Abstract
Introduction:
Televised direct-to-consumer advertising (DTCA) for prescription drugs is controversial, especially for tobacco cessation products such as varenicline, given safety concerns that arose only after its market approval. We aim to quantify the extent to which DTCA influenced varenicline use.
Methods:
We linked monthly DTCA television ratings with monthly prescription data from IMS Health’s National Prescription Audit across top 75 media markets in 2006–2009. We used Poisson models with Generalized Estimating Equations to analyze effects of exposures to DTCA for both varenicline and nicotine replacement therapies on rate of dispensed varenicline prescriptions among smokers, controlling for population characteristics and varenicline-related events.
Results:
Varenicline prescriptions increased dramatically following DTCA launch and declined sharply after safety risks were publicized and US Food and Drug Administration (FDA) issued an advisory. DTCA had significant impact on new prescription dispensing in the subsequent month: before the FDA advisory, one additional exposure to varenicline DTCA was associated with a 1.8% (rate ratio [RR] = 1.018 [1.015–1.021]) higher rate of new prescriptions; no effect was observed after the advisory (RR = 1.000 [0.997–1.003]). Prior to the advisory, cross-product effects of nicotine replacement therapy advertising on varenicline prescribing were negligible (RR = 1.002 [0.999–1.004]); after the advisory, effects were positive (RR = 1.015 [1.012–1.019]).
Conclusions:
DTCA for varenicline had a significant impact on varenicline prescribing when the drug’s safety profile was not well characterized, supporting arguments to limit DTCA for newly approved products whose real-world safety is unclear.
Implications:
We examined the fluctuations in varenicline use in association with DTCA for varenicline and other tobacco cessation aids. To our knowledge this is the first study to quantify the effects of televised DTCA for varenicline and other tobacco cessation aids on varenicline prescription dispensing. We believe that understanding these relationships is critical for formulating effective public health policy and interventions.
Introduction
Televised direct-to-consumer advertising (DTCA) for prescription drugs is permitted in only two countries—the United States and New Zealand. DTCA’s impact on consumer health care has been a controversial topic debated by US policymakers, medical professionals, and the pharmaceutical industry since the US Food and Drug Administration (FDA) relaxed regulations on televised DTCA in 1997. Proponents assert that DTCA educates patients about available treatment options and can encourage appropriate use of drugs to treat high-priority illness. 1 Critics argue that such advertising increases drug prices and creates demand for unnecessary, potentially harmful treatments. 2 , 3 Generally DTCA starts early in the product life cycle whereas information regarding adverse events and effectiveness continues to emerge; thus advertising for new products may carry even greater risk than advertising for products later in their life cycle.
DTCA that promotes pharmaceutical cigarette smoking cessation aids is of particular interest to the public health community because it remains unclear whether such products or their advertising encourage long-term quitting. 4–6 Smoking remains the leading preventable cause of death in the United States, and advertising for cessation products will benefit public health to the extent that it inspires successful quit attempts. 7 Yet advertising for cessation drugs also may be harmful if it leads consumers to use products that are relatively ineffective or that have serious side effects. 3 While some evidence suggests that smoking cessation pharmaceuticals demonstrate clinical efficacy 8 and their promotion is related to increased sales of such products, 9 other evidence questions whether their promotion is associated with quitting at the population level. 10 , 11 Further research has suggested that advertising for cessation drugs may become a sort of “get out of jail free card” by creating a false impression of the ease with which smokers may quit at a later time. 12 Despite the mixed findings regarding the impact of DTCA for smoking cessation drugs on quit attempts and sales volume, such advertising has nonetheless represented the dominant form of smoking-related messaging on television in the United States: pharmaceutical cessation aids advertising comprised 47% of smoking-related television ads aired in the top 75 media markets in 1999–2007. 10 , 13
The effects of DTCA on the prescribing and use of smoking cessation pharmacotherapies are of interest to policymakers as the products have become a central component of recent US health care reform at both the federal and state levels. For example, policy initiatives such as the Health Information Technology for Economic and Clinical Health (HITECH) Act and the Affordable Care Act have prioritized evidence-based cessation treatments in efforts to reduce smoking prevalence and its associated health care costs. 14 , 15 At the state level, tobacco cessation is high priority because Medicaid recipients smoke at higher rates than the general population. 16 To date little is known about the effects of these policies on prescribing and/or use of pharmaceutical cessation aids particularly in low-income populations.
Varenicline (marketed in the United States as Chantix) was approved by the FDA to be used for smoking cessation in 2006. Soon thereafter Chantix became the first and only non-nicotine smoking cessation medication advertised on US television. Televised ad campaigns launched in December 2006, the first of which was a “help-seeking” campaign that did not name the brand but directed consumers to a website which provided information about Chantix. 17 Help-seeking ads do not include risk and benefit information. In contrast, branded ads must detail major risks of the named product. 18–20 Branded ad campaigns for varenicline began in 2007, which was early in the product’s life cycle when adverse events were likely still to be detected. Subsequently in 2007 Chantix was linked to a high-profile shooting in which a Dallas musician was killed 21 and to multiple other incidences of violence and aggression. 22 Based on these reports and additional scientific evidence, the FDA issued a public health advisory in 2008 23 and required a “black box” warning on the drug in 2009 24 advising of the drug’s association with serious neuropsychiatric side effects including depression, hostility and suicidal ideation.
