variables and proportion for categoric variables. Paired t-tests were used to compare mean heart rates between any two points of time. Oxygen saturation being not normally distributed, was tested with Wilcoxon signed-rank test. McNemar's test with the Yates continuity correction of 0.5, was used to compare number of events of bradycardia, tachycardia hypoxia, and apnea 1 hour before and 1 hour after the procedure. A sample size of 133 neonates was required to detect an effect size of 31%16 in heart rate at end of 1 hour, with 90% power and a two-sided alpha error of 5%, allowing provision for a nonresponse rate of 20%. Vassarstats16 was used for analysis and GPower17 for sample size calculation.
Results
Of the 140 infants in the study, 86 (61.43%) were male and 54 (38.57%) were female. The mean birth weight of the cohort was 1368 (SD 289.2) g (range, 650–2026 g) and mean gestational age was 30.61 (SD 2.44) weeks (range, 26–35 weeks). The mean postmenstrual age (PMA) at which the imaging was performed was 39.74 (SD 4.75) weeks (range, 30–52 weeks).
Systemic Safety
The mean baseline heart rate was 147.03 (SD 21.13) and range of 115 (80–195) beats per minute (bpm) and the mean baseline oxygen saturation was 94.54 ± 4.42% and range 20 (80–100). During the procedure, there was a mean increase in the heart rate (156.71 [SD 23.68], range of 115 [5–200]; paired t-test, P < 0.0001), which started reducing at the end of 5 minutes (150.23 [SD 20.38], range 105 [93–198]; paired t-test, P < 0.0001) following the procedure and came back to baseline (148.07 [SD 19.28], range 103 [90–193]; paired t-test, P = 0.11) at the 60-minute recording.
The oxygen saturation decreased marginally during the procedure (92.59 [SD 5.31], range 25 [75 –100]; Wilcoxon signed-rank test P < 0.0001), recovered after 5 minutes (93.35 [SD 4.63], range 21 [79–100], P < .0001), and returned to the baseline (94.34 [SD 4.23], range 20 [80–100]; P = 0.054), at the end of the 60-minute period. The difference between the study points is summarized in Table 3.
Table 3.
Mean Differences Between Two Time Points of Examination
| Measure and Test |
Time |
Mean |
Mean Difference (CI) From Baseline |
| Heart ratea | Baseline | 147.03 | |
| During | 156.71 | 9.68 (7.53–11.83)* | |
| 5 min after procedure | 150.23 | 3.20 (1.75–4.65)* | |
| 60 min after procedure | 148.07 | 1.04 (2.33 to −0.24) | |
| Oxygen saturationb | Baseline | 94.54 | |
| During | 92.59 | −1.94 (−1.60 to −2.28)* | |
| 5 min after procedure | 93.35 | −1.19 (−0.90 to −1.47)* | |
| 60 min after procedure | 94.34 | −0.19 (−0.00 to −0.38) |
Paired t-test.
Wilcoxon signed-rank test.
P < 0.05.
The number of episodes of bradycardia, as defined by a drop-in heart rate less than 80, during the procedure was 9 (6.4%). The number of episodes of tachycardia (heart rate >200 bpm sustained for >30 seconds) during the procedure was 3 (2.14%). Number of episodes of bradycardia an hour prior to the procedure (3, 2.14%) compared with an hour after (5, 3.6%) showed no difference (McNemar's paired t-test with Yates continuity correction, P =0.68); similarly, tachycardia was seven (5%) and nine (6.4%), respectively (P = 0.54).
As per the first definition of hypoxia (oxygen concentration of <80% ≥30 seconds), the number of episodes in the 1-hour period before the procedure was four (2.9%) as compared with two (1.4%) in the period between immediately after procedure to 1 hour after (McNemar's test with Yates continuity correction, P = 0.45). As per the second definition (drop in oxygen >20% of baseline; the last reading before procedure was baseline and the last reading at end of 60 minutes after procedure for comparison) there were no episodes of hypoxia.
Summary of Systemic Safety
To summarize the systemic safety results, none of the measured changes were clinically significant and none required any intervention. Hypoxia was seen in no infants at the end of procedure or at the end of 1 hour after the procedure. No screening session had to be stopped due to compromised systemic safety or critically altered as decided clinically by the attending anesthetist who monitored the event. No other systemic study adverse events were recorded at any time interval of the study period.