The extent to which levels of varenicline DTCA exposure, combined with varenicline safety-related events, may have contributed to increases and declines in the popularity of varenicline remains unknown. In addition, we hypothesize that advertising for over-the-counter nicotine replacement therapy (NRT) cessation aids may also have contributed to varenicline use, since DTCA has been shown to affect sales for the product’s broader therapeutic class. 25 We examine the fluctuations in varenicline use in association with DTCA for nicotine-based and non-nicotine-based cessation aids. While studies of DTCA’s impact often rely on national expenditures data, 26 our study accounts for varied intensities of advertising exposures across media markets. We linked Nielsen television ratings for varenicline and NRT product advertising with prescription dispensing data from the IMS Health National Prescription Audit (NPA) database in order to characterize the effects of DTCA on dispensed prescriptions for varenicline. Obtaining a prescription for a smoking cessation drug typically represents a direct precursor to a quit attempt, and such data can help clarify the relationship between DTCA and cessation behavior. 27 Understanding these relationships is critical to formulating effective public health policy and interventions.
Methods
Data
We purchased Nielsen television ratings for varenicline advertising and NRT advertising from each of the top 75 US designated market areas (DMAs) for 2006–2009. DMAs are composed of contiguous counties typically centered in and near large cities, wherein the population receives very similar television programming. DTCA is measured using gross ratings points, a standard metric for quantifying advertising intensity that reflects the product of the percentage of the target audience reached multiplied by frequency of exposures. For instance, an ad with 100 gross ratings points per month can be interpreted as having been seen one time per month by 100% of the audience. We summed gross ratings points within the same DMA and month, then divided this value by 100 to obtain the average number of times an advertisement for varenicline and NRT products aired for television households (“exposures”).
We used the IMS Health NPA to obtain monthly dispensed prescription data for varenicline. The NPA collects prescription dispensing data from approximately 47 500 pharmacies and projects to a universe of approximately 62 500 pharmacies. Prescriptions from all payers including private insurance, Medicare, Medicaid, and self-pay are captured. IMS categorizes claims into two categories: new prescriptions, which reflect the initial claim for a specific medicine; and refills, which reflect additional dispensations of an existing prescription. The new and total (new plus refills) prescriptions dispensed at retail pharmacies were linked to the exposures to DTCA for smoking cessation aids by DMAs and months. We used monthly number of dispensed new prescriptions in each DMA as the primary outcome.
Media market-level population characteristics may confound the association of DTCA and prescription dispensing. We therefore extracted annual estimates of median income, physician density, Medicaid-eligible population, Medicare-eligible population and total population in each DMA from the Health Resources and Services Administration’s Area Resource File. The Area Resource File provides annual county-level data on a variety of measures related to health care services. We aggregated these data from the county level to the DMA level. The Selected Metropolitan/Micropolitan Area Risk Trends of Behavioral Risk Factor Surveillance System (SMART BRFSS) provides the annual smoking prevalence for each metropolitan/ micropolitan statistical area (MMSA). We used the SMART data to derive the number of smokers in each DMA. MMSAs are closely related to DMAs geographically. We mapped most MMSAs to DMAs and used state-level prevalence for the DMAs that did not have matching MMSAs. For a few DMAs located at the borders of two or more states and having no MMSA-level data, we used population-weighted average of state-level prevalence. We derived the annual number of insured individuals per DMA from the Small Area Health Insurance Estimates. The US Census Bureau’s Small Area Health Insurance Estimates provides annual model-based estimates of health insurance coverage under 65 years old for all counties, and we aggregated these data from the county to the DMA level. We obtained 2005–2009 5-year estimates of median age and white population (%) for each county from the American Community Survey and aggregated to the DMA level. Annual estimates for those variables were not available for all counties forming the 75 DMAs, and median age and white population do not substantially change year by year.
Tobacco control policies are related to smoking prevalence. 28 The state-level annual average price of a pack of cigarettes, excluding generic brands, was compiled based on the Tax Burden on Tobacco Vol. 49. 29 The cigarette prices include state and federal taxes on cigarettes and were adjusted for inflation using the consumer price index. Smoke-free air (SFA) index was compiled based on the American Nonsmokers’ Rights Foundation’s Tobacco Control Policy Database to capture state smoke-free policies in three venues (private workplaces, restaurants, and bars), taking into account any preemption laws. The coding scheme for each venue was: 0 = no SFA laws, 1 = some smoking restrictions, 2 = smoking bans with exemptions, and 3 = comprehensive smoking bans without exemptions. We constructed DMA-level cigarette prices and SFA index by calculating population-weighted average of state-level data. We linked the contextual factors to the number of dispensed prescriptions by DMAs and years.
Analysis
To examine the overall pattern of new prescriptions and advertising in 2006–2009, we computed monthly rates of new prescription dispensation per 1 000 000 smokers for each DMA and monthly levels of exposures to varenicline advertising (help-seeking and branded) and to NRT advertising.
We used the Poisson models with Generalized Estimating Equation 30 to examine the association of ad exposures and new varenicline prescription rates. The models account for lack of independence between monthly dispensed prescriptions nested within DMAs and use estimated total smokers within DMAs as an offset. We linked the prescriptions dispensed in a current month with ad exposures of a prior month to reflect that it may take a few weeks to a month after seeing the ads before a smoker visits a doctor’s office to obtain a prescription. Media coverage on the shooting and side effects in September 2007 and the FDA advisory issued in February 2008 may have adversely affected varenicline prescribing; we used linear spline to model temporal trends allowing the pattern to change in those 2 months. 31 The model equation is presented in the Supplementary Appendix .
We began with all contextual factors in modeling, but eligible for Medicare (%), median income, and under poverty (%) were excluded due to their high correlations with one another and with other covariates ( Supplementary Table A ). Medicare-eligible population was highly correlated with median age ( r = 0.85), moderately correlated with median income ( r = −0.55) and white population ( r = 0.46). Median income was correlated with physician density ( r = 0.52), insured population ( r = 0.42), and white population ( r = −0.40). SFA was also excluded because of its correlation with cigarette price. We further examined how DTCA exposure contributed to increases in new prescriptions before the FDA advisory and to declines afterwards by including an indicator of two periods (post-advisory and pre-advisory) and its interaction with DTCA exposures in the model. We also conducted sensitivity analysis of total prescription rates (new + refills). All analyses were conducted using SAS/STAT software version 9.4.
Results
Trends in Varenicline Prescriptions
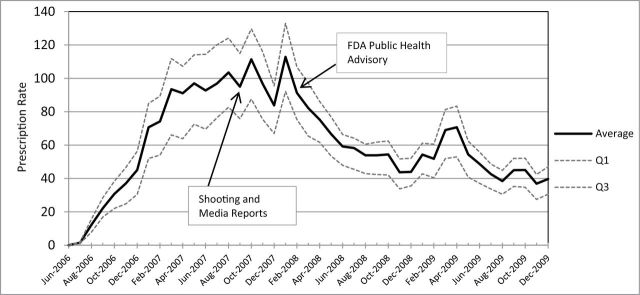
Sharp increases in varenicline use were observed after the 2006 market launch, followed by declines in use. Monthly rates of new varenicline prescriptions dispensed per 1 000 000 smokers in June 2006–December 2009 over 75 DMAs are presented in Figure 1 . The average rate sharply increased after the launch of varenicline and peaked at approximately 112 per 1 000 000 smokers in October 2007 and January 2008. After FDA issued the public health advisory in February, the average rate dropped to below 60 in 6 months. Monthly totals of new prescription dispensations over 75 DMAs showed a very similar pattern with about 450 000 prescriptions dispensed in October 2007 and January 2008 before dropping to 208 000 by August 2008 (data not shown). The band of 25th to 75th percentiles ( Figure 1 ) indicates that dispensed prescription rates had more variation over time than across DMAs. On average, across all DMAs, the monthly new prescription rate per million smokers was 61.5 ( Table 1 ), and roughly 10.4 million new prescriptions in total were dispensed during the study period.
Figure 1.
Rates of dispensed new prescriptions for varenicline at retail pharmacies per 1 000 000 smokers in 2006–2009. Monthly average, first quartile (Q1), and third quartiles (Q3) over 75 market areas. Source: IMS Health National Prescription Audit.
Table 1.
Summary Statistics of Dependent Variable and Predictors in 2006–2009
| Variables | N | Mean | SD |
|---|---|---|---|
| DMA × monthly a varying variables | |||
| Chantix new Rx b count | 3225 | 3224.21 | 3215.45 |
| Chantix total Rx count | 3225 | 3939.33 | 3864.79 |
| Chantix new Rx rate (/10 5 smokers) | 3225 | 6.15 | 3.87 |
| Chantix total Rx rate (/10 5 smokers) | 3225 | 7.56 | 4.71 |
| Chantix ad exposure (/100 GRPs) | 3225 | 3.60 | 3.99 |
| Help-seeking | 3225 | 2.37 | 3.31 |
| Branded | 3225 | 1.22 | 2.37 |
| NRT ad exposure (/100 GRPs) | 3225 | 5.52 | 4.13 |
| DMA × yearly varying covariates | |||
| Insured population under 65 years (%) | 300 | 82.56 | 4.76 |
| Medicaid eligible (%) | 300 | 19.04 | 5.05 |
| Physician density (/10 5 ) | 300 | 226.74 | 51.54 |
| Cigarette price (×10¢/pack) c,d | 300 | 49.01 | 8.67 |
| DMA varying covariates | |||
| Median age (%) | 75 | 36.99 | 2.38 |
| White population (%) | 75 | 77.23 | 11.57 |
DMA = designated market area; GRP = gross ratings point; NRT = nicotine replacement therapy.
a 43 months in June 2006–December 2009.
b Dispensed prescription, source: IMS National Health Prescription Audit.
c State and federal tax included, Consumer Price Index adjusted (base year = 2009).
d Population-weighted average of state-level data.