Ocular Safety
Ocular adverse events noted were transient redness in two eyes (0.7%) of two infants, which resolved spontaneously after 3 and 4 hours, respectively. No cases of conjunctival laceration, corneal abrasion, eye infection, hyphema, retinal, or vitreous hemorrhages were noted in any study infant.
The technicians did not face any difficulty in imaging infants on the Neo and were able to capture the regions of interest with ease comparable to the RetCam. All sessions complied with the previously published protocols with respect to steps and duration. No session took over 3 minutes.
Case Illustration of Image Comparison Between Neo and RetCam
The square Neo images were cropped from its original square to resemble the rectangular images of the RetCam. An ongoing comparative study between the two devices will be subsequently reported.
In case 1 (Fig. 2), an infant born at 32-weeks gestation with 1530 g, imaged at 36 + 3-weeks PMA, shows a regressing stage 2 ROP in zone 2 anterior on the ‘Neo' (top panel) and on the RetCam (lower panel).
Figure 2.
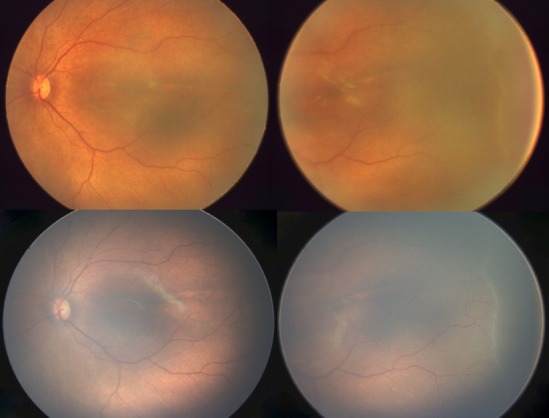
An infant born at 32-weeks gestation at 1530 g, imaged at 36 + 3-weeks PMA, shows a regressing stage 2 ROP in zone 2 anterior on the ‘Neo' (top panel) and on the RetCam (lower panel).
In case 2 (Fig. 3), immature retina imaged at 34-weeks PMA in an infant born at 1760 g and 31-weeks gestation on the ‘Neo' (left) and on the RetCam (right).
Figure 3.
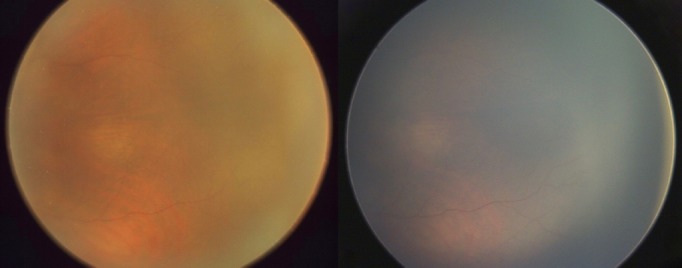
Immature retina imaged at 34-weeks PM) in an infant born at 1760 g and 31-weeks gestation on the ‘Neo' (left) and on the RetCam (right).
In case 3 (Fig. 4), mature retina imaged in an infant born at 1100 g and 28 weeks showing vessels up to the ora serrata at 42-weeks PMA on the ‘Neo' (left) and the RetCam (right).
Figure 4.
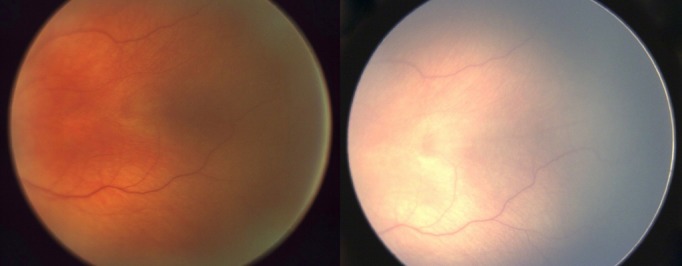
Mature retina imaged in an infant born at 1100 g and 28 weeks showing vessels up to the ora serrata at 42 weeks PMA on the ‘Neo' (left) and the RetCam (right).
Discussion
In the current scenario in India and other middle-income countries, the number of ROP specialists required to screen the increasing number of premature infants is grossly inadequate. Onsite, binocular indirect ophthalmoscopy performed by an ROP specialist, has several limitations in these low human resource settings. These include, the scarcity of trained specialists, lack of objectivity, lack of photodocumentation, medicolegal concerns, low or no reimbursement, remote or poorly accessible locations of neonatal care centers, and ergonomic and logistic difficulties.18 For these reasons, a majority of semiurban and rural neonatal care units in India have no ROP screening programs.