Exposures to DTCA for Varenicline and NRT
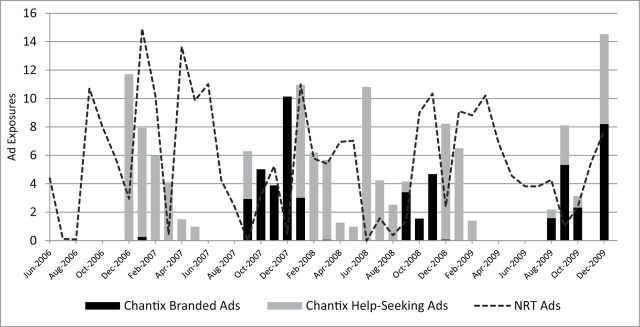
Figure 2 displays the pattern of exposures to televised advertising for varenicline (bars) and NRT (dotted lines) during the study period. The average monthly exposures to varenicline ads were broken down between branded ads (black bars) and help-seeking ads (gray bars). TV advertising for varenicline started with help-seeking ads in December 2006. Help-seeking ads were dominant until summer of 2007 with a decreasing trend; this period coincides with a dramatic increase in prescription rates. The branded ads were dominant in the last quarter of 2007 when new prescription rates reached the highest and fluctuated widely. After FDA announced the public health advisory, help-seeking ads were aired across many months, although branded ads were aired primarily in a few later months of each year. Overall, more help-seeking ads were aired than branded ads ( Table 1 ). Also NRT ads (5.5 mean monthly exposures) were more frequently aired than varenicline ads (3.6 mean monthly exposures) on average.
Figure 2.
Monthly direct-to-consumer advertising (DTCA) exposures for Chantix and over-the-counter nicotine replacement therapy (NRT) products in 2006–2009. Monthly exposures (gross ratings point/100) averaged over 75 media markets were broken down by branded full-disclosure ads and help-seeking ads for Chantix.
Effect of DTCA on Varenicline Prescriptions
Table 2 presents the crude rate ratios (RRs) of dispensed new varenicline prescriptions among smokers for one ad exposure, estimated using Poisson models. We examined the summed exposures to both branded and help-seeking ads for varenicline and the exposures to each type of ad. Table 2 also presents the relationship between ad exposures and rates of dispensed new varenicline prescriptions among smokers adjusting for contextual factors. The magnitude of effects is smaller than those of crude RRs, indicating that variation in the prescription data is partially explained by contextual factors. One exposure to varenicline ads was associated with a 1.5% higher rate of dispensed new prescriptions in the subsequent month across the study period (RR = 1.015 [1.013–1.018]). The association of exposure to Chantix branded ads with prescription rates was stronger than was the association of exposure to help-seeking ads; the prescription rate was 3.4% higher for each exposure to branded ads (RR = 1.034 [1.032–1.037]) while 0.9% higher for each exposure to help-seeking ads (RR = 1.009 [1.006–1.011]). The smaller effect of help-seeking ads appear to reflect the period between December 2006 and summer 2007 when the prescription rates rapidly increased as the exposures to help-seeking ads decreased; and the post-advisory period when more help-seeking ads were used, but the prescription rates decreased and did not recover to their previous state. Exposure to NRT ads was also positively associated with varenicline prescription dispensing rates, although to a smaller degree than exposure to varenicline ads: 0.8% to 0.9% higher prescription rate for each exposure. A 10-cent change in cigarette price was associated with 1.6% higher rate of prescription, controlling for ad exposures and other factors. DMAs with higher percentages of white population and insured population tended to have higher prescription rates, while the percentage of Medicaid-eligible population was negatively related to prescription rates.
Table 2.
Association of Varenicline New Prescription Rates, Exposure to Varenicline Advertising (Total, Branded, Help-Seeking) and Nicotine Replacement Therapy (NRT) Advertising Adjusting for Contextual Factors a
| Variables | Crude association | Adjusted association | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Chantix ads | By types of Chantix ads | ||||||||
| RR b | 95% CI c | P c | RR b | 95% CI c | P c | RR b | 95% CI c | P c | |
| Chantix total ads | 1.047 | (1.044–1.050) | <.0001 | 1.015 | (1.013–1.018) | <.0001 | — | — | — |
| Chantix branded ads | 1.059 | (1.053–1.065) | <.0001 | — | — | — | 1.034 | (1.032–1.037) | <.0001 |
| Chantix help-seeking ads | 1.032 | (1.030–1.034) | <.0001 | — | — | — | 1.009 | (1.006–1.011) | <.0001 |
| NRT ads | 1.006 | (1.004–1.009) | <.0001 | 1.009 | (1.007–1.011) | <.0001 | 1.008 | (1.006–1.010) | <.0001 |
| Insured (%) | 1.013 | (0.999–1.027) | .064 | 1.013 | (0.999–1.027) | .070 | |||
| Medicaid (%) | 0.979 | (0.964–0.993) | .005 | 0.978 | (0.964–0.993) | .004 | |||
| Physician (/10 5 ) | 1.000 | (0.999–1.001) | .704 | 1.000 | (0.999–1.001) | .707 | |||
| Median age (y) | 1.011 | (0.990–1.034) | .301 | 1.012 | (0.990–1.034) | .300 | |||
| Cigarette price (/pack) | 1.015 | (1.009–1.022) | <.0001 | 1.016 | (1.010–1.022) | <.0001 | |||
| White (%) | 1.020 | (1.013–1.028) | <.0001 | 1.020 | (1.013–1.028) | <.0001 | |||
a Results from Poisson Generalized Estimating Equation models with first-order autoregressive correlation structure within designated market areas.
b RR = rate ratio.
c 95% confidence intervals and P values were computed using the robust variance estimation.
Effect of Safety Signals on Impact of Varenicline DTCA
Varenicline prescription rates greatly fluctuated shortly after widespread media coverage regarding its potential link with neuropsychiatric events and quickly declined after the FDA public health advisory. We examined whether and how DTCA exposure contributed to prescribing before and after the advisory. The interaction effects of DTCA exposures and pre-post advisory periods were significant ( P < .0001). In the pre-advisory period, one more exposure to varenicline ads was associated with a 1.8% higher rate of dispensed new prescriptions (RR = 1.018 [1.015–1.021]) and exposure to NRT ads was not significantly associated (RR = 1.002 [0.999–1.004]). On the contrary, in the post-advisory period, the prescription dispensing rate was 1.5% higher for each exposure to NRT ads in the previous month (RR = 1.015 [1.012–1.019]) and not associated with exposure to varenicline ads (RR = 1.000 [0.997–1.003]).
Sensitivity Analyses
Sensitivity analysis of dispensed total prescription rates (new + refills) showed similar results: RR = 1.014 (1.011–1.016) for one more exposure to varenicline ads, and RR = 1.009 (1.007–1.011) for one more exposure to NRT ads adjusting for the contextual factors. Analysis including the indicator of pre-/post-advisory periods showed similar results ( Supplementary Table B ) as well.
Discussion
We examined the relationship between varenicline DTCA and dispensed new prescriptions for varenicline, as well as the cross-product relationship between NRT DTCA and dispensed new prescriptions for varenicline. We found positive relationships between DTCA and dispensed prescriptions. We also showed that significant events related to varenicline safety and the ensuing FDA advisories were associated with lower prescription rates and mitigated the effects of varenicline DTCA in subsequent time periods. Interestingly, we showed that prior to the FDA advisory, NRT DTCA was not related to varenicline prescriptions, but after the advisory exposure to NRT DTCA was positively associated with varenicline prescriptions.
These findings are important because the impact of DTCA for specific medications varies based on the safety and effectiveness of the medications being advertised. In the case of varenicline, there is evidence supporting the clinical efficacy of the drug, 8 , 32 but DTCA was launched before the product’s safety and effectiveness among the general population in real world setting had been well characterized and relevant studies were published. 22 , 33 , 34 Our findings demonstrate that varenicline DTCA was positively associated with prescription increases during a period of emerging safety concerns. Further, the positive effect of NRT DTCA on varenicline prescriptions after the advisory supports prior work indicating that DTCA may increase demand for pharmaceuticals across an entire class of drugs. However, the lack of a cross-product effect prior to the advisory may suggest that branded advertising is an important source of product information if there is little other information available about a given medication early in the productʼs life cycle.
Our findings may also imply income-related disparity in access to evidence-based smoking cessation aids. Prescription rates were significantly lower in areas with higher Medicaid-eligible population, although smoking rates tend to be higher in such low-income populations. A study based on the International Tobacco Control Four Country Survey in 2004–2011 reported a similar demographic pattern: smokers who used varenicline to quit were likely to be non-Latino white, more educated, and have higher income. 35 The authors inferred that these findings might suggest existence of disparity in access to evidence-based cessation methods. Low-income populations tend to be motivated to quit by immediate health problems. 36 Research is needed to examine whether the effect of DTCA is stronger or weaker in low-income populations.
Evidence regarding the effects of pharmaceutical advertising on cessation behavior is mixed: advertising for smoking cessation aids has been positively associated with NRT sales volume, 9 although it was found to be associated with fewer attempts to quit in another study. 10 Obtaining a prescription for a smoking cessation drug is a better precursor to actual cessation behavior than self-reports of quit attempts, 37 but it remains unclear to what extent recipients of dispensed prescriptions were successful in their attempts or how DTCA for cessation drugs may influence quit attempts among those who do not seek and receive prescriptions. Help-seeking ads may have particular impact in spurring quit attempts in cases where consumers do not seek or receive prescriptions. Further research should investigate the impact of DTCA on successful quit attempts, and whether and how evidence-based cessation methods such as varenicline influence that relationship.