In some regions of the world, wide-field imaging provides an adjunctive role to the existing model, by assisting the ROP specialist who is performing on-site exams to confirm or document his or her findings. Tele-ROP on the other hand, is the practical use of wide-field imaging in a formal telemedicine network and has the potential of providing ROP care where there are few or no specialists, ensuring the continuity of care of these infants. This has been made possible for several decades with the availability of the current gold standard, the RetCam imaging systems (Clarity MSI).19–23
In India, tele-ROP has been successfully employed for over a decade to screen in remote rural centers.7,8,10,12,24 The scalability of this program has been limited owing to the high costs incurred in its setting up. This requires a public and private partnership to make it financially and logistically sustainable.6,13 Despite government funding, the high cost of the camera limits the number of units that can be used within a state or district.8 This leaves a vast majority of remote centers unscreened.
The Neo was developed to be able to fill this void of low-cost infant retinal devices and took 36 months of multiple prototype validation. Some of the modifications related to improving the ergonomic design, portability, light source, patented liquid-lens systems, weight, and the size. After the device secured successful safety certificates for biomedical parameters it was used for clinical validation. This manuscript focuses on the systemic and ocular adverse effects on 140 infants who underwent Neo imaging.
Using standard definitions of systemic study parameters (Table 2), we measured observed changes during, 5 minutes postimaging and an hour after the imaging session. These were compared with available studies of systemic changes using RetCam imaging.25,26,27 The heart rate increased by approximately 10 bpm during the procedure, which settled down to an increase of 3 bpm by the end of 5 minutes and an increase of 1 bpm at the end of 1 hour. Our results are consistent with Mukherjee et al.25 who also found a mean increase of 13 bpm during the procedure and Mehta et al.27 who found 12 bpm during the procedure. Both these studies reported a reduction in the heart rate by 10 minutes. Our results at the end of 60 minutes, compares well with Mehta et al.27 who found that the heart rate settled to baseline at end of 30 minutes and differs from Mukherjee et al.25 who reported a reduction in the heart rate by 5 bpm at end of 30 minutes. Wade et al.28 reported 0.7% tachycardia compared with our 2.1%. However, in their study, RetCam imaging was used only as an adjunct over indirect ophthalmoscopy and was stopped as soon as the heart rate fluctuated.
From a clinical perspective, bradycardia is of concern to the treating neonatologist who is often responsible for monitoring the baby during ROP screening. We found that 3.6% of our infants had bradycardia. This was significantly lower than 11.9% reported by Mukherjee et al.25 Studies that recorded bradycardia using indirect ophthalmoscopy have also reported higher levels of 24%29 and 31%.30 The relatively short duration of our imaging session and the lesser systemic stress as we did not use scleral indentation could have contributed to the lower incidence of bradycardia in our series.31
The mean oxygen concentration drop in our study was 1.9%, which is lower than previous reports of 3%25 and 7%.30 This is probably due to their longer screening duration and a smaller sample size (n = 15), respectively, in these studies. Desaturation was observed in 8.6% of examinations with the RetCam.32 The lack of uniform definitions across studies makes comparisons difficult. Mukherjee et al.25 reported 1.5% examinations with hypoxia when more than 20% of the base line was used as a cut-off. We had no episodes of hypoxia in the 1-hour period after the imaging session with the Neo. However, it must be mentioned that our study cohort was uniformly Asian Indian preterm infants. Infants undergoing ROP screening in our cohort are heavier and older compared with their counterparts in the Western world. This may limit the influence of generalizability of our results to other such population cohorts.
To summarize the systemic safety results, no baby required any extra intervention for correction of any of the systemic changes recorded during or after the procedure suggesting the systemic safety of the Neo. Similarly, ocular safety was established by the presence of only two babies showing transient conjunctival hyperemia after the procedure with no other adverse ocular or adnexal effect during or after the procedure.
Future Directions
After the safety profile of the Neo was established, the ethics committee approved a larger multicenter study that would compare the usability and image quality between the Neo and the RetCam using real-world metrics required in tele-ROP algorithms. The significant financial advantage is likely to promote the use of this device in low-volume neonatal units, which could previously not afford the RetCam. Furthermore, the smaller size and improved portability could allow the Neo to be taken to remote rural centers more easily than the RetCam. The potential advantage of smaller vehicles required to transport the Neo could also add to this cost benefit. A cost-benefit study comparing the direct and indirect costs between the two devices using real-world expenses in a tele-ROP model of screening will be required to explore its full potential.