While this study offers evidence that DTCA for varenicline—both help-seeking and branded—affects prescription volume, several questions remain. First, diagnostic information is not provided in NPA data; thus we do not know whether varenicline prescribing represents appropriate use of the medication. Further research should incorporate patient-level clinical information. Second, while this study found that higher levels of varenicline prescribing corresponded with increases in DTCA, we did not examine trends in quit intentions or attempts. We cannot assess whether the observed increases in prescription volume represented new quit attempts inspired by the advertising campaign, or whether individuals who were already planning or attempting to quit switched to varenicline rather than pursuing other cessation methods. Third, our analyses did not evaluate the impact of insurance coverage for specific smoking cessation therapies on DTCA effects. Coverage for cessation products by private insurance plans has changed with recent health care policy reforms. Fourth, we did not account for physician-targeted promotion for varenicline. Although office-based detailing and distribution of free samples may influence varenicline prescribing, such data are available only at the national level. Therefore we could not examine their effects on geographically varying prescription volume.
Finally, we did not characterize the content of specific advertisements for this study, and thus cannot speak to how messaging styles of individual ads, including their presentation of risk information, may have affected consumers’ quit attempts or information-seeking behaviors. For example, as new medication risks emerge, FDA requires updating risk information provided in televised DTCA. Such requirements, to the extent that they changed advertising content, may have influenced its effect. 38 These requirements may also have affected the use of help-seeking campaigns. Since help-seeking ads do not provide risk information, their deployment has been previously described as a potential method of “skirting” risk disclosure. 20 This method may be especially appealing to pharmaceutical companies in the context of multiple major medication risks or heightened consumer awareness of risks, such as after the issuance of public health advisories. Indeed, we noted a marked shift from branded ads to help-seeking ads around the public health advisory, and only help-seeking ads were aired for several months.
Conclusions
This study suggests that demand and prescribing for varenicline rapidly increased following the launch of DTCA for the product and then declined quickly in the wake of media coverage and FDA advisories questioning product safety. Our findings revealed that DTCA for varenicline contributed to increases in prescription volume at a time when the drug’s real world risk-benefit profile was not well characterized. 3 Arguments have been raised in support of more stringent regulations on DTCA to be composed by a body independent of the pharmaceutical industry. 39 Suggestions for policy change have included imposing a moratorium or complete ban on DTCA, requirement of FDA pre-clearance for advertisements, requirement of comparative effectiveness disclosure in advertisements and detailed plan for safety communication, and the development of regulations for online advertising. 39–43 Our findings also support proposed policies to restrict DTCA for newly approved drugs, thus delaying advertising launch to allow time to monitor real-world use and conduct adequate safety studies. 40–42
Funding
This manuscript was supported by the National Heart, Lung and Blood Institute (R01HL107345). The funding source had no role in the design and conduct of the study, analysis or interpretation of the data, preparation or final approval of the manuscript prior to publication.
Declaration of Interests
GCA is Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee, serves as a paid consultant to IMS Health, and serves on an IMS Health scientific advisory board. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Supplementary Material
Acknowledgments
Authors would like to thank Hy Tran for preparing data from many sources, Melissa Buenger for literature review, and Jidong Huang for helpful comments regarding cigarette price and SFA data. We also thank the reviewers for their insightful comments that led us to greatly improve this article.
References