Conclusion
To conclude, this report demonstrates the systemic and ophthalmic safety of a newly introduced wide-field, infant retinal camera, the Neo, for use in preterm infants who are at risk for ROP. The device has some promising novel features, which include the liquid-lens systems to dynamically focus the image, an LED light source, an integrated lens hand-piece, which is ergonomically lighter and more portable. The most distinct advantage of the device is the cost. Further studies comparing the Neo head-to-head with the RetCam in the real world and cost-utility assessments are necessary to validate the same.
Supplementary Material
Acknowledgments
The authors thank Padmanaba Holla, Manjunath Chandrashekaraiah, and Ujjal Datta from Forus Health, and Sivakumar Munusamy and Narasimha Krishnan from KIDROP for imaging these infants.
Funding from Grant-in-aid by Department of Biotechnology, BIRAC (Biotechnology Industry Research Assistance Council), Government of India, New Delhi.
The authors alone are responsible for the content and writing of the paper. The Neo device was provided by the manufacturer for the purpose of the study.
Disclosure: A. Vinekar, None; S.V. Rao, Founder and Director, Forus (E); S. Murthy, Forus Health, Bangalore, India (C); C. Jayadev, None; M.R. Dogra, None; A. Verma, None; B. Shetty, None
References
- 1.The global action report on preterm birth born too soon. 2017 Available at: http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf Accessed June 20,
- 2.Sanghi G, Dogra MR, Katoch D, Gupta A. Demographic profile of infants with stage 5 retinopathy of prematurity in North India: implications for screening. Ophthalmic Epidemiol. 2011;18:72–74. doi: 10.3109/09286586.2010.551575. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C, Rahi J, Eckstein M, O'Sullivan J, Foster A. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350:12–14. doi: 10.1016/S0140-6736(97)01107-0. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert C, Fielder A, Gordillo L, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115:e518–e25. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 5.Report in the Supreme Court of India, Civil Appellate Jurisdiction. 20152018 Available at: http://supremecourtofindia.nic.in/FileServer/2015-07-02_1435823185.pdf Accessed February 7,
- 6.Vinekar A. IT-enabled innovation to prevent infant blindness in rural India: the KIDROP experience. J Indian Bus Res. 2011;3:98–102. [Google Scholar]
- 7.Vinekar A, Avadhani K, Dogra M, et al. A novel, low-cost method of enrolling infants at risk for retinopathy of prematurity in centers with no screening program: the REDROP study. Ophthalmic Epidemiol. 2012;19:317–321. doi: 10.3109/09286586.2012.698358. [DOI] [PubMed] [Google Scholar]
- 8.Vinekar A, Jayadev C, Mangalesh S, Shetty B, Vidyasagar D. Role of tele-medicine in retinopathy of prematurity screening in rural outreach centers in India - a report of 20, 214 imaging sessions in the KIDROP program. Semin Fetal Neonatal Med. 2015;20:335–345. doi: 10.1016/j.siny.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Trese MT. What is the real gold standard for ROP screening? Retina. 2008;28(3 Suppl):S1–S2. doi: 10.1097/IAE.0b013e31816a5587. [DOI] [PubMed] [Google Scholar]
- 10.Vinekar A, Gilbert C, Dogra M, et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014;62:41–49. doi: 10.4103/0301-4738.126178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hungi B, Vinekar A, Datti N, et al. Retinopathy of prematurity in a rural neonatal intensive care unit in South India–a prospective study. Indian J Pediatr. 2012;79:911–915. doi: 10.1007/s12098-012-0707-y. [DOI] [PubMed] [Google Scholar]
- 12.Vinekar A, Jayadev C, Mangalesh S, et al. Initiating retinopathy of prematurity screening before discharge from the neonatal care unit: effect on enrolment in rural India. Indian Pediatr. 2016;53(Suppl 2):S107–S111. [PubMed] [Google Scholar]
- 13.Karnataka Internet Assisted Diagnosis of Retinopathy of Prematurity (KIDROP): program evaluation based on CDC guidelines. 20152018 Available at: http://www.paediatrics.uwa.edu.au/__data/assets/pdf_file/0008/2816783/KIDROP-report-2015.pdf Accessed February 7,