- 1. Almasi EA, Stafford RS, Kravitz RL, Mansfield PR . What are the public health effects of direct-to-consumer drug advertising? PLOS Med . 2006. ; 3 ( 3 ): 284 – 288 . doi: 10.1371/journal.pmed.0030145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frosch DL, Grande D, Tarn DM, Kravitz RL . A decade of controversy: balancing policy with evidence in the regulation of prescription drug advertising . Am J Public Health . 2010. ; 100 ( 1 ): 24 – 32 . doi: 10.2105/AJPH.2008.153767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brody H, Light DW . The inverse benefit law: how drug marketing undermines patient safety and public health . Am J Public Health . 2011. ; 101 ( 3 ): 399 – 404 . doi: 10.2105/AJPH.2010.199844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fucito LM, Bars MP, Forray A, et al. . Addressing the evidence for FDA nicotine replacement therapy label changes: a policy statement of the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco . Nicotine Tob Res . 2014. ; 16 ( 7 ): 909 – 914 . doi: 10.1093/ntr/ntu087 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilpin EA, Messer K, Pierce JP . Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res . 2006. ; 8 ( 5 ): 661 – 669 . doi: 10.1080/14622200600910801 . [DOI] [PubMed] [Google Scholar]
- 6. Kotz D, Brown J, West R . Prospective cohort study of the effectiveness of varenicline versus nicotine replacement therapy for smoking cessation in the “real world.” BMC Public Health . 2014. ; 14 ( 1 ): 1163 . doi: 10.1186/1471-2458-14-1163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avery R, Kenkel D, Lillard DR, Mathios A . Private Profits and Public Health: Does Advertising Smoking Cessation Products Encourage Smokers to Quit? Cambridge, MA: : National Bureau of Economic Research; ; 2006. . www.nber.org/papers/w11938 . Accessed November 14, 2014 . doi: 10.3386/w11938 . [Google Scholar]
- 8. Cahill K, Stead LF, Lancaster T . Nicotine receptor partial agonists for smoking cessation . Cochrane Database Syst Rev . 2009. ; 3 : CD006103 . doi: 10.1002/14651858.CD006103.pub6 . [DOI] [PubMed] [Google Scholar]
- 9. Tauras JA, Chaloupka FJ, Emery S . The impact of advertising on nicotine replacement therapy demand . Soc Sci Med . 2005. ; 60 ( 10 ): 2351 – 2358 . doi: 10.1016/j.socscimed.2004.10.007 . [DOI] [PubMed] [Google Scholar]
- 10. Emery S, Kim Y, Choi YK, Szczypka G, Wakefield M, Chaloupka FJ . The effects of smoking-related television advertising on smoking and intentions to quit among adults in the United States: 1999–2007 . Am J Public Health . 2012. ; 102 ( 4 ): 751 – 757 . doi: 10.2105/AJPH.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wakefield MA, Spittal MJ, Yong H-H, Durkin SJ, Borland R . Effects of mass media campaign exposure intensity and durability on quit attempts in a population-based cohort study . Health Educ Res . 2011. ; 26 ( 6 ): 988 – 997 . doi: 10.1093/her/cyr054 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolton LE, Cohen JB, Bloom PN . Does marketing products as remedies create “Get Out of Jail Free Cards”? J Consum Res . 2006. ; 33 ( 1 ): 71 – 81 . [Google Scholar]
- 13. Wakefield M, Szczypka G, Terry-McElrath Y, et al. . Mixed messages on tobacco: comparative exposure to public health, tobacco company- and pharmaceutical company-sponsored tobacco-related television campaigns in the United States, 1999–2003 . Addiction . 2005. ; 100 ( 12 ): 1875 – 1883 . doi: 10.1111/j.1360-0443.2005.01298.x . [DOI] [PubMed] [Google Scholar]
- 14. HITECH Answers . Smoking and meaningful use: Meaningful Use Objectives . 2011. . www.hitechanswers.net/smoking-and-meaningful-use/ . Accessed November 25, 2014 .
- 15. Kofman M, Dunton K, Senkewicz MB . Implementation of Tobacco Cessation Coverage under the Affordable Care Act: Understanding How Private Health Insurance Policies Cover Tobacco Cessation Treatments . Washington, DC: : Health Policy Institute, Georgetown University; ; 2012. . www.tobaccofreekids.org/pressoffice/2012/georgetown/coveragereport.pdf . Accessed November 25, 2014 . [Google Scholar]
- 16. Centers for Disease Control and Prevention . National Center for Health Statistics. National Health Interview Survey, 2008 Provisional Data . Atlanta, GA: : Centers for Disease Control and Prevention; ; 2009. . [Google Scholar]
- 17. Kamal KM, Desselle SP, Rane P, Parekh R, Zacker C . Content analysis of FDA warning letters to manufacturers of pharmaceuticals and therapeutic biologicals for promotional violations . Drug Inf J . 2009. ; 43 ( 4 ): 385 – 393 . doi: 10.1177/009286150904300401 . [Google Scholar]
- 18. Arnold DG . The ethics of direct-to-consumer pharmaceutical advertising . In: Arnold DG , ed. Ethics and the Business of Biomedicine . New York, NY: : Cambridge University Press; ; 2009. : 131 – 149 . [Google Scholar]
- 19. Calfee JE . Public policy issues in direct-to-consumer advertising of prescription drugs . J Public Policy Mark . 2002. ; 21 ( 2 ): 174 – 193 . doi: 10.1509/jppm.21.2.174.17580 . [Google Scholar]
- 20. Morris LA, Mazis MB, Brinberg D . Risk disclosures in televised prescription drug advertising to consumers . J Public Policy Mark . 1989. ; 8 : 64 – 80 . [Google Scholar]
- 21. Grabell M, Eiserer T . Girlfriend of slain musician cites anti-smoking drug . The Dallas Morning News . September 5, 2007. . [Google Scholar]
- 22. Moore TJ, Glenmullen J, Furberg CD . Thoughts and acts of aggression/violence toward others reported in association with varenicline . Ann Pharmacother . 2010. ; 44 ( 9 ): 1389 – 1394 . doi: 10.1345/aph.1P172 . [DOI] [PubMed] [Google Scholar]