- 14.Vinekar A, Dogra MR, Jayadev C, Murthy S, Rao SV, Shetty B. Evaluation of a new, low-cost, portable, wide-field, digital, retinal camera, “Neo” for screening infants for retinopathy of prematurity – a prospective, multi-center, validation report in Asian Indian infants. Invest Ophthalmol Vis Sci. 2016;57(12) [Google Scholar]
- 15.Pejaver R, Bilagi A, Vinekar A, Bilagi P. National Neonatology Foundation's evidence based clinical practice guidelines for retinopathy of prematurity, NNF India, guidelines. 2017 January 2010. Available at: https://www.scienceopen.com/document?vid=3bfe12b1-64da-41ad-ae31-55cdf1d2a12e Accessed June 20,
- 16.Lowry R. vassarstats.net. Available at: http://vassarstats.net/ Accessed February 7. 2018
- 17.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 18.Vinekar A, Jayadev C, Shetty B. Telemedicine in retinopathy of prematurity. Adv Ophthalmol Optom. 2016;1:193–210. al. et. [Google Scholar]
- 19.Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity. Ophthalmology. 2003;110:2113–2117. doi: 10.1016/S0161-6420(03)00831-5. [DOI] [PubMed] [Google Scholar]
- 20.Dai S, Chow K, Vincent A. Efficacy of wide-field digital retinal imaging for retinopathy of prematurity screening. Clin Exp Ophthalmol. 2011;39:23–29. doi: 10.1111/j.1442-9071.2010.02399.x. [DOI] [PubMed] [Google Scholar]
- 21.Fijalkowski N, Zheng LL, Henderson MT, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic Surgery, Lasers Imaging Retin. 2014;45:106–113. doi: 10.3928/23258160-20140122-01. [DOI] [PubMed] [Google Scholar]
- 22.Quinn GE, Ying G, Daniel E, et al. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014;132:1178–1184. doi: 10.1001/jamaophthalmol.2014.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz B, Spasovska K, Elflein H, Schneider N. Wide-field digital imaging based telemedicine for screening for acute retinopathy of prematurity (ROP). Six-year results of a multicentre field study. Graefes Arch Clin Exp Ophthalmol. 2009;247:1251–1262. doi: 10.1007/s00417-009-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinekar A, Jayadev C, Bauer N. Need for telemedicine in retinopathy of prematurity in middle-income countries. JAMA Ophthalmol. 2015;133:360–361. doi: 10.1001/jamaophthalmol.2014.4913. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee AN, Watts P, Al-Madfai H, Manoj B, Roberts D. Impact of retinopathy of prematurity screening examination on cardiorespiratory indices a comparison of indirect ophthalmoscopy and retcam imaging. Ophthalmology. 2006;113:1547–1552. doi: 10.1016/j.ophtha.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell AJ, Green A, Jeffs D a, Roberson PK. Physiologic effects of retinopathy of prematurity screening examinations. Adv Neonatal Care. 2011;11:291–297. doi: 10.1097/ANC.0b013e318225a332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta M, Adams GGW, Bunce C, Xing W, Hill M. Pilot study of the systemic effects of three different screening methods used for retinopathy of prematurity. Early Hum Dev. 2005;81:355–360. doi: 10.1016/j.earlhumdev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Wade KC, Pistilli M, Baumritter A, et al. Safety of retinopathy of prematurity examination and imaging in premature infants. J Pediatr. 2015;167:994–1000.e2. doi: 10.1016/j.jpeds.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laws DE, Morton C, Weindling M, Clark D, Clark D, Laws D. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol. 1996;80:425–428. doi: 10.1136/bjo.80.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke WN, Hodges E, Noel LP, Roberts D, Coneys M. The oculocardiac reflex during ophthalmoscopy in premature infants. Am J Ophthalmol. 1985;99:649–651. doi: 10.1016/s0002-9394(14)76029-5. [DOI] [PubMed] [Google Scholar]
- 31.Belda S, Pallás CR, de la Cruz J, Tejada P. Screening for retinopathy of prematurity: is it painful? Neonatology. 2004;86:195–200. doi: 10.1159/000079542. [DOI] [PubMed] [Google Scholar]
- 32.Moral-Pumarega M, Caserío-Carbonero S, De-La-Cruz-Bértolo J, Tejada-Palacios P, Lora-Pablos D, Pallás-Alonso CR. Pain and stress assessment after retinopathy of prematurity screening examination: indirect ophthalmoscopy versus digital retinal imaging. BMC Pediatr. 2012;12:132. doi: 10.1186/1471-2431-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