- 23. U.S. Food and Drug Administration . FDA Issues Public Health Advisory on Chantix . Washington, DC: : U.S. Food and Drug Administration; ; 2008. . www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116849.htm . Accessed November 14, 2014 . [Google Scholar]
- 24. U.S. Food and Drug Administration . Boxed Warning on Serious Mental Health Events to be Required for Chantix and Zyban . Washington, DC: : FDA; ; 2009. . www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm170100.htm . Accessed November 20, 2014 . [Google Scholar]
- 25. Rosenthal MB, Berndt ER, Donohue JM, Epstein AM, Frank RG . Demand Effects of Recent Changes in Prescription Drug Promotion . National Bureau of Economic Research, Inc; ; 2003. : 1 – 26 . http://ideas.repec.org/h/nbr/nberch/9862.html . Accessed April 1, 2013 . [Google Scholar]
- 26. Kornfield R, Alexander GC, Qato DM, Kim Y, Hirsch JD, Emery SL . Trends in exposure to televised prescription drug advertising, 2003–2011 . Am J Prev Med . 2014. ; 48 ( 5 ): 575 – 579 . doi: 10.1016/j.amepre.2014.12.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perry JE, Cox AD, Cox D . Direct-to-consumer drug advertisements and the informed patient: a legal, ethical, and content analysis . Am Bus Law J . 2013. ; 50 ( 4 ): 729 – 778 . doi: 10.1111/ablj.12019 . [Google Scholar]
- 28. Chaloupka F, Levy D, Huang J . The Impact of Tax and Smoke-Free Air Policy Changes . Robert Wood Johnson Foundation; ; 2011. . www.rwjf.org/content/rwjf/en/research-publications/find-rwjf-research/2011/03/preface/the-impact-of-tax-and-smoke-free-air-policy-changes.html . Accessed October 8, 2012 . [Google Scholar]
- 29. Centers for Disease Control and Prevention . The Tax Burden on Tobacco Volume 49, 1970–2014 . Atlanta, GA: : Centers for Disease Control and Prevention; ; 2015. . https://chronicdata.cdc.gov/Policy/The-Tax-Burden-on-Tobacco-Volume-49-1970-2014/7nwe-3aj9 . Accessed June 29, 2015 . [Google Scholar]
- 30. Liang K-Y, Zeger SL . Longitudinal data analysis using generalized linear models . Biometrika . 1986. ; 73 ( 1 ): 13 – 22 . [Google Scholar]
- 31. Fitzmaurice GM, Laird NM, Ware JH . Applied Longitudinal Analysis . 1 st ed. New York, NY: : John Wiley & Sons; ; 2004. . [Google Scholar]
- 32. Gonzales D, Rennard SI, Nides M, et al. . Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial . JAMA . 2006. ; 296 ( 1 ): 47 – 55 . doi: 10.1001/jama.296.1.47 . [DOI] [PubMed] [Google Scholar]
- 33. Gibbons RD, Mann JJ . Varenicline, smoking cessation, and neuropsychiatric adverse events . Am J Psychiatry . 2013. ; 170 ( 12 ): 1460 – 1467 . doi: 10.1176/appi.ajp.2013.12121599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cahill K, Stevens S, Perera R, Lancaster T . Pharmacological interventions for smoking cessation: an overview and network meta-analysis . Cochrane Database Syst Rev . 2013. ; 5 : CD009329 . doi: 10.1002/14651858.CD009329.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kasza KA, Cummings KM, Carpenter MJ, Cornelius ME, Hyland AJ, Fong GT . Use of stop-smoking medications in the United States before and after the introduction of varenicline . Addiction . 2015. ; 110 ( 2 ): 346 – 355 . doi: 10.1111/add.12778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vangeli E, West R . Sociodemographic differences in triggers to quit smoking: Findings from a national survey . Tob Control . 2008. ; 17 ( 6 ): 410 – 415 . doi: 10.1136/tc.2008.025650 . [DOI] [PubMed] [Google Scholar]
- 37. Hammond D, Reid JL, Driezen P, et al. . Smokers’ use of nicotine replacement therapy for reasons other than stopping smoking: findings from the ITC Four Country Survey . Addiction . 2008. ; 103 ( 10 ): 1696 – 1703 . doi: 10.1111/j.1360-0443.2008.02320.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kopp SW, Bang H-K . Benefit and risk information in prescription drug advertising . Health Mark Q . 2000. ; 17 ( 3 ): 39 – 56 . doi: 10.1300/J026v17n03_04 . [DOI] [PubMed] [Google Scholar]
- 39. Connors AL . Big bad pharma: an ethical analysis of physician-directed and consumer-directed marketing tactics . Albany Law Rev . 2009. ; 73 : 243 – 282 . [PubMed] [Google Scholar]
- 40. Shuchman M . Drug risks and free speech—Can Congress ban consumer drug ads? N Engl J Med . 2007. ; 356 ( 22 ): 2236 – 2239 . doi: 10.1056/NEJMp078080 . [DOI] [PubMed] [Google Scholar]
- 41. Donohue JM, Cevasco M, Rosenthal MB . A decade of direct-to-consumer advertising of prescription drugs . N Engl J Med . 2007. ; 357 ( 7 ): 673 – 681 . doi: 10.1056/NEJMsa070502 . [DOI] [PubMed] [Google Scholar]
- 42. Ventola CL . Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? Pharm Ther . 2011. ; 36 ( 10 ): 669 – 684 . www.ncbi.nlm.nih.gov/pmc/articles/PMC3278148/pdf/ptj3610669.pdf . Accessed June 25, 2015 . [PMC free article] [PubMed] [Google Scholar]
- 43. Liang BA, Mackey T . Reforming direct-to-consumer advertising . Nat Biotech . 2011. ; 29 ( 5 ): 397 – 400 . doi: 10.1038/nbt.1865 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.